Current Perspectives on Non-Metastatic Male Breast Cancer: Genetics, Biology, and Treatment Advances: A Systematic Review
Simple Summary
Abstract
1. Introduction
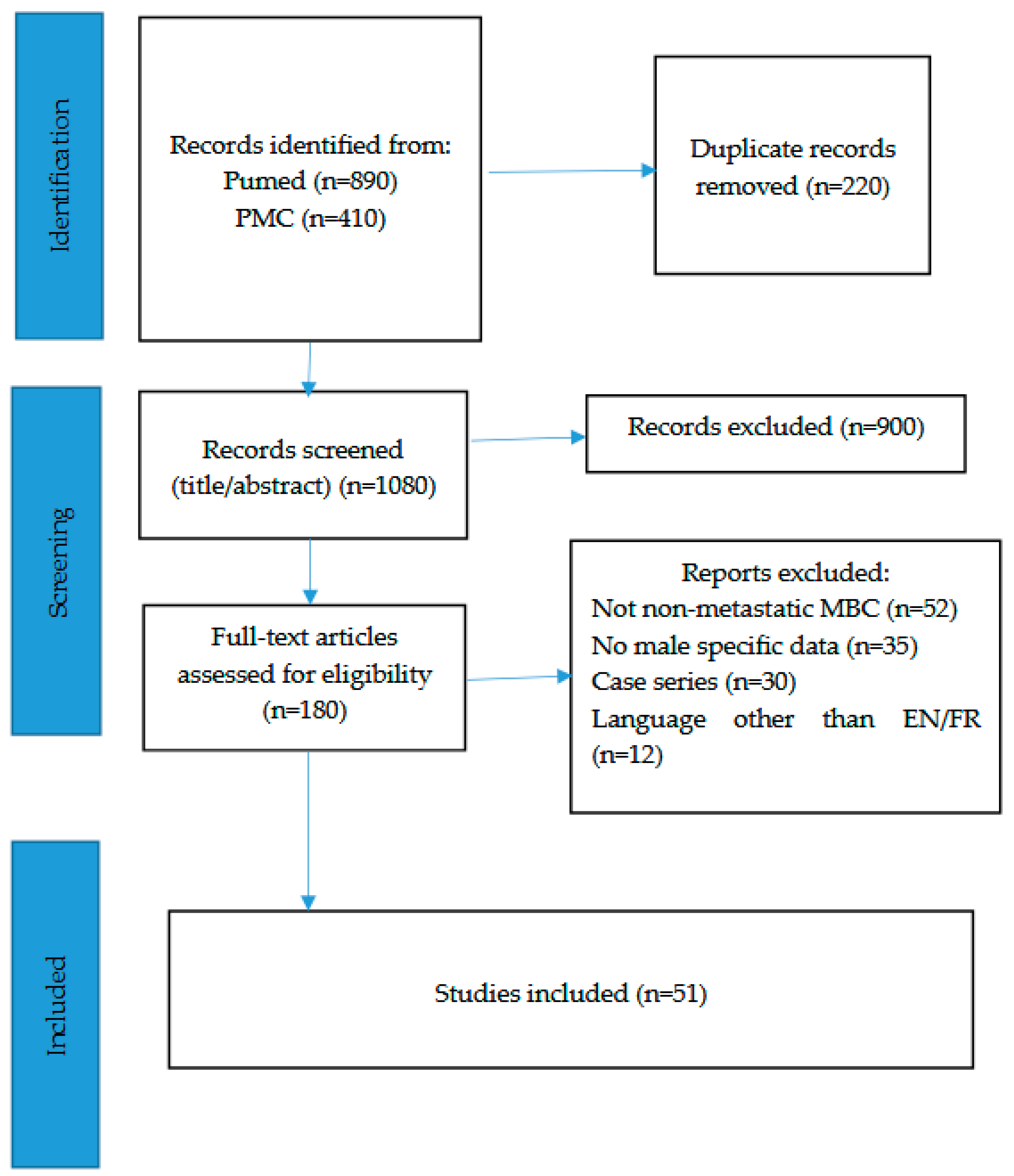
2. Materials and Methods
3. Results
3.1. Genetic Alterations Shaping Male Breast Cancer Risk
3.1.1. High-Penetrance Genes
3.1.2. Moderate-Penetrance Genes
3.1.3. Low-Penetrance Alleles and Susceptibility Loci
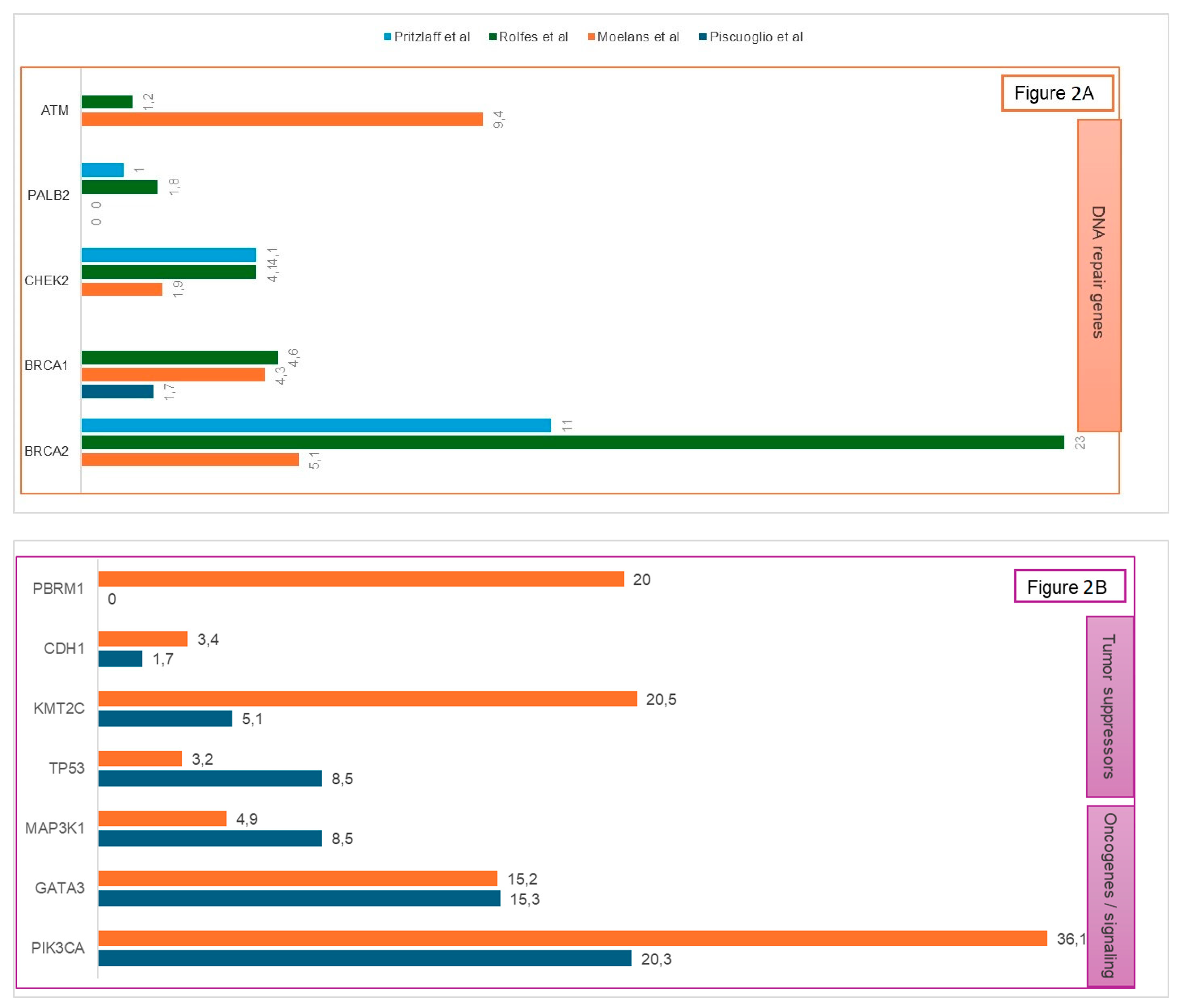
3.1.4. Somatic Alterations
3.1.5. Multigene Panels and Extended Spectrum
3.1.6. Epigenetic Alterations
3.2. Molecular Pathways and Clinicopathological Correlates in Male Breast Cancer
3.2.1. Histology and Hormone Receptor Status
3.2.2. Androgen Receptor (AR) Expression
3.2.3. HER2 Overexpression
3.2.4. Molecular Subtypes
3.2.5. Tumor Microenvironment and Lipid Metabolism
3.2.6. Emerging Biomarkers
3.2.7. Clinical Presentation and Prognostic Factors
3.3. Treatment Advances in Non-Metastatic Male Breast Cancer
3.3.1. Is Breast-Conserving Surgery a Safe and Feasible Alternative to Mastectomy?
3.3.2. Axillary Staging: Sentinel Lymph Node Biopsy (SLNB) Performance for Male Breast Cancer
3.3.3. Adjuvant Radiotherapy in Male Breast Cancer
3.3.4. Adjuvant Endocrine Treatment: Survival Impact and Compliance Challenges in Male Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, P.; Shao, Q.; Jiang, D.; Zhao, Y.; Zeng, X. Global, Regional, and National Burden of Male Breast Cancer, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Cancer Med. 2025, 14, e70632. [Google Scholar] [CrossRef]
- Panorama des Cancers en France—Édition 2024. Available online: https://www.cancer.fr/catalogue-des-publications/panorama-des-cancers-en-france-edition-2024 (accessed on 16 June 2025).
- Black Men Have Higher Incidence Rates for All Types of Breast Cancer. Available online: https://www.cancer.org/research/acs-research-highlights/breast-cancer-research-highlights/risk-factors-and-prevention-studies-breast-cancer/black-men-have-higher-incidence-rates-for-all-types-of-breast-cancer.html (accessed on 16 June 2025).
- NCCN. Clinical Pratice Guideline in Oncology; Breast Cancer: Chicago, IL, USA, 2024. [Google Scholar]
- Gwark, S.; Kim, J.; Chung, I.Y.; Kim, H.J.; Ko, B.S.; Lee, J.W.; Son, B.H.; Ahn, S.H.; Lee, S.B. Survival pattern in male breast cancer: Distinct from female breast cancer. Front. Oncol. 2024, 14, 1392592. [Google Scholar] [CrossRef]
- Corrigan, K.L.; Mainwaring, W.; Miller, A.B.; Lin, T.A.; Jethanandani, A.; Espinoza, A.F.; Piotrowski, M.; Fuller, C.D.; Stauder, M.C.; Shaitelman, S.F.; et al. Exclusion of Men from Randomized Phase III Breast Cancer Clinical Trials. Oncologist 2020, 25, e990–e992. [Google Scholar] [CrossRef]
- Hassett, M.J.; Somerfield, M.R.; Baker, E.R.; Cardoso, F.; Kansal, K.J.; Kwait, D.C.; Plichta, J.K.; Ricker, C.; Roshal, A.; Ruddy, K.J.; et al. Management of Male Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1849–1863. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Rolfes, M.; Borde, J.; Möllenhoff, K.; Kayali, M.; Ernst, C.; Gehrig, A.; Sutter, C.; Ramser, J.; Niederacher, D.; Horváth, J.; et al. Prevalence of Cancer Predisposition Germline Variants in Male Breast Cancer Patients: Results of the German Consortium for Hereditary Breast and Ovarian Cancer. Cancers 2022, 14, 3292. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Lakhani, S.R.; Ottini, L.; Fox, S.B. The cancer genetics and pathology of male breast cancer. Histopathology 2016, 68, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pritzlaff, M.; Summerour, P.; McFarland, R.; Li, S.; Reineke, P.; Dolinsky, J.S.; Goldgar, D.E.; Shimelis, H.; Couch, F.J.; Chao, E.C.; et al. Male breast cancer in a multi-gene panel testing cohort: Insights and unexpected results. Breast Cancer Res. Treat. 2017, 161, 575–586. [Google Scholar] [CrossRef]
- Janatová, M.; Borecká, M.; Zemánková, P.; Matějková, K.; Nehasil, P.; Černá, L.; Černá, M.; Dušková, P.; Doležalová, T.; Foretová, L.; et al. Genetic Predisposition to Male Breast Cancer. Folia Biol. 2024, 70, 274–284. [Google Scholar] [CrossRef]
- Valentini, V.; Bucalo, A.; Conti, G.; Celli, L.; Porzio, V.; Capalbo, C.; Silvestri, V.; Ottini, L. Gender-Specific Genetic Predisposition to Breast Cancer: BRCA Genes and Beyond. Cancers. 2024. Available online: https://consensus.app/papers/genderspecific-genetic-predisposition-to-breast-cancer-conti-ottini/f90a6fde1b7d5ed58b1fbf61274719ad/ (accessed on 27 August 2025).
- Campos, F.A.B.; Rouleau, E.; Torrezan, G.T.; Carraro, D.M.; Casali da Rocha, J.C.; Mantovani, H.K.; da Silva, L.R.; Osório, C.A.B.d.T.; Sanches, S.M.; Caputo, S.M.; et al. Genetic Landscape of Male Breast Cancer. Cancers 2021, 13, 3535. [Google Scholar] [CrossRef]
- Rizzolo, P.; Zelli, V.; Silvestri, V.; Valentini, V.; Zanna, I.; Bianchi, S.; Masala, G.; Spinelli, A.M.; Tibiletti, M.G.; Russo, A.; et al. Insight into genetic susceptibility to male breast cancer by multigene panel testing: Results from a multicenter study in Italy. Int. J. Cancer 2019, 145, 390–400. [Google Scholar] [CrossRef]
- Chatterji, S.; Krzoska, E.; Thoroughgood, C.W.; Saganty, J.; Liu, P.; Elsberger, B.; Abu-Eid, R.; Speirs, V. Defining genomic, transcriptomic, proteomic, epigenetic, and phenotypic biomarkers with prognostic capability in male breast cancer: A systematic review. Lancet Oncol. 2023, 24, e74–e85. [Google Scholar] [CrossRef]
- Moelans, C.B.; de Ligt, J.; van der Groep, P.; Prins, P.; Besselink, N.J.M.; Hoogstraat, M.; ter Hoeve, N.D.; Lacle, M.M.; Kornegoor, R.; van der Pol, C.C.; et al. The molecular genetic make-up of male breast cancer. Endocr. Relat. Cancer 2019, 26, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Szwiec, M.; Tomiczek-Szwiec, J.; Kluźniak, W.; Wokołorczyk, D.; Osowiecka, K.; Sibilski, R.; Wachowiak, M.; Gronwald, J.; Gronwald, H.; Lubiński, J.; et al. Genetic predisposition to male breast cancer in Poland. BMC Cancer 2021, 21, 975. [Google Scholar] [CrossRef] [PubMed]
- Piscuoglio, S.; Ng, C.K.; Murray, M.P.; Guerini-Rocco, E.; Martelotto, L.G.; Geyer, F.C.; Bidard, F.-C.; Berman, S.; Fusco, N.; Sakr, R.A.; et al. The Genomic Landscape of Male Breast Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4045–4056. [Google Scholar] [CrossRef]
- Rizzolo, P.; Silvestri, V.; Tommasi, S.; Pinto, R.; Danza, K.; Falchetti, M.; Gulino, M.; Frati, P.; Ottini, L. Male breast cancer: Genetics, epigenetics, and ethical aspects. Ann. Oncol. 2013, 24 (Suppl. S8), viii75–viii82. [Google Scholar] [CrossRef]
- Wen, W.; Zhao, S.; Jiang, Y.; Ou, C.; Guo, C.; Jia, Z.; Li, J.; Huang, Y.; Xu, H.; Pu, P.; et al. Genome sequencing enhances the diagnostic yield and expands the genetic landscape of male breast cancer. Genet. Med. Open. 2025, 3, 101899. [Google Scholar] [CrossRef] [PubMed]
- Bucalo, A.; Conti, G.; Valentini, V.; Capalbo, C.; Bruselles, A.; Tartaglia, M.; Bonanni, B.; Calistri, D.; Coppa, A.; Cortesi, L.; et al. Male breast cancer risk associated with pathogenic variants in genes other than BRCA1/2: An Italian case-control study. Eur. J. Cancer 2023, 188, 183–191. [Google Scholar] [CrossRef]
- Chamseddine, R.S.; Wang, C.; Yin, K.; Wang, J.; Singh, P.; Zhou, J.; Robson, M.E.; Braun, D.; Hughes, K.S. Penetrance of male breast cancer susceptibility genes: A systematic review. Breast Cancer Res. Treat. 2022, 191, 31–38. [Google Scholar] [CrossRef]
- Silvestri, V.; Rizzolo, P.; Zelli, V.; Valentini, V.; Zanna, I.; Bianchi, S.; Tibiletti, M.G.; Varesco, L.; Russo, A.; Tommasi, S.; et al. A possible role of FANCM mutations in male breast cancer susceptibility: Results from a multicenter study in Italy. Breast 2018, 38, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-N.; Geng, J.-S.; Liu, T.; Zhong, Z.-B.; Liu, Y.; Xia, B.-S.; Ji, H.-F.; Li, X.-M.; Zhang, G.-Q.; Ren, Y.-L.; et al. Long CAG repeat sequence and protein expression of androgen receptor considered as prognostic indicators in male breast carcinoma. PLoS ONE 2012, 7, e52271. [Google Scholar] [CrossRef]
- Maguire, S.; Perraki, E.; Tomczyk, K.; Jones, M.E.; Fletcher, O.; Pugh, M.; Winter, T.; Thompson, K.; Cooke, R.; kConFab Consortium; et al. Common Susceptibility Loci for Male Breast Cancer. J. Natl. Cancer Inst. 2021, 113, 453–461. [Google Scholar] [CrossRef]
- Deb, S.; Do, H.; Byrne, D.; Jene, N.; kConFab Investigators; Dobrovic, A.; Fox, S.B. PIK3CA mutations are frequently observed in BRCAX but not BRCA2-associated male breast cancer. Breast Cancer Res. 2013, 15, R69. [Google Scholar] [CrossRef]
- Johansson, I.; Killander, F.; Linderholm, B.; Hedenfalk, I. Molecular profiling of male breast cancer–lost in translation? Int. J. Biochem. Cell Biol. 2014, 53, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Kornegoor, R.; Verschuur-Maes, A.H.J.; Buerger, H.; Hogenes, M.C.H.; de Bruin, P.C.; Oudejans, J.J.; van der Groep, P.; Hinrichs, B.; van Diest, P.J. Molecular subtyping of male breast cancer by immunohistochemistry. Mod. Pathol. 2012, 25, 398–404. [Google Scholar] [CrossRef]
- Humphries, M.P.; Rajan, S.S.; Honarpisheh, H.; Cserni, G.; Dent, J.; Fulford, L.; Jordan, L.B.; Jones, J.L.; Kanthan, R.; Litwiniuk, M.; et al. Characterisation of male breast cancer: A descriptive biomarker study from a large patient series. Sci. Rep. 2017, 7, 45293. [Google Scholar] [CrossRef]
- Fentiman, I.S. The biology of male breast cancer. Breast 2018, 38, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.A.; Slaets, L.; Cardoso, F.; Giordano, S.H.; Tryfonidis, K.; van Diest, P.J.; Dijkstra, N.H.; Schröder, C.P.; van Asperen, C.J.; Linderholm, B.; et al. Pathological characterisation of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur. J. Cancer 2017, 82, 219–227. [Google Scholar] [CrossRef]
- Chavez-Macgregor, M.; Clarke, C.A.; Lichtensztajn, D.; Hortobagyi, G.N.; Giordano, S.H. Male breast cancer according to tumor subtype and race: A population-based study. Cancer 2013, 119, 1611–1617. [Google Scholar] [CrossRef]
- Shaaban, A.M.; Ball, G.R.; Brannan, R.A.; Cserni, G.; Di Benedetto, A.; Dent, J.; Fulford, L.; Honarpisheh, H.; Jordan, L.; Jones, J.L.; et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res. Treat. 2012, 133, 949–958. [Google Scholar]
- Scatena, C.; Scarpitta, R.; Innocenti, L.; Miccoli, M.; Biancotti, R.; Diodati, L.; Ghilli, M.; Naccarato, A.G. Androgen receptor expression inversely correlates with histological grade and N stage in ER+/PgRlow male breast cancer. Breast Cancer Res. Treat. 2020, 182, 55–65. [Google Scholar]
- Shang, J.; Miao, J.; Niu, S.; Sun, X.; Liu, Y. Redefining therapeutic landscapes: Clinicopathological insights into low and ultra-low HER2 expression in male breast cancer. Diagn. Pathol. 2025, 20, 43. [Google Scholar] [CrossRef]
- Silvestri, V.; Valentini, V.; Bucalo, A.; Conti, G.; Manzella, L.; Turchetti, D.; Russo, A.; Capalbo, C.; Ottini, L. HER2-Low Expression in Male Breast Cancer: Results from a Multicenter Series in Italy. Cancers 2024, 16, 548. [Google Scholar] [CrossRef]
- Lyu, B.; Zhao, S.; Wang, H.; Gong, S.; Wang, B. HER2 expression and pathway status in male breast cancer patients: Results of an integrated analysis among 6150 patients. Sci. Rep. 2025, 15, 3354. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.H.; Afonso, N.; Abreu, P.H.; Menezes, F.; Lopes, P.; Henrique, R.; Pereira, D.; Lopes, C. Male breast cancer: Looking for better prognostic subgroups. Breast 2016, 26, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, L.; Wang, Z.; Gu, D.; Zhu, M.; Cai, Y.; Li, L.; Tang, J.; Huang, B.; Bosco, B.; et al. Single-cell transcriptome analysis indicates fatty acid metabolism-mediated metastasis and immunosuppression in male breast cancer. Nat. Commun. 2023, 14, 5590. [Google Scholar] [PubMed]
- André, S.; Pinto, A.E.; Silva, G.L.; Silva, F.; Serpa, J.; Félix, A. Male Breast Cancer-Immunohistochemical Patterns and Clinical Relevance of FASN, ATF3, and Collagen IV. Breast Cancer Basic Clin. Res. 2021, 15, 11782234211002496. [Google Scholar]
- Accomasso, F.; Actis, S.; Minella, C.; Rosso, R.; Granaglia, C.; Ponzone, R.; Biglia, N.; Bounous, V.E. Clinical, Pathological, and Prognostic Features of Male Breast Cancer: A Multicenter Study. Curr. Oncol. 2023, 30, 9860–9871. [Google Scholar] [CrossRef]
- Cardoso, F.; Bartlett, J.M.S.; Slaets, L.; van Deurzen, C.H.M.; van Leeuwen-Stok, E.; Porter, P.; Linderholm, B.; Hedenfalk, I.; Schröder, C.; Martens, J.; et al. Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 2018, 29, 405–417. [Google Scholar] [CrossRef]
- Sanguinetti, A.; Polistena, A.; Lucchini, R.; Monacelli, M.; Galasse, S.; Avenia, S.; Triola, R.; Bugiantella, W.; Cirocchi, R.; Rondelli, F.; et al. Male breast cancer, clinical presentation, diagnosis and treatment: Twenty years of experience in our Breast Unit. Int. J. Surg. Case Rep. 2016, 20, 8–11. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Qi, P.; Wo, Y.; Pang, Y.; Xu, Q.; Xu, M.; Huang, S.; Wang, Q. Gene expression profiling for the diagnosis of male breast cancer. BMC Cancer 2024, 24, 1584. [Google Scholar] [CrossRef]
- Leone, J.P.; Freedman, A.R.; Leone, J.; Tolaney, S.M.; Vallejo, C.T.; Leone, B.A.; Winer, E.P.; Lin, N.U.; Hassett, M.J. Survival in male breast cancer over the past 3 decades. J. Natl. Cancer Inst. 2023, 115, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Huang, T.W.; Tam, K.W. Treatment of male breast cancer: Meta-analysis of real-world evidence. Br. J. Surg. 2021, 108, 1034–1042. [Google Scholar]
- Den, J.; Nelson, N.; Khanipov, K.; Klimberg, V.S. Breast Conservation Surgery for Breast Cancer in Men. J. Am. Coll. Surg. 2025, 240, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Zaenger, D.; Rabatic, B.M.; Dasher, B.; Mourad, W.F. Is Breast Conserving Therapy a Safe Modality for Early-Stage Male Breast Cancer? Clin. Breast Cancer 2016, 16, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Elmi, M.; Sequeira, S.; Azin, A.; Elnahas, A.; McCready, D.R.; Cil, T.D. Evolving surgical treatment decisions for male breast cancer: An analysis of the National Surgical Quality Improvement Program (NSQIP) database. Breast Cancer Res. Treat. 2018, 171, 427–434. [Google Scholar] [CrossRef]
- Sauder, C.A.M.; Bateni, S.B.; Davidson, A.J.; Nishijima, D.K. Breast Conserving Surgery Compared With Mastectomy in Male Breast Cancer: A Brief Systematic Review. Clin. Breast Cancer 2020, 20, e309–e314. [Google Scholar] [CrossRef]
- Bakalov, V.; Jayakrishnan, T.T.; Abel, S.; Hilton, C.; Rusia, B.; Wegner, R.E. The use of adjuvant radiation therapy in male breast cancer and its impact on outcomes. Cancer Treat. Res. Commun. 2021, 27, 100359. [Google Scholar] [CrossRef]
- Weir, J.; Zhao, Y.D.; Herman, T.; Algan, Ö. Clinicopathologic Features and Radiation Therapy Utilization in Patients with Male Breast Cancer: A National Cancer Database Study. Breast Cancer Basic Clin. Res. 2018, 12, 1178223418770687. [Google Scholar] [CrossRef]
- De La Cruz, L.M.; Thiruchelvam, P.T.R.; Shivani, J.; Trina, J.; Blankenship, S.A.; Fisher, C.S. Saving the Male Breast: A Systematic Literature Review of Breast-Conservation Surgery for Male Breast Cancer. Ann. Surg. Oncol. 2019, 26, 3939–3944. [Google Scholar] [CrossRef] [PubMed]
- Fogh, S.; Kachnic, L.A.; Goldberg, S.I.; Taghian, A.G.; Powell, S.N.; Hirsch, A.E. Localized therapy for male breast cancer: Functional advantages with comparable outcomes using breast conservation. Clin. Breast Cancer 2013, 13, 344–349. [Google Scholar] [CrossRef]
- Giordano, S.H.; Temin, S.; Chandarlapaty, S.; Crews, J.R.; Esteva, F.J.; Kirshner, J.J.; Krop, I.E.; Levinson, J.; Lin, N.U.; Modi, S.; et al. Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2736–2740. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Hernandez-Boussard, T.; Wapnir, I.L. Outcomes of partial mastectomy in male breast cancer patients: Analysis of SEER, 1983–2009. Ann. Surg. Oncol. 2013, 20, 1545–1550. [Google Scholar] [CrossRef]
- Bateni, S.B.; Davidson, A.J.; Arora, M.; Daly, M.E.; Stewart, S.L.; Bold, R.J.; Canter, R.J.; Sauder, C.A.M. Is Breast Conserving Therapy Appropriate for Male Breast Cancer Patients? A National Cancer Database Analysis. Ann. Surg. Oncol. 2019, 26, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Parpex, G.; Ottaviani, M.; Lorphelin, H.; Mezzadri, M.; Marchand, E.; Cahen-Doidy, L.; Benifla, J.L.; Huchon, C.; Mimoun, C. Accuracy of sentinel lymph node biopsy in male breast cancer: Systematic review and meta-analysis. Breast 2024, 75, 103703. [Google Scholar] [CrossRef]
- Spinaci, S.; Arecco, L.; Anedda, A.; Martino, L.; Firpo, E.; Ghilli, M.; Lambertini, M.; Ferrarazzo, G. Treatments of Interest in Male Breast Cancer: An Umbrella Review. J. Pers. Med. 2025, 15, 66. [Google Scholar] [CrossRef]
- Koukouras, D.; Spyropoulos, C.; Zygomalas, A.; Tzoracoleftherakis, E. Sentinel node biopsy in male breast carcinoma: Is the “female” approach justified? Eur. J. Gynaecol. Oncol. 2012, 33, 255–256. [Google Scholar]
- Gentilini, O.; Chagas, E.; Zurrida, S.; Intra, M.; De Cicco, C.; Gatti, G.; Silva, L.; Renne, G.; Cassano, E.; Veronesi, U. Sentinel lymph node biopsy in male patients with early breast cancer. Oncologist 2007, 12, 512–515. [Google Scholar] [CrossRef]
- Maráz, R.; Boross, G.; Pap-Szekeres, J.; Markó, L.; Rajtár, M.; Ambrózay, É.; Bori, R.; Cserni, G. The role of sentinel node biopsy in male breast cancer. Breast Cancer 2016, 23, 85–91. [Google Scholar] [CrossRef]
- Şimşek, O.; Belli, A.; Aydoğan, F.; Karataş, A.; Canbay, E.; Kepil, N.; Selcukbiricik, F.; Celik, V.; Uras, C. Combination Technique Is Superior to Dye Alone in Identification of the Sentinel Lymph Node in Male Breast Cancer. Am. Surg. 2018, 84, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ruan, Y.; Wang, J.; Qiao, J.; Liu, Z. Trends and efficacy of omitting axillary lymph node dissection in early-stage male breast cancer with limited nodal involvement: A population-based cohort study. Cancer Med. 2024, 13, e70243. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Feng, K.; Wei, Y.; Wang, K.; Yang, C.; Zhao, S.; Liu, J.; Meng, X.; Li, Y.; Du, C.; et al. Evaluation of Male Breast Cancer and the Application of Sentinel Lymph Node Biopsy: A Multicenter Retrospective Study. Oncologist 2023, 28, e1170–e1178. [Google Scholar] [CrossRef]
- Jardel, P.; Vignot, S.; Cutuli, B.; Creisson, A.; Vass, S.; Barranger, E.; Thariat, J. Should Adjuvant Radiation Therapy Be Systematically Proposed for Male Breast Cancer? A Systematic Review. Anticancer. Res. 2018, 38, 23–31. [Google Scholar] [CrossRef]
- Colciago, R.R.; Lancellotta, V.; De Santis, M.C.; Bonzano, E.; De Rose, F.; Rocca, E.L.; Meduri, B.; Pasinetti, N.; Prisco, A.; Gennari, A.; et al. The role of radiation therapy in the multidisciplinary management of male breast cancer: A systematic review and meta-analysis on behalf of the Clinical Oncology Breast Cancer Group (COBCG). Crit. Rev. Oncol. Hematol. 2024, 204, 104537. [Google Scholar] [CrossRef]
- Forster, T.; Köhler, C.; El Shafie, R.; Weykamp, F.; König, L.; Arians, N.; Adeberg, S.; Michel, L.; Smetanay, K.; Golatta, M.; et al. Adjuvant Radiation Therapy for Male Breast Cancer-A Rare Indication? Cancers 2020, 12, 3645. [Google Scholar] [CrossRef]
- Cutuli, B.; Lacroze, M.; Dilhuydy, J.M.; Velten, M.; De Lafontan, B.; Marchal, C.; Resbeut, M.; Graic, Y.; Campana, F.; Moncho-Bernier, V.; et al. Male breast cancer: Results of the treatments and prognostic factors in 397 cases. Eur. J. Cancer 1995, 31, 1960–1964. [Google Scholar] [CrossRef]
- Eggemann, H.; Ignatov, A.; Stabenow, R.; von Minckwitz, G.; Röhl, F.W.; Hass, P.; Costa, S.-D. Male breast cancer: 20-year survival data for post-mastectomy radiotherapy. Breast Care 2013, 8, 270–275. [Google Scholar] [CrossRef]
- Asgharian, M.; Moslemi, D.; Nikbakht, H.A.; Jahani, M.A.; Bijani, A.; Mehdizadeh, H. Male breast cancer: A 32-year retrospective analysis in radiation therapy referral center in northern Iran. Ann. Med. Surg. 2024, 86, 5756–5761. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, C.L.; Goodman, D.; Lannigan, A. A systematic literature review of the management, oncological outcomes and psychosocial implications of male breast cancer. Eur. J. Surg. Oncol. 2022, 48, 2104–2111. [Google Scholar] [CrossRef]
- Yadav, B.S.; Sharma, S.C.; Singh, R.; Dahiya, D.; Ghoshal, S. Male breast cancer: Outcome with adjuvant treatment. J. Cancer Res. Ther. 2020, 16, 1287–1293. [Google Scholar] [CrossRef]
- Venigalla, S.; Carmona, R.; Guttmann, D.M.; Jain, V.; Freedman, G.M.; Clark, A.S.; Shabason, J.E. Use and Effectiveness of Adjuvant Endocrine Therapy for Hormone Receptor-Positive Breast Cancer in Men. JAMA Oncol. 2018, 4, e181114. [Google Scholar] [CrossRef]
- Popa-Nimigean, V.; Ahmed, M. Current state of surgical management for male breast cancer. Transl. Cancer Res. 2019, 8 (Suppl. S5), S457–S462. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.C.; Domchek, S.; Parmigiani, G.; Chen, S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 2007, 99, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.S.; Huda, F.; Misra, S.; Amulya, K.R.; Raj, N.; Karn, S.; Basu, S. Male Breast Cancer: An Updated Review of Patient Characteristics, Genetics, and Outcome. Int. J. Breast Cancer 2024, 2024, 9003572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cheng, H.; He, D.; Zhang, Y.; Chai, Y.; Song, A.; Sun, G. Decoding male breast cancer: Epidemiological insights, cutting-edge treatments, and future perspectives. Discov. Oncol. 2025, 16, 360. [Google Scholar] [CrossRef]
- Huang, A.; Li, D.; Fan, Z.; Chen, J.; Zhang, W.; Wu, W. Long-term trends in the incidence of male breast cancer and nomogram for predicting survival in male breast cancer patients: A population-based epidemiologic study. Sci. Rep. 2025, 15, 2027. [Google Scholar] [CrossRef]
- Sun, W.; Cheng, M.; Zhou, H.; Huang, W.; Qiu, Z. Nomogram Predicting Cause-Specific Mortality in Nonmetastatic Male Breast Cancer: A Competing Risk Analysis. J. Cancer 2019, 10, 583–593. [Google Scholar] [CrossRef]
- Fentiman, I.S. Surgical options for male breast cancer. Breast Cancer Res. Treat. 2018, 172, 539–544. [Google Scholar] [CrossRef]
- Suami, H.; Pan, W.R.; Mann, G.B.; Taylor, G.I. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: A human cadaver study. Ann. Surg. Oncol. 2008, 15, 863–871. [Google Scholar] [CrossRef]
| OR [IC95%] | ||||||
|---|---|---|---|---|---|---|
| Gene | Chromosome | Inheritance | Pathogenic Variants | Clinical Syndrome | Pritzlaff et al. [12] | Janatova et al. [13] |
| High penetrance | ||||||
| BRCA2 | 13q12-13 | AD | LOF mutations | HBOC | 13,9 (5–22) | 44,0 (2–92) |
| BRCA1 | 17q21 | AD | LOF mutations | HBOC | 1,8 (0,3–6,8) | 5,8 (0–14) |
| TP53 * | 17p13 | AD | Missense/LOF mutation | Li-fraumeni | - | - |
| PTEN * | 10q23 | AD | LOF mutations | Cowden | - | - |
| STK11 * | 19 | AD | LOF mutations | Peutz Jeghers | - | - |
| Moderate or Low penetrance | ||||||
| CHEK2 | 22q11 | AD | 1100delC | Li-fraumeni variant | 2,4 (1,3,4,9) | 5,0 (1,3,8–12) |
| ATM | 11q22-23 | AR/AD | Truncating/missense variants | HC: breast cancer risk; BA: Ataxia–Telangiectasia | 1,4 (0,1,3–5) | 1,3 (0–7) |
| PALB2 | 16p22 | AD AR | Truncating variants | HBOC Syndrome Fanconi | 6,6 (1,7–21) | 8,3 (2–27) |
| Study (Reference) | Kornegoor et al. [30] | Vermeulen et al. [33] | Moelans et al. [18] | Piscuoglio et al. [20] | |
|---|---|---|---|---|---|
| Histological type (%) - | Ductal Lobular Others | 95 2 3 | >90 <10 | 90 10 | NR |
| Grade (%) | 1 2 3 | 19 43 38 | 19 42 39 | NR | NR |
| ER−/ER+ | (%) | 1/99 | 1/99 | 4/96 | 0/100 |
| PR−/PR+ | (%) | 18/82 | 18/82 | 34/66 | NR |
| HER2−/HER2+ | (%) | 95/5 | 95/5 | 98/2 | 97/3 |
| LN−/LN+ | (%) | 54/46 | 54/46 | NR | NR |
| Ki-67 | (Low/High) | 79/21 | NR | NR | NR |
| Subtypes (%) | Luminal A like Luminal B like Basal like HER2 | 75 21 4 0 | 81 13 6 0 | 50 46 4 0 | 29 71 |
| First Autor (Reference) | Survival Data | ||
|---|---|---|---|
| BCS | Mastectomy | ||
| 5-Year DSS | Cloyd et al. [58] | 87% | 88% |
| Zaenger et al. [50] | 100% | 97.3% | |
| 5-Year OS | Den et al. [49] | 84% | 86% |
| Zaenger et al. [50] | 97% | 95% | |
| Bateni et al. [59] | 41% | 40% | |
| Cloyd et al. [58] | 66% | 70% | |
| Fogh et al. [56] | 7 of 8 | 34 of 34 | |
| 10-Year-OS | Bateni et al. [59] | 6% | 5% |
| Cloyd et al. [58] | 47% | 46% | |
| Fogh et al. [56] | 6 of 8 | 23 of 34 | |
| First Authors, Year, Source | Study Design | N (SLNB) | Identification Rate (IR) | False-Negative Rate (FNR) |
|---|---|---|---|---|
| Parpex, 2024 [60] | Meta-analysis, no heterogeneity | 164 (IR)/50 (FNR) | 99.0% | 0% |
| Koukouras, 2012 [62] | Retrospective study | 11 | 100% | 0% |
| Gentilini, 2007 [63] | Retrospective study | 32 | 100% | 0% |
| Maràz, 2016 [64] | Retrospective study | 16 | 100% | 3/12 |
| Lin, 2011 [48] | Meta-analysis | 213 | 97.4% | 7.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melan, K.; Loap, P.; Kirova, Y. Current Perspectives on Non-Metastatic Male Breast Cancer: Genetics, Biology, and Treatment Advances: A Systematic Review. Cancers 2025, 17, 3270. https://doi.org/10.3390/cancers17193270
Melan K, Loap P, Kirova Y. Current Perspectives on Non-Metastatic Male Breast Cancer: Genetics, Biology, and Treatment Advances: A Systematic Review. Cancers. 2025; 17(19):3270. https://doi.org/10.3390/cancers17193270
Chicago/Turabian StyleMelan, Kathleen, Pierre Loap, and Youlia Kirova. 2025. "Current Perspectives on Non-Metastatic Male Breast Cancer: Genetics, Biology, and Treatment Advances: A Systematic Review" Cancers 17, no. 19: 3270. https://doi.org/10.3390/cancers17193270
APA StyleMelan, K., Loap, P., & Kirova, Y. (2025). Current Perspectives on Non-Metastatic Male Breast Cancer: Genetics, Biology, and Treatment Advances: A Systematic Review. Cancers, 17(19), 3270. https://doi.org/10.3390/cancers17193270






