Advanced Biliary Tract Cancer: Exploration of Third-Line and Other Therapeutic Areas After Failure of Chemotherapy Alone
Abstract
Simple Summary
Abstract
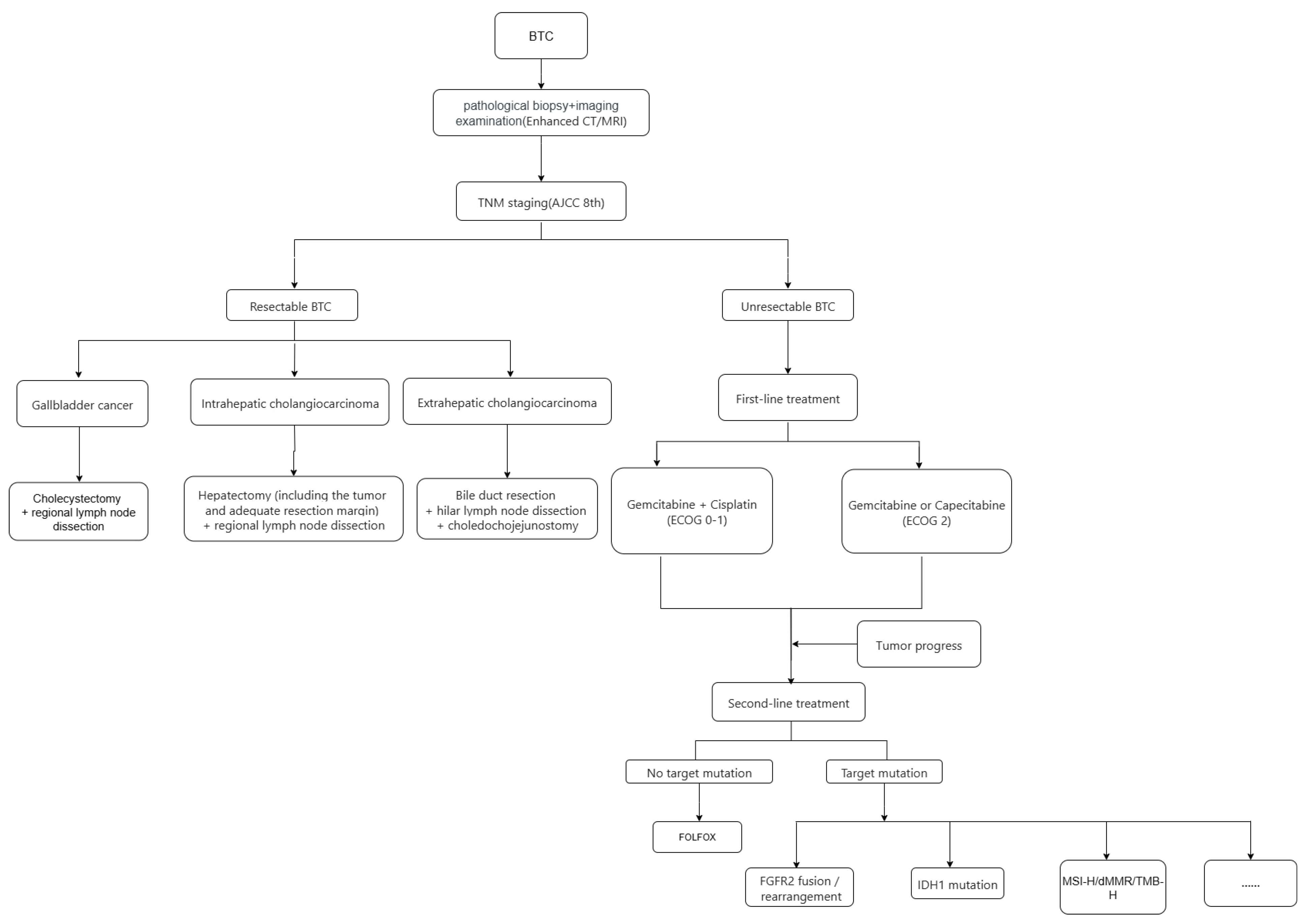
1. Introduction
2. Tumor Biomarkers
3. Limitations
4. First-Line and Second-Line Treatment
5. Identification of Therapeutic Targets
6. Clinical Trials in Treatment-Resistant Settings
6.1. Chemotherapy
6.2. Targeted Therapy
6.3. Immunotherapy
6.4. Combination of Targeted Therapy and Immunotherapy
6.5. Novel Therapeutic Approaches
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qurashi, M.; Vithayathil, M.; Khan, S.A. Epidemiology of cholangiocarcinoma. Eur. J. Surg. Oncol. 2025, 51, 107064. [Google Scholar] [CrossRef]
- Huang, J.; Patel, H.K.; Boakye, D.; Chandrasekar, V.T.; Koulaouzidis, A.; Lucero-Prisno, D.E., III; Ngai, C.H.; Pun, C.N.; Bai, Y.; Lok, V.; et al. Worldwide distribution, associated factors, and trends of gallbladder cancer: A global country-level analysis. Cancer Lett. 2021, 521, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Pan, G.; Chen, X. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer 2021, 127, 2238–2250. [Google Scholar] [CrossRef]
- GBD 2019 Adolescent and Young Adult Cancer Collaborators. The global burden of adolescent and young adult cancer in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022, 23, 27–52. [Google Scholar] [CrossRef]
- Vogel, A.; Ducreux, M.; on behalf of the ESMO Guidelines Committee. ESMO Clinical Practice Guideline Interim Update on the Management of Biliary Tract Cancer. ESMO Open 2025, 10, 104003. [Google Scholar] [CrossRef]
- Chapman, M.H.; Sandanayake, N.S.; Andreola, F.; Dhar, D.K.; Webster, G.J.; Dooley, J.S.; Pereira, S.P. Circulating CYFRA 21-1 is a Specific Diagnostic and Prognostic Biomarker in Biliary Tract Cancer. J. Clin. Exp. Hepatol. 2011, 1, 6–12. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 108–122. [Google Scholar] [CrossRef] [PubMed]
- Zavattari, C.; Tommasi, A.; Moi, L.; Canale, M.; Po, A.; Sabato, C.; Vega-Benedetti, A.F.; Ziranu, P.; Puzzoni, M.; Lai, E.; et al. HOXD8 hypermethylation as a fully sensitive and specific biomarker for biliary tract cancer detectable in tissue and bile samples. Br. J. Cancer 2022, 126, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Feng, M.; Zhou, N.; Zhang, S.; Yi, C.; Gou, H. DNA damage response and repair gene mutations predict clinical outcomes in biliary tract cancer. Cancer 2025, 131, e35726. [Google Scholar] [CrossRef]
- Kearney, J.F.; Weighill, D.; Yeh, J.J. Promise of bile circulating tumor DNA in biliary tract cancers. Cancer 2023, 129, 1643–1645. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, C.; Yu, X.-X.; Wu, C.; Jia, H.-L.; Gao, X.-M.; Yang, J.-M.; Wang, C.-Q.; Luo, Q.; Zhu, Y.; et al. Osteopontin promotes metastasis of intrahepatic cholangiocarcinoma through recruiting MAPK1 and mediating Ser675 phosphorylation of β-Catenin. Cell Death Dis. 2018, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.K.; Sheel, A.; Rahman, S.; Esnakula, A.; Manne, A. Understanding the Clinical Significance of MUC5AC in Biliary Tract Cancers. Cancers 2023, 15, 433. [Google Scholar] [CrossRef]
- Bai, X.; Huang, J.; Jin, Y.; Chen, J.; Zhou, S.; Dong, L.; Han, X.; He, X. M6A RNA methylation in biliary tract cancer: The function roles and potential therapeutic implications. Cell Death Discov. 2024, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qiu, Z.; Huang, H.; Xiao, X.; Du, F.; Ji, J.; Xu, X.; Jiang, X.; Wang, Y.; Gao, C. Alterations in genomic features and the tumour immune microenvironment predict immunotherapy outcomes in advanced biliary tract cancer patients. Br. J. Cancer 2025, 132, 1072–1082. [Google Scholar] [CrossRef]
- Rohan, T.; Cechova, B.; Matkulcik, P.; Straka, M.; Zavadil, J.; Eid, M.; Uher, M.; Dostal, M.; Andrasina, T. Prognostic factors for survival in patients with advanced cholangiocarcinoma treated with percutaneous transhepatic drainage. Sci. Rep. 2025, 15, 2172. [Google Scholar] [CrossRef]
- Kang, M.J.; Lim, J.; Han, S.-S.; Park, H.M.; Kim, S.-W.; Lee, W.J.; Woo, S.M.; Kim, T.H.; Won, Y.-J.; Park, S.-J. Distinct prognosis of biliary tract cancer according to tumor location, stage, and treatment: A population-based study. Sci. Rep. 2022, 12, 10206. [Google Scholar] [CrossRef]
- Thanasukarn, V.; Prajumwongs, P.; Muangritdech, N.; Loilome, W.; Namwat, N.; Klanrit, P.; Wangwiwatsin, A.; Charoenlappanit, S.; Jaresitthikunchai, J.; Roytrakul, S.; et al. Discovery of novel serum peptide biomarkers for cholangiocarcinoma recurrence through MALDI-TOF MS and LC–MS/MS peptidome analysis. Sci. Rep. 2025, 15, 2582. [Google Scholar] [CrossRef]
- Kefas, J.; Bridgewater, J.; Vogel, A.; Stein, A.; Primrose, J. Adjuvant therapy of biliary tract cancers. Ther. Adv. Med. Oncol. 2023, 15, 17588359231163785. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Barmpounakis, P.; Demiris, N.; Jah, A.; Spiers, H.V.; Talukder, S.; Martin, J.L.; Gibbs, P.; Harper, S.J.; Huguet, E.L.; et al. Surgical outcomes of gallbladder cancer: The OMEGA retrospective, multicentre, international cohort study. eClinicalMedicine 2023, 59, 101951. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Khan, S.; Koerkamp, B.G.; Roessler, S.; Andersen, J.B.; Raggi, C.; Lleo, A.; Nault, J.-C.; Calderaro, J.; Gabbi, C.; et al. Mapping the landscape of biliary tract cancer in Europe: Challenges and controversies. Lancet Reg. Health Eur. 2025, 50, 101171. [Google Scholar] [CrossRef] [PubMed]
- Van Valckenborgh, E.; Hébrant, A.; Antoniou, A.; Van Hoof, W.; Van Bussel, J.; Pauwels, P.; Salgado, R.; Van Doren, W.; Waeytens, A.; Van den Bulcke, M. Roadbook for the Implementation of Next-Generation Sequencing in Clinical Practice in Oncology and Hemato-Oncology in Belgium. Arch. Public Health 2018, 76, 49. [Google Scholar] [CrossRef]
- Casolino, R.; Braconi, C.; Prete, M.; Romero, F.G.; Castro, R.; Banales, J.; Cardinale, V.; Morement, H.; Milne, J.; Krawczyk, M.; et al. Bridging the equity gap in patient education: The biliary tract cancer BABEL project. Lancet Oncol. 2022, 23, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281, Erratum in N. Engl. J. Med. 2010, 363, 198. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.-J.; Ozaka, M.; Verslype, C.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865, Erratum in Lancet 2023, 402, 964. https://doi.org/10.1016/S0140-6736(23)01904-9; Erratum in Lancet 2024, 403, 1140. https://doi.org/10.1016/S0140-6736(24)00545-2. [Google Scholar] [CrossRef]
- Wei, F. Second-line FOLFOX chemotherapy for advanced biliary tract cancer. Lancet Oncol. 2021, 22, E284. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tu, X.; Zhao, P.; Jiang, W.; Liu, L.; Tong, Z.; Zhang, H.; Yan, C.; Fang, W.; Wang, W. A randomised phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br. J. Cancer 2018, 119, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Lengelé, A.; Bekolo, W.; Hendlisz, A. FOLFIRI as second-line treatment of metastatic biliary tract cancer patients. Autops. Case Rep. 2019, 9, e2019087. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684, Erratum in Lancet Oncol. 2024, 25, e3. https://doi.org/10.1016/S1470-2045(23)00642-3. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2 -Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807, Erratum in Lancet Oncol. 2020, 21, e462. https://doi.org/10.1016/S1470-2045(20)30547-7; Erratum in Lancet Oncol. 2024, 25, e61. https://doi.org/10.1016/S1470-2045(24)00013-5. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, M.; Wang, L.; Li, L.; Lei, J.H.; Zhou, J.; Chen, J.; Wu, Y.; Miao, K.; Deng, C.-X. The heterogeneity of signaling pathways and drug responses in intrahepatic cholangiocarcinoma with distinct genetic mutations. Cell Death Dis. 2024, 15, 34. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, L.; Jia, Z.; Liu, L.; Gu, A.; Liu, Z.; Zhu, Q.; Zuo, Y.; Yang, M.; Wang, S.; et al. Full-length transcriptome atlas of gallbladder cancer reveals trastuzumab resistance conferred by ERBB2 alternative splicing. Signal Transduct. Target. Ther. 2025, 10, 54. [Google Scholar] [CrossRef]
- Jung, D.E.; Seo, M.-K.; Jo, J.H.; Kim, K.; Kim, C.; Kang, H.; Park, S.B.; Lee, H.S.; Kim, S.; Song, S.Y. PUM1-TRAF3 fusion protein activates non-canonical NF-κB signaling via rescued NIK in biliary tract cancer. NPJ Precis. Oncol. 2024, 8, 170. [Google Scholar] [CrossRef]
- Trakoonsenathong, R.; Kunprom, W.; Aphivatanasiri, C.; Yueangchantuek, P.; Pimkeeree, P.; Sorin, S.; Khawkhiaw, K.; Chiu, C.-F.; Okada, S.; Wongkham, S.; et al. Liraglutide exhibits potential anti-tumor effects on the progression of intrahepatic cholangiocarcinoma, in vitro and in vivo. Sci. Rep. 2024, 14, 13726. [Google Scholar] [CrossRef]
- Zhen, Y.; Liu, K.; Shi, L.; Shah, S.; Xu, Q.; Ellis, H.; Balasooriya, E.R.; Kreuzer, J.; Morris, R.; Baldwin, A.S.; et al. FGFR inhibition blocks NF-ĸB-dependent glucose metabolism and confers metabolic vulnerabilities in cholangiocarcinoma. Nat. Commun. 2024, 15, 3805. [Google Scholar] [CrossRef] [PubMed]
- Angerilli, V.; Sacchi, D.; Rizzato, M.; Gasparello, J.; Ceccon, C.; Sabbadin, M.; Niero, M.; Bergamo, F.; Cillo, U.; Franzina, C.; et al. Claudin 18.2: A promising actionable target in biliary tract cancers. ESMO Open 2025, 10, 105049. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Noh, Y.-K.; Son, B.K.; Kim, D.-H.; Min, K.-W.; Chae, S.W.; Kim, H.S.; Kwon, M.J.; Pyo, J.S.; Byun, Y. Activated cancer-associated fibroblasts correlate with poor survival and decreased lymphocyte infiltration in infiltrative type distal cholangiocarcinoma. Sci. Rep. 2025, 15, 20644. [Google Scholar] [CrossRef]
- Hyung, J.; Kim, I.; Kim, K.-P.; Ryoo, B.-Y.; Jeong, J.H.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.-S.; et al. Treatment with Liposomal Irinotecan Plus Fluorouracil and Leucovorin for Patients with Previously Treated Metastatic Biliary Tract Cancer: The Phase 2b NIFTY Randomized Clinical Trial. JAMA Oncol. 2023, 9, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Yamada, I.; Morizane, C.; Okusaka, T.; Mizusawa, J.; Kataoka, T.; Ueno, M.; Ikeda, M.; Okano, N.; Todaka, A.; Shimizu, S.; et al. The clinical outcomes of combination chemotherapy in elderly patients with advanced biliary tract cancer: An exploratory analysis of JCOG1113. Sci. Rep. 2022, 12, 987. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Elez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.K.; Tam, V.C.; McNamara, M.G.; Jang, R.; Hedley, D.; Chen, E.; Dhani, N.; Tang, P.; Sim, H.-W.; O’Kane, G.M.; et al. Randomised, Phase II study of selumetinib, an oral inhibitor of MEK, in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer. Br. J. Cancer 2022, 127, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.-Y.; Hanna, D.L.; Kang, Y.-K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Xu, J.; Bai, Y.; Sun, H.; Bai, C.; Jia, R.; Li, Y.; Zhang, W.; Liu, L.; Huang, C.; Guan, M.; et al. A Single-Arm, Multicenter, Open-Label Phase 2 Trial of Surufatinib in Patients with Unresectable or Metastatic Biliary Tract Cancer. Cancer 2021, 127, 3975–3984. [Google Scholar] [CrossRef]
- Boilève, A.; Hilmi, M.; Gougis, P.; Cohen, R.; Rousseau, B.; Blanc, J.-F.; Ben Abdelghani, M.; Castanié, H.; Dahan, L.; Tougeron, D.; et al. Triplet Combination of Durvalumab, Tremelimumab, and Paclitaxel in Biliary Tract Carcinomas: Safety Run-in Results of the Randomized IMMUNOBIL PRODIGE 57 Phase II Trial. Eur. J. Cancer 2021, 143, 55–63. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients with Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef]
- Patel, S.P.; Guadarrama, E.; Chae, Y.K.; Dennis, M.J.; Ferri, W.A., Jr.; George, T.J.; Liao, C.-Y.; Powers, B.C.; Ryan, C.W.; Sharon, E.; et al. SWOG 1609 Cohort 48: Anti-CTLA-4 and Anti-PD-1 for Advanced Gallbladder Cancer. Cancer 2024, 130, 2918–2927. [Google Scholar] [CrossRef]
- Li, J.; Zhou, S.; Xu, X.; Zheng, Q.; Zhang, F.; Luo, C.; Li, D.; Sun, X.; Han, Z.; Wu, W.; et al. Sintilimab and anlotinib with gemcitabine plus cisplatin in advanced biliary tract cancer: SAGC a randomized phase 2 trial. Nat. Commun. 2025, 16, 5559. [Google Scholar] [CrossRef]
- Yi, L.; Pan, H.; Ning, Z.; Xu, L.; Zhang, H.; Peng, L.; Liu, Y.; Yang, Y.; Si, W.; Wang, Y.; et al. Clinical and biomarker analyses of SHR-1701 combined with famitinib in patients with previously treated advanced biliary tract cancer or pancreatic ductal adenocarcinoma: A phase II trial. Signal Transduct. Target. Ther. 2024, 9, 347. [Google Scholar] [CrossRef]
- Jackson, S.S.; Pfeiffer, R.M.; Liu, Z.; Anderson, L.A.; Tsai, H.-T.; Gadalla, S.M.; Koshiol, J. Association Between Aspirin Use and Biliary Tract Cancer Survival. JAMA Oncol. 2019, 5, 1802–1804, Erratum in JAMA Oncol. 2019, 5, 1811. https://doi.org/10.1001/jamaoncol.2019.5657. [Google Scholar] [CrossRef]
- Yamazaki, H.; Shibuya, K.; Kimoto, T.; Suzuki, M.; Murakami, M.; Terashima, K.; Okimoto, T.; Iizumi, T.; Sakurai, H.; Wakatsuki, M.; et al. Updated Japanese multicenter registry study evaluates the efficacy and safety of proton beam therapy for treating extrahepatic cholangiocarcinoma. Sci. Rep. 2025, 15, 23250. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Hirose, Y.; Takihara, H.; Okuda, A.; Ling, Y.; Tajima, Y.; Shimada, Y.; Ichikawa, H.; Takizawa, K.; Sakata, J.; et al. Unveiling microbiome profiles in human inner body fluids and tumor tissues with pancreatic or biliary tract cancer. Sci. Rep. 2022, 12, 8766. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Fukui, S.; Oosuka, K.; Ikeda, K.; Kobayashi, M.; Shimada, Y.; Nakazawa, Y.; Nishiura, Y.; Suga, D.; Moritani, I.; et al. Biliary microbiome profiling via 16 S rRNA amplicon sequencing in patients with cholangiocarcinoma, pancreatic carcinoma and choledocholithiasis. Sci. Rep. 2025, 15, 16966. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.C.; Kilgour, E.; Jacobs, T.; Lamarca, A.; Hubner, R.A.; Valle, J.W.; McNamara, M.G. Potential influence of the microbiome environment in patients with biliary tract cancer and implications for therapy. Br. J. Cancer 2021, 126, 693–705. [Google Scholar] [CrossRef]
| Regimen | Applicable Population | Design | mOS | Hazard Ratio p Value | mPFS | Hazard Ratio p Value | ORR | p Value | Source |
|---|---|---|---|---|---|---|---|---|---|
| Durvalumab + GEM + DDP | U/M | Durvalumab + GEM + DDP GEM + DDP | 12.9 m 11.3 m | HR = 0.80 p = 0.021 | 7.2 m 5.7 m | HR = 0.75 p = 0.001 | 26.7% 18.7% | p = 0.007 | TOPAZ-1 |
| Pembrolizumab + GEM + DDP | U/M | Pembrolizumab + GEM + DDP GEM + DDP | 12.7 m 10.9 m | HR = 0.83 p = 0.0034 | 6.3 m 5.8 m | HR = 0.86 p = 0.0038 | 29.0% 24.0% | p = 0.0029 | KEYNOTE-966 |
| GEM + DDP | NI | GEM + DDP GEM | 11.7 m 8.1 m | HR = 0.64 p < 0.001 | 8.0 m 5.0 m | HR = 0.63 p < 0.001 | 26.1% 15.5% | p = 0.006 | ABC-02 |
| Targeted Therapy | Patients with alterations * | Refer to Table 2 | |||||||
| Biomarker | Regimen | Study Population | Treatment Group | mDoR | mPFS | mOS | ORR | DCR | Trial |
|---|---|---|---|---|---|---|---|---|---|
| MSI-H/dMMR | Pembrolizumab * | Pre-treated Patients | SA | N | 4.1 m | 23.5 m | 39.60% | - | KEYNOTE-158 |
| NTRK Gene Fusion | Larotrectinib * | 49.3 m | 35.4 m | N | 75.00% | - | LOXO-TRK-01,NAVIGATE | ||
| Entrectinib * | 20.0 m | 13.8 m | 33.8 m | 63.00% | - | STARTRK-1/2,ALKA-372-001 | |||
| FGFR2 Fusion/ Rearrangement | Pemigatinib * | 7.5 m | 6.9 m | 21.1 m | 35.50% | 82.00% | FIGHT-202 | ||
| Futibatinib * | 9.5 m | 8.9 m | 20.0 m | 41.70% | 82.50% | FOENIX-CCA2 | |||
| Infigratinib | 5.0 m | 7.3 m | 12.2 m | 23.10% | 84.30% | CBGJ398X2204 | |||
| IDH1 Mutation | Ivosidenib * | Ivosidenib PB | - | 2.7 m 1.4 m (HR = 0.37, p < 0.0001) | 10.3 m 7.5 m (HR = 0.49, p < 0.0001) | 2.4% p = 0.283 | 53.2% p < 0.0001 | ClarlDHy | |
| BRAF V600E Mutation | Dabrafenib * + Trametinib * | SA | 8.7 m | 8.9 m | 13.5 m | 51% | 79% | ROAR |
| Treatment Population | Treatment Strategy | Regimen | mOS | Hazard Ratio p Value | mPFS | Hazard Ratio p Value | ORR | Trail |
|---|---|---|---|---|---|---|---|---|
| With actionable targets | Refer to Table 2 | |||||||
| Without actionable targets | FOLFOX | FOLFOX +ASC ASC | 6.2 m 5.3 m | HR = 0.69 p = 0.031 | 4.0 m 2.0 m | HR = 0.63 p = 0.006 | 5% 0 | ABC-06 |
| FOLFIRI | Irinotecan +Leucovorin +5-FU | 10.6 m | - | 4.6 m | - | 15% | Phase II single-arm | |
| Nab-Paclitaxel +Gemcitabine | Nab-Paclitaxel +Gemcitabine | 7-9 m | - | 3-5 m | - | 10-20% | Retrospective analysis | |
| Capecitabine | Capecitabine | 5.3 m | - | 2.3 m | - | 5.60% | Retrospective analysis | |
| S-1 | S-1 | 7.6 m | - | 2.7 m | - | 10% | Phase II single-arm | |
| Clinical Trail | N | Regimen | mOS | Hazard Ratio p Value | mPFS | Hazard Ratio p Value | ORR | p Value |
|---|---|---|---|---|---|---|---|---|
| chemotherapy | ||||||||
| NIFTY Trial Jaewon Hyung, Ilhwan Kim et al. JAMA Oncol. 2023 [40] | 174 | nal-IRI + FU/LV PB | 8.6 m 5.3 m | HR = 0.68 p = 0.02 | 3.9 m 1.6 m | HR: 0.51 p < 0.001 | 19.3% 2.3% | p < 0.001 |
| targeted therapy | ||||||||
| FIGHT-202 Trial Abou-Alfa GK et al. Lancet Oncol. 2020 [29] | 146 | pemigatinib | 21.1 m | - | 6.9 m | - | 35.50% | - |
| FOENIX-CCA2 Trial Goyal L et al. N. Engl. J. Med. 2023 [30] | 103 | futibatinib | 20.0 m | - | 8.9 m | - | 41.70% | - |
| ClarIDHy Goyal L et al. N. Engl. J. Med. 2023 [30] | 185 | Ivosidenib PB | 10.3 m 7.5 m | HR = 0.49 p < 0.0001 | 2.7 m 1.4 m | HR: 0.37 p < 0.0001 | 2.4% 0 | - |
| ROAR Trial Vivek Subbiah et al. Lancet Oncol. 2020 [42] | 43 | Dabrafenib+ trametinib | 13.5 m | - | 8.9 m | - | 51.00% | - |
| Zanidatamab-related Funda Meric-Bernstam et al. Lancet Oncol. 2022 [44] | 86 | zanidatamab | - | - | 5.4 m | - | 37.00% | - |
| Surufatinib-related Jianming Xu et al. Cancer 2021 [46] | 39 | surufatinib | 6.9 m | - | 3.7 m | - | 0.00% | - |
| immunotherapy | ||||||||
| Nivolumab-related Richard D. Kim et al. JAMA Oncol. 2020 [48] | 54 | nivolumab | 14.24 m | - | 3.68 m | - | 22.00% | - |
| SWOG 1609 cohort 48 Sandip P. Patel et al. Cancer 2024 [50] | 19 | Nivolumab+ ipilimumab | 7 m | - | 1.8 m | - | 16.00% | - |
| immunotherapy combined with chemotherapy | ||||||||
| IMMUNOBIL PRODIGE 57 Trial Alice Boilève et al. Eur. J. Cancer 2021 [47] | 20 | Durvalumab + tremelimumab + paclitaxel PB | - | - | - | - | 10.00% | - |
| immunotherapy combined with targeted therapy | ||||||||
| SAGC Trial Jaewon Hyung, Ilhwan Kim et al. JAMA Oncol. 2023 [40] | 80 | Sintilimab + anlotinib + GC GC | 13.2 m 13.7 m | HR: 1.04 p = 0.895 | 8.5 m 6.3 m | HR: 0.48 p = 0.005 | 51.4% 29.4% | p = 0.033 |
| SHR-1701 combined with famitinib Lixia Yi et al. Signal Transduct. Target. Ther. 2024 [52] | 27 | SHR-1701+ famitinib | 16.0 m | - | 5.1 m | - | 28.00% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Yi, C. Advanced Biliary Tract Cancer: Exploration of Third-Line and Other Therapeutic Areas After Failure of Chemotherapy Alone. Cancers 2025, 17, 3268. https://doi.org/10.3390/cancers17193268
Ma L, Yi C. Advanced Biliary Tract Cancer: Exploration of Third-Line and Other Therapeutic Areas After Failure of Chemotherapy Alone. Cancers. 2025; 17(19):3268. https://doi.org/10.3390/cancers17193268
Chicago/Turabian StyleMa, Li, and Cheng Yi. 2025. "Advanced Biliary Tract Cancer: Exploration of Third-Line and Other Therapeutic Areas After Failure of Chemotherapy Alone" Cancers 17, no. 19: 3268. https://doi.org/10.3390/cancers17193268
APA StyleMa, L., & Yi, C. (2025). Advanced Biliary Tract Cancer: Exploration of Third-Line and Other Therapeutic Areas After Failure of Chemotherapy Alone. Cancers, 17(19), 3268. https://doi.org/10.3390/cancers17193268




