Implementing a Geriatric Assessment-Guided Rehabilitation Care Model in Community Oncology Care: Feasibility and Impact on Patient-Reported and Performance-Based Outcomes †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
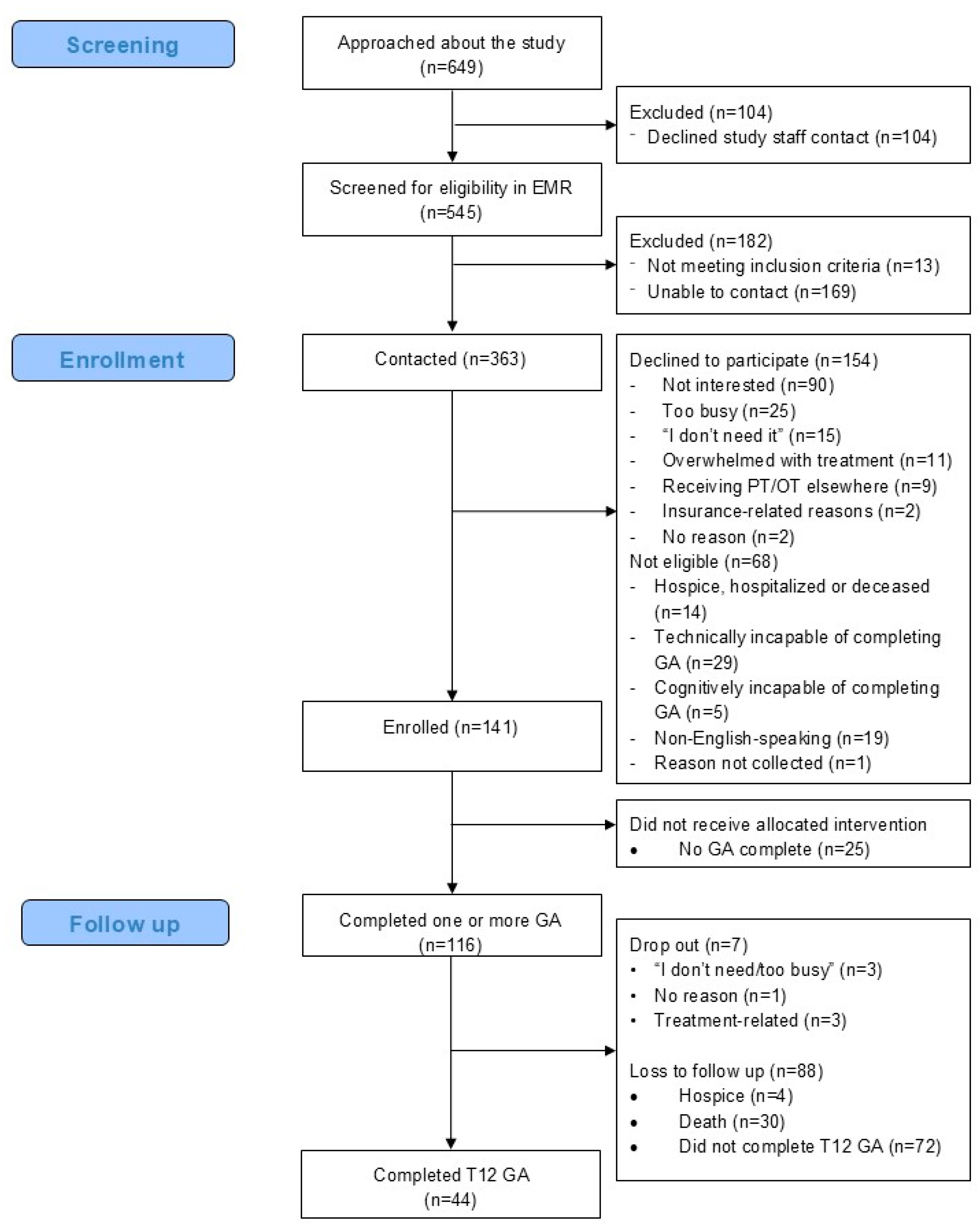
2.3. Recruitment and Enrollment
2.4. Data Collection
2.5. Intervention
2.6. Implementation Strategies
2.7. Feasibility Outcomes
2.8. Acceptability Outcomes
2.9. Patient-Reported and Performance-Based Outcomes
2.10. Statistical Analyses
3. Results
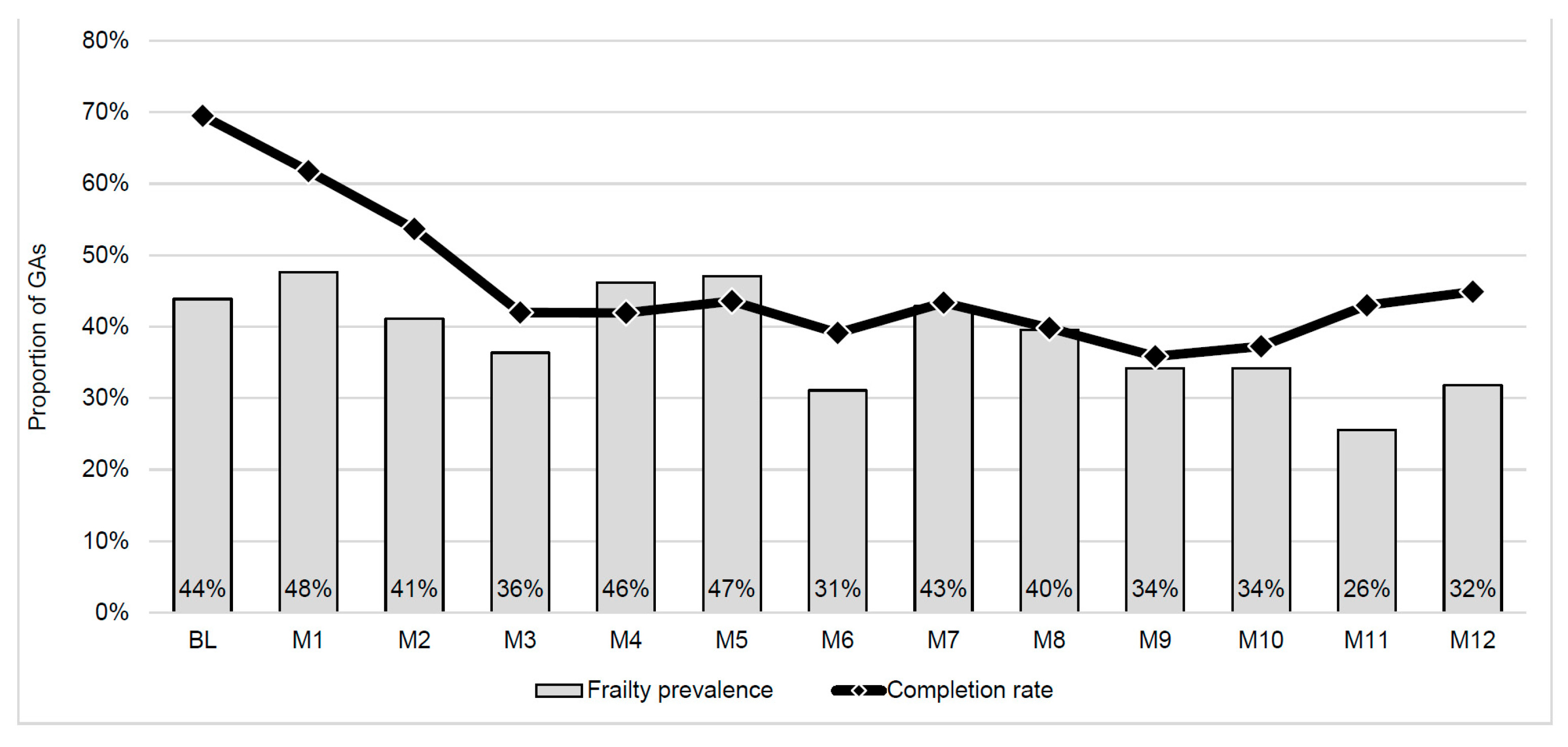
3.1. Feasibility
3.2. Participant Acceptability
3.3. Rehabilitation Outcomes
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goede, V. Frailty and cancer: Current perspectives on assessment and monitoring. Clin. Interv. Aging 2023, 18, 505–521. [Google Scholar] [CrossRef]
- Williams, G.R.; Deal, A.M.; Sanoff, H.K.; Nyrop, K.A.; Guerard, E.J.; Pergolotti, M.; Shachar, S.S.; Reeve, B.B.; Bensen, J.T.; Choi, S.K.; et al. Frailty and health-related quality of life in older women with breast cancer. Support. Care Cancer 2019, 27, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; Rosko, A.E.; Spakowicz, D.J.; Hammer, M.J.; Von Ah, D. Associations of frailty with symptoms, and HRQOL in older cancer survivors after cancer treatments: A systematic review and meta-analyses. Qual. Life Res. 2024, 33, 583–598. [Google Scholar] [CrossRef]
- Mozessohn, L.; Li, Q.; Liu, N.; Leber, B.; Khalaf, D.; Sabloff, M.; Christou, G.; Yee, K.; Chodirker, L.; Parmentier, A.; et al. Impact of Frailty on Health Care Resource Utilization and Costs of Care in Myelodysplastic Syndromes. JCO Oncol. Pract. 2023, 19, e559–e569. [Google Scholar] [CrossRef]
- Min, G.J.; Cho, B.S.; Park, S.-S.; Park, S.; Jeon, Y.-W.; Shin, S.H.; Yahng, S.A.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; et al. Physical and Psychological Impairments as Practical Frailty Markers in Elderly AML Fit for Intensive Chemotherapy; Interim Data of a Prospective Cohort Study. Blood 2019, 134, 3831. [Google Scholar] [CrossRef]
- Dale, W.; Klepin, H.D.; Williams, G.R.; Alibhai, S.M.H.; Bergerot, C.; Brintzenhofeszoc, K.; Hopkins, J.O.; Jhawer, M.P.; Katheria, V.; Loh, K.P.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Systemic Cancer Therapy: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 4293–4312. [Google Scholar] [CrossRef]
- Williams, G.R.; Deal, A.M.; Jolly, T.A.; Alston, S.M.; Gordon, B.B.E.; Olajide, O.A.; Taylor, W.C.; Messino, M.J.; Muss, H.B. Feasibility of geriatric assessment in community oncology clinics. J. Geriatr. Oncol. 2014, 5, 245–251. [Google Scholar] [CrossRef]
- Williams, G.R.; Weaver, K.E.; Lesser, G.J.; Dressler, E.; Winkfield, K.M.; Neuman, H.B.; Kazak, A.E.; Carlos, R.; Gansauer, L.J.; Kamen, C.S.; et al. Capacity to provide geriatric specialty care for older adults in community oncology practices. Oncologist 2020, 25, 1032–1038. [Google Scholar] [CrossRef]
- Giri, S.; Mir, N.; Al-Obaidi, M.; Clark, D.; Kenzik, K.M.; McDonald, A.; Young-Smith, C.; Paluri, R.; Nandagopal, L.; Gbolahan, O.; et al. Use of Single-Item Self-Rated Health Measure to Identify Frailty and Geriatric Assessment-Identified Impairments Among Older Adults with Cancer. Oncologist 2022, 27, e45–e52. [Google Scholar] [CrossRef]
- Giri, S.; Al-Obaidi, M.; Weaver, A.; Kenzik, K.M.; McDonald, A.; Clark, D.; Young-Smith, C.; Paluri, R.; Nandagopal, L.; Gbolahan, O.; et al. Association Between Chronologic Age and Geriatric Assessment-Identified Impairments: Findings from the CARE Registry. J. Natl. Compr. Cancer Netw. 2021, 19, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Segaux, L.; Broussier, A.; Oubaya, N.; Leissing-Desprez, C.; Laurent, M.; Naga, H.; Fromentin, I.; David, J.P.; Bastuji-Garin, S. Several frailty parameters highly prevalent in middle age (50–65) are independent predictors of adverse events. Sci. Rep. 2021, 11, 8774. [Google Scholar] [CrossRef]
- Park, J.; Look, K.A. Health Care Expenditure Burden of Cancer Care in the United States. Inquiry 2019, 56, 0046958019880696. [Google Scholar] [CrossRef]
- Al Obaidi, M.; Giri, S.; Mir, N.; Kenzik, K.; McDonald, A.M.; Smith, C.Y.; Gbolahan, O.B.; Paluri, R.K.; Bhatia, S.; Williams, G.R. Use of self-rated health to identify frailty and predict mortality in older adults with cancer. Results from the care study. J. Clin. Oncol. 2020, 38, 12046. [Google Scholar] [CrossRef]
- van Abbema, D.L.; van den Akker, M.; Janssen-Heijnen, M.L.; van den Berkmortel, F.; Hoeben, A.; de Vos-Geelen, J.; Buntinx, F.; Kleijnen, J.; Tjan-Heijnen, V.C.G. Patient- and tumor-related predictors of chemotherapy intolerance in older patients with cancer: A systematic review. J. Geriatr. Oncol. 2019, 10, 31–41. [Google Scholar] [CrossRef]
- Sikorskii, A.; Tam, S.; Given, B.; Given, C.W.; Adjei Boakye, E.; Zatirka, T.; Nair, M.; Su, W.-T.K.; Jogunoori, S.; Watson, P.; et al. Thresholds in PROMIS scores anchored to subsequent unscheduled health service use among people diagnosed with cancer. JCO Oncol. Pract. 2024, 20, 1391–1400. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Shun, S.-C.; Lai, Y.-H.; Liang, J.-T.; Tsauo, J.-Y. Comparison of the Effects of a Supervised Exercise Program and Usual Care in Patients with Colorectal Cancer Undergoing Chemotherapy. Cancer Nurs. 2014, 37, E21–E29. [Google Scholar] [CrossRef] [PubMed]
- Moullin, J.C.; Dickson, K.S.; Stadnick, N.A.; Rabin, B.; Aarons, G.A. Systematic review of the Exploration, Preparation, Implementation, Sustainment (EPIS) framework. Implement. Sci. 2019, 14, 1. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Rabin, B.; Aarons, G.A. CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Rabin, B.; Aarons, G.A. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Williams, G.R.; Kenzik, K.M.; Parman, M.; Al-Obaidi, M.; Francisco, L.; Rocque, G.B.; McDonald, A.; Paluri, R.; Navari, R.M.; Nandagopal, L.; et al. Integrating geriatric assessment into routine gastrointestinal (GI) consultation: The Cancer and Aging Resilience Evaluation (CARE). J. Geriatr. Oncol. 2019, 11, 270–273. [Google Scholar] [CrossRef]
- Pergolotti, M.; Wood, K.C.; Hidde, M.; Tendig, T.D.; Ronnen, E.A.; Giri, S.; Williams, G.R. Geriatric assessment-identified impairments and frailty in adults with cancer younger than 65: An opportunity to optimize oncology care. J. Geriatr. Oncol. 2024, 15, 101751. [Google Scholar] [CrossRef]
- Guerard, E.J.; Deal, A.M.; Williams, G.R.; Jolly, T.A.; Wood, W.A.; Muss, H.B. Construction of a frailty index for older adults with cancer using a geriatric assessment. J. Clin. Oncol. 2015, 33, 9535. [Google Scholar] [CrossRef]
- Giri, S.; Al-Obaidi, M.; Harmon, C.; Clark, D.; Ubersax, C.; Dai, C.; Young-Smith, C.; Outlaw, D.; Gbolahan, O.; Khushman, M.; et al. Patient-reported geriatric assessment-based frailty index among older adults with gastrointestinal malignancies. J. Am. Geriatr. Soc. 2023, 71, 136–144. [Google Scholar] [CrossRef]
- Harris, L.K.; Skou, S.T.; Juhl, C.B.; Jäger, M.; Bricca, A. Recruitment and retention rates in randomised controlled trials of exercise therapy in people with multimorbidity: A systematic review and meta-analysis. Trials 2021, 22, 396. [Google Scholar] [CrossRef]
- Reynolds, S.A.; O’Connor, L.; McGee, A.; Kilcoyne, A.Q.; Connolly, A.; Mockler, D.; Guinan, E.; O’Neill, L. Recruitment rates and strategies in exercise trials in cancer survivorship: A systematic review. J. Cancer Surviv. 2024, 18, 1233–1242. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
- Pergolotti, M.; Wood, K.; Kendig, T. Pragmatic test-retest and concurrent reliability of three PROMIS® measures in breast cancer rehabilitation. Arch. Phys. Med. Rehabil. 2024, 105, e63. [Google Scholar] [CrossRef]
- Pergolotti, M.; Wood, K.C.; Kendig, T.D.; Mayo, S. Impact of Real-World Outpatient Cancer Rehabilitation Services on Health-Related Quality of Life of Cancer Survivors across 12 Diagnosis Types in the United States. Cancers 2024, 16, 1927. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.X.M.; Park, J.; Lee, J.; Jung, Y.-S.; Chang, Y.; Cho, H. Utility of the Patient-Reported Outcomes Measurement Information System (PROMIS) to measure primary health outcomes in cancer patients: A systematic review. Support. Care Cancer 2020, 29, 1723–1739. [Google Scholar] [CrossRef]
- Jensen, R.E.; Moinpour, C.M.; Potosky, A.L.; Lobo, T.; Hahn, E.A.; Hays, R.D.; Cella, D.; Smith, A.W.; Wu, X.C.; Keegan, T.H.M.; et al. Responsiveness of 8 PROMIS® Measures in a Large, Community-Based Cancer Study Cohort. Cancer 2017, 123, 327. [Google Scholar] [CrossRef]
- Terwee, C.B.; Peipert, J.D.; Chapman, R.; Lai, J.S.; Terluin, B.; Cella, D.; Griffith, P.; Mokkink, L.B. Minimal important change (MIC): A conceptual clarification and systematic review of MIC estimates of PROMIS measures. Qual. Life Res. 2021, 30, 2729–2754. [Google Scholar] [CrossRef]
- Labott, B.K.; Bucht, H.; Morat, M.; Morat, T.; Donath, L. Effects of Exercise Training on Handgrip Strength in Older Adults: A Meta-Analytical Review. Gerontology 2019, 65, 686–698. [Google Scholar] [CrossRef]
- Fisher, M.I.; Lee, J.; Davies, C.C.; Geyer, H.; Colon, G.; Pfalzer, L. Oncology section EDGE task force on breast cancer outcomes: A systematic review of outcome measures for functional mobility. Rehabil. Oncol. 2015, 33, 19–31. [Google Scholar] [CrossRef]
- Zanini, A.; Crisafulli, E.; D’Andria, M.; Gregorini, C.; Cherubino, F.; Zampogna, E.; Azzola, A.; Spanevello, A.; Chetta, A. Minimal clinically important difference in 30 second sit-to-stand test after pulmonary rehabilitation in patients with COPD. Eur. Respir. J. 2018, 52, OA5199. [Google Scholar] [CrossRef]
- Abbade, L.P.F.; Abbade, J.F.; Thabane, L. Introducing the CONSORT extension to pilot trials: Enhancing the design, conduct and reporting of pilot or feasibility trials. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.A.; Almeida, T.D.N.; Andrade, G.S.; Ribeiro, A.S.; Rêgo, A.S.; da Silva Dias, R.; Ferreira, P.R.; Penha, L.R.L.N.; de Oliveira Pires, F.; Dibai-Filho, A.V.; et al. Reliability and Accuracy of 2-Minute Step Test in Active and Sedentary Lean Adults. J. Manip. Physiol. Ther. 2021, 44, 120–127. [Google Scholar] [CrossRef]
- Lund, C.M.; Vistisen, K.K.; Olsen, A.P.; Bardal, P.; Schultz, M.; Dolin, T.G.; Rønholt, F.; Johansen, J.S.; Nielsen, D.L. The effect of geriatric intervention in frail older patients receiving chemotherapy for colorectal cancer: A randomised trial (GERICO). Clin. Study Br. J. Cancer 2021, 124, 1949–1958. [Google Scholar] [CrossRef]
- Ørum, M.; Eriksen, S.V.; Gregersen, M.; Jensen, A.R.; Jensen, K.; Meldgaard, P.; Nordsmark, M.; Damsgaard, E.M. The impact of a tailored follow-up intervention on comprehensive geriatric assessment in older patients with cancer—A randomised controlled trial. J. Geriatr. Oncol. 2021, 12, 41–48. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.L.; Kim, H.; Soto-Perez-De-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric Assessment-Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults with Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef]
- Mohile, S.G.; Mohamed, M.R.; Xu, H.; Culakova, E.; Loh, K.P.; Magnuson, A.; Flannery, M.A.; Obrecht, S.; Gilmore, N.; Ramsdale, E.; et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): A cluster-randomised study. Lancet 2021, 398, 1894–1904. [Google Scholar] [CrossRef]
- Mohile, S.G.; Epstein, R.M.; Hurria, A.; Heckler, C.E.; Canin, B.; Culakova, E.; Duberstein, P.; Gilmore, N.; Xu, H.; Plumb, S.; et al. Communication with Older Patients with Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2020, 6, 196. [Google Scholar] [CrossRef]
- Ørum, M.; Jensen, K.; Gregersen, M.; Meldgaard, P.; Damsgaard, E.M. Impact of comprehensive geriatric assessment on short-term mortality in older patients with cancer-a follow-up study. Eur. J. Cancer 2019, 116, 27–34. [Google Scholar] [CrossRef]
- Brick, R.; Natori, A.; Moreno, P.I.; Molinares, D.; Koru-Sengul, T.; Penedo, F.J. Predictors of cancer rehabilitation medicine referral and utilization based on the Moving Through Cancer physical activity screening assessment. Support. Care Cancer 2023, 31, 216. [Google Scholar] [CrossRef] [PubMed]
- Pergolotti, M.; Alfano, C.M.; Cernich, A.N.; Yabroff, K.R.; Manning, P.R.; de Moor, J.S.; Hahn, E.E.; Cheville, A.L.; Mohile, S.G. A health services research agenda to fully integrate cancer rehabilitation into oncology care. Cancer 2019, 125, 3908–3916. [Google Scholar] [CrossRef] [PubMed]
- Pergolotti, M.; Deal, A.M.; Lavery, J.; Reeve, B.B.; Muss, H.B. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. J. Geriatr. Oncol. 2015, 6, 194–201. [Google Scholar] [CrossRef]
- Dale, W.; Williams, G.R.; RMacKenzie, A.; Soto-Perez-de-Celis, E.; Maggiore, R.J.; Merrill, J.K.; Katta, S.; Smith, K.T.; Klepin, H.D. How Is Geriatric Assessment Used in Clinical Practice for Older Adults with Cancer? A Survey of Cancer Providers by the American Society of Clinical Oncology. JCO Oncol. Pract. 2021, 17, 336–344. [Google Scholar] [CrossRef]
- Sum, G.; Nicholas, S.O.; Nai, Z.L.; Ding, Y.Y.; Tan, W.S. Health outcomes and implementation barriers and facilitators of comprehensive geriatric assessment in community settings: A systematic integrative review. BMC Geriatr. 2022, 22, 379. [Google Scholar] [CrossRef] [PubMed]
- Pergolotti, M.; Deal, A.M.; Williams, G.R.; Bryant, A.L.; McCarthy, L.; Nyrop, K.A.; Covington, K.R.; Reeve, B.B.; Basch, E.; Muss, H.B. A randomized controlled trial of outpatient CAncer REhabilitation for older adults: The CARE Program. Contemp. Clin. Trials 2015, 44, 89–94. [Google Scholar] [CrossRef]
- Penna, G.B.; Otto, D.M.; da Silva, T.C.; Pedroni, A.S.; Macagnan, F.E. Physical rehabilitation for the management of cancer-related fatigue during cytotoxic treatment: A systematic review with meta-analysis. Support. Care Cancer 2023, 31, 129. [Google Scholar] [CrossRef]
- Brick, R.; Lyons, K.D.; Bender, C.; Eilers, R.; Ferguson, R.; Pergolotti, M.; Toto, P.; Skidmore, E.; Leland, N.E. Factors influencing utilization of cancer rehabilitation services among older breast cancer survivors in the USA: A qualitative study. Support. Care Cancer 2022, 30, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Santa Mina, D.; Lyons, K.D.; Robb, K.; Silver, J.K. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J. Clin. 2020, 71, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Bohlke, K.; Alfano, C.M. Exercise, Diet, and Weight Management During Cancer Treatment: ASCO Guideline Summary and Q&A. JCO Oncol. Pract. 2022, 18, OP2200277. [Google Scholar] [CrossRef]
- National Accreditation for Breast Centers (NAPBC). Optimal Resources for Breast Cancer: 2024 Standards; NAPBC: Chicago, IL, USA, 2024. [Google Scholar]
- American College of Surgeons. Optimal Resources for Cancer Care: 2020 Standards; American College of Surgeons: Chicago, IL, USA, 2020. [Google Scholar]
- Lin, K.Y.; Cheng, H.C.; Yen, C.J.; Hung, C.H.; Haung, Y.T.; Yang, H.L.; Cheng, W.T.; Tsai, K.L. Effects of Exercise in Patients Undergoing Chemotherapy for Head and Neck Cancer: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 1291. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Vasan, S.; Luo, J.; Binder, A.M.; Rowan, S.; Chlebowski, T.; Quesenberry, C.; Banack, H.R.; Caan, B.J.; Paskett, E.D.; et al. Long-term Trajectories of Physical Function Decline in Women with and Without Cancer. JAMA Oncol. 2023, 9, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Soto-Perez-de-Celis, E.; Allred, J.B.; Cohen, H.J.; Arsenyan, A.; Ballman, K.; Le-Rademacher, J.; Jatoi, A.; Filo, J.; Mandelblatt, J.; et al. Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J. Am. Geriatr. Soc. 2019, 67, 920–927. [Google Scholar] [CrossRef]
| All Enrolled (n = 141) | Completed at Least 1 GA (n = 116) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age | 63.64 ± 10.81 | 64.15 ± 10.83 | 0.227 |
| Sex | 0.416 | ||
| Male | 52, 36.9% | 41, 35.3% | |
| Female | 89, 63.1% | 75, 64.7% | |
| Race | 0.871 | ||
| White | 88, 62.4% | 77, 66.4% | |
| Black | 14, 9.9% | 12, 10.3% | |
| Asian | 6, 4.26% | 5, 4.3% | |
| Other | 9, 6.38% | 7, 6.0% | |
| Unknown or missing | 24, 17.0% | 15, 12.9% | |
| Ethnicity (Hispanic/Latino) | 8, 5.7% | 7, 7.1% | 0.947 |
| Cancer diagnosis | |||
| Cancer type | 0.179 | ||
| Breast | 49, 34.8% | 43, 37.1% | |
| Head and neck | 4, 2.8% | 3, 2.6% | |
| Hematologic | 25, 17.7% | 20, 17.2% | |
| Gynecologic | 9, 6.4% | 7, 6.0% | |
| Genitourinary | 7, 5.0% | 7, 6.0% | |
| Gastrointestinal | 28, 19.9% | 22, 9.0% | |
| Lung | 16, 11.3% | 13, 11.2% | |
| Other | 3, 2.1% | 0, 0% | |
| Stage | 0.646 | ||
| 0 to 2 | 44, 31.2% | 38, 32.76 | |
| 3 to 4 | 37, 26.2% | 61, 52.59% | |
| Unknown | 23, 16.3% | 14.66% | |
| Recurrence (at time of recruitment) | 24, 17.0% | 21, 18.3% | 0.452 |
| Treatment information | |||
| First line therapy (Yes) | 101, 71.6% | 82, 70.7% | 0.593 |
| Number of cycles planned | 11.66 ± 7.72 | 11.70 ± 7.88 | 0.892 |
| Completed all cycles | 58, 41.1% | 47, 40.5% | 0.748 |
| Started new therapy during study | 75, 53.2% | 59, 50.9% | 0.324 |
| n, % or Median (IQR) | |
|---|---|
| Service type | |
| Physical therapy (PT) | 39, 84.8% |
| Occupational therapy (OT) | 1, 2.2% |
| Multidiscipline (PT/OT) | 5, 10.9% |
| Speech | 1, 2.2% |
| Insurance type | |
| Private | 27 (57.4%) |
| Medicare/Medicaid/Medicare Advantage | 19 (41.3%) |
| Rehabilitation needs a | |
| Falls | 27, 21.6% |
| Mobility | 20, 16.0% |
| Pain | 18, 14.4% |
| Weakness | 16, 12.8% |
| Fatigue | 12, 9.6% |
| Limited ROM | 12, 9.6% |
| ADL limitation | 10, 8.0% |
| Neuropathy | 5, 4.0% |
| Other | 3, 2.4% |
| Lymphedema | 2, 1.6% |
| Length of stay | 17.84 (5.96 to 20.71) |
| Visits attended | 12.00 (5.50 to 25.25) |
| Discharge reason | |
| Program complete/goals met | 15, 32.6% |
| Patient choice | 11, 23.9% |
| Hospitalized | 7, 15.2% |
| Surgery | 3, 6.0% |
| Hospice | 3, 6.0% |
| Physician discretion due to disease progression Unknown | 1, 2.0% 6, 13% |
| Question | Average Rating (SD) | % “Agreed” |
|---|---|---|
| The monthly evaluation was simple to complete online | 4.70 ± 0.63 | 97.73% |
| The amount of time it took me to complete the online monthly evaluation was appropriate | 4.57 ± 0.97 | 95.45% |
| The online monthly evaluation was helpful to monitor for potential side effects of cancer treatment | 4.16 ± 1.24 | 86.36% |
| I feel the online monthly evaluation helped me to have access to multidisciplinary care when I needed it | 3.50 ± 1.72 | 72.72% |
| I would recommend the online monthly evaluation to others who are starting a new cancer treatment | 4.16 ± 1.31 | 88.64% |
| The online monthly evaluation was an added benefit of receiving my care | 4.07 ± 1.45 | 81.82% |
| I feel that my therapist(s) used my monthly evaluation to individualize my care * | 3.79 ± 1.38 | 87.50% |
| Specific Domains Measured | Initial Evaluation Mean, SD | Final Evaluation Mean, SD | Change Mean, SD | Change T-Score | Percent Achieving MIC | |
|---|---|---|---|---|---|---|
| Global physical health | Overall health, ability to perform daily activities, fatigue, pain. | 39.38, 7.12 | 43.30, 7.48 | +3.92, 6.86 | 3.18 * | 58.10% |
| Global mental health | Mood, ability to think, feelings of anxiety or depression, satisfaction with social activities and relationships. | 45.76, 7.98 | 49.03, 7.17 | +3.27, 7.89 | 2.31 * | 54.80% |
| Physical function | Ability to do household chores, walk up/down stairs, walk at least 15 min, and run errands. | 37.73, 8.35 | 40.79, 8.00 | +3.06, 7.12 | 2.40 * | 54.80% |
| Ability to participate in social roles and activities | Ability to do leisure and family activities, work, and activities with friends. | 45.18, 8.11 | 47.49, 7.47 | +2.31, 6.82 | 1.89 * | 41.90% |
| n | Initial Evaluation Mean, SD | Discharge Mean, SD | Change Mean, SD | Change T-Score | MIC Value | Percent Achieving MIC | |
|---|---|---|---|---|---|---|---|
| Mobility | |||||||
| Timed Up-and-Go (seconds) | 27 | 15.16, 6.23 | 12.81, 5.31 | −2.34, 4.15 | 2.93 * | −1 s [35] | 48.1% |
| Aerobic capacity | |||||||
| Six-minute walk test (feet) | 9 | 641.67, 375.77 | 863.44, 347.61 | +221.78, 235.5 | 2.83 * | +65.6 feet [35] | 88.9% |
| Two-minute step test (step repetitions) | 5 | 57.00, 20.25 | 67.80, 19.72 | +10.80, 5.45 | 4.43 * | +9.61 [38] | 100% |
| Strength | |||||||
| Handgrip strength (lbs.) | 19 | 45.00, 12.31 | 47.52, 13.64 | +2.52, 11.86^ | 0.93 | +3.53 lbs [34] | 47.4% |
| Five times sit-to-stand (seconds) | 19 | 18.26, 5.67 | 15.44, 6.33 | −2.82, 3.3 | 3.72 * | −2.3 s [35] | 63.2% |
| 30 s sit-to-stand (repetitions) | 12 | 10.00, 4.49 | 12.50, 4.93 | 2.50, 4.81 | 1.80 * | +2 repetitions [36] | 58.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergolotti, M.; Wood, K.C.; Hidde, M.; Kendig, T.D.; Meehan, D.; Hutzayluk, K.; Newell, A.M.; Bertram, J.; Lightner, A.; Mayo, S.; et al. Implementing a Geriatric Assessment-Guided Rehabilitation Care Model in Community Oncology Care: Feasibility and Impact on Patient-Reported and Performance-Based Outcomes. Cancers 2025, 17, 3274. https://doi.org/10.3390/cancers17193274
Pergolotti M, Wood KC, Hidde M, Kendig TD, Meehan D, Hutzayluk K, Newell AM, Bertram J, Lightner A, Mayo S, et al. Implementing a Geriatric Assessment-Guided Rehabilitation Care Model in Community Oncology Care: Feasibility and Impact on Patient-Reported and Performance-Based Outcomes. Cancers. 2025; 17(19):3274. https://doi.org/10.3390/cancers17193274
Chicago/Turabian StylePergolotti, Mackenzi, Kelley C. Wood, Mary Hidde, Tiffany D. Kendig, Deanna Meehan, Katie Hutzayluk, Alaina M. Newell, Jessica Bertram, Ashley Lightner, Stacye Mayo, and et al. 2025. "Implementing a Geriatric Assessment-Guided Rehabilitation Care Model in Community Oncology Care: Feasibility and Impact on Patient-Reported and Performance-Based Outcomes" Cancers 17, no. 19: 3274. https://doi.org/10.3390/cancers17193274
APA StylePergolotti, M., Wood, K. C., Hidde, M., Kendig, T. D., Meehan, D., Hutzayluk, K., Newell, A. M., Bertram, J., Lightner, A., Mayo, S., Hedaya, A., Giri, S., & Williams, G. R. (2025). Implementing a Geriatric Assessment-Guided Rehabilitation Care Model in Community Oncology Care: Feasibility and Impact on Patient-Reported and Performance-Based Outcomes. Cancers, 17(19), 3274. https://doi.org/10.3390/cancers17193274






