Tumour-on-Chip Models for the Study of Ovarian Cancer: Current Challenges and Future Prospects
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
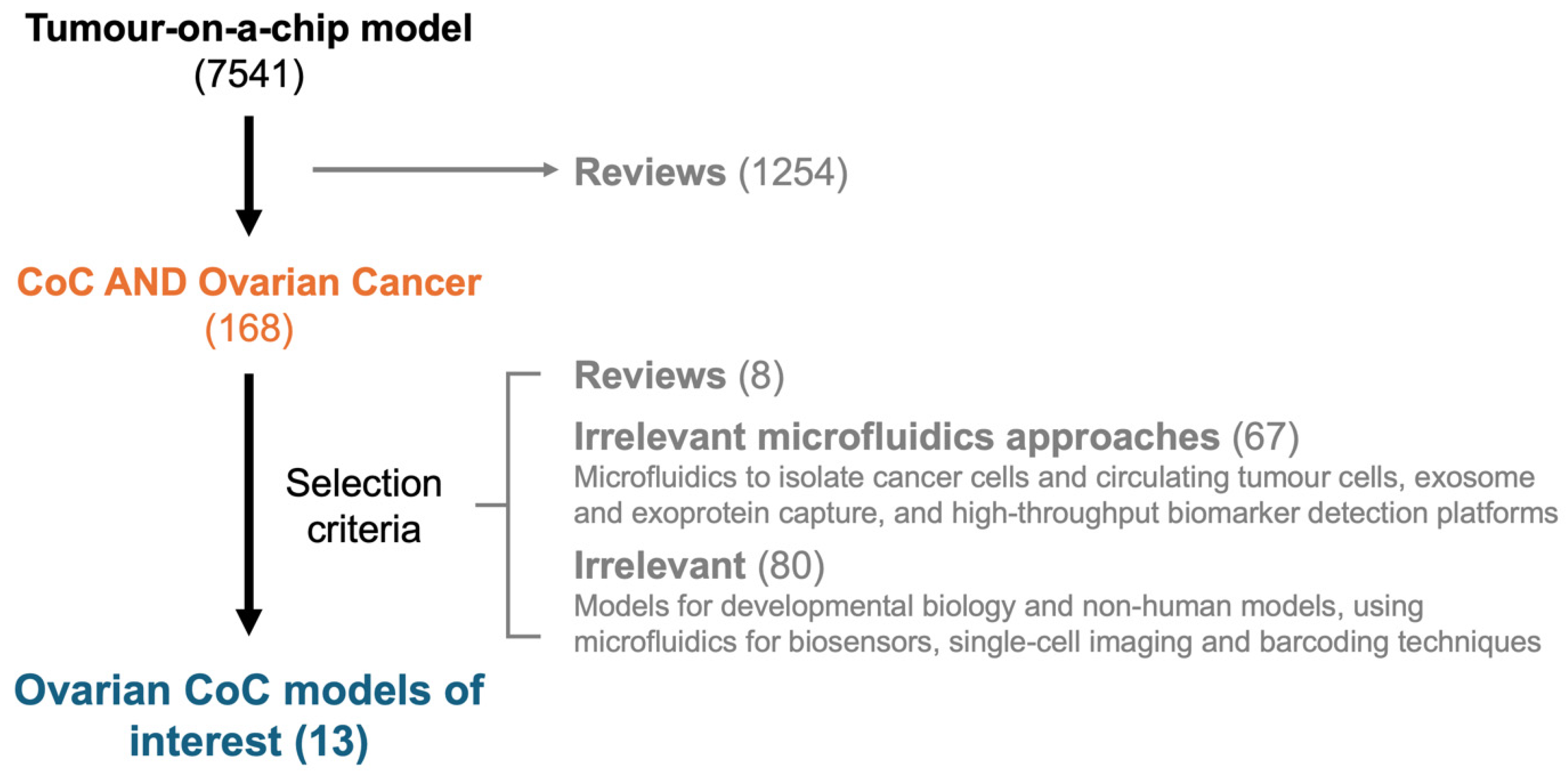
2.1. Literature Review Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
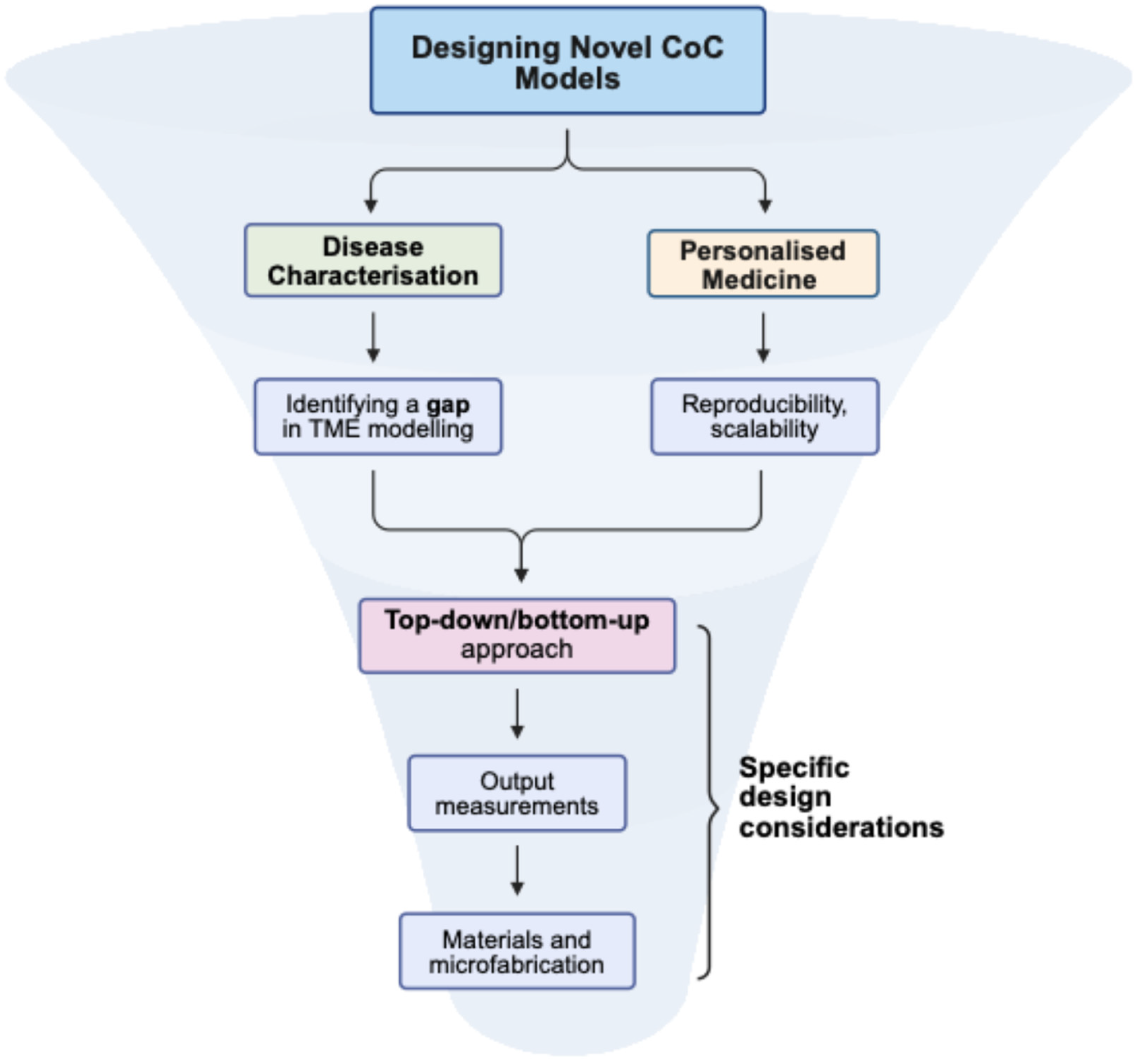
2.4. Design Considerations
3. Results
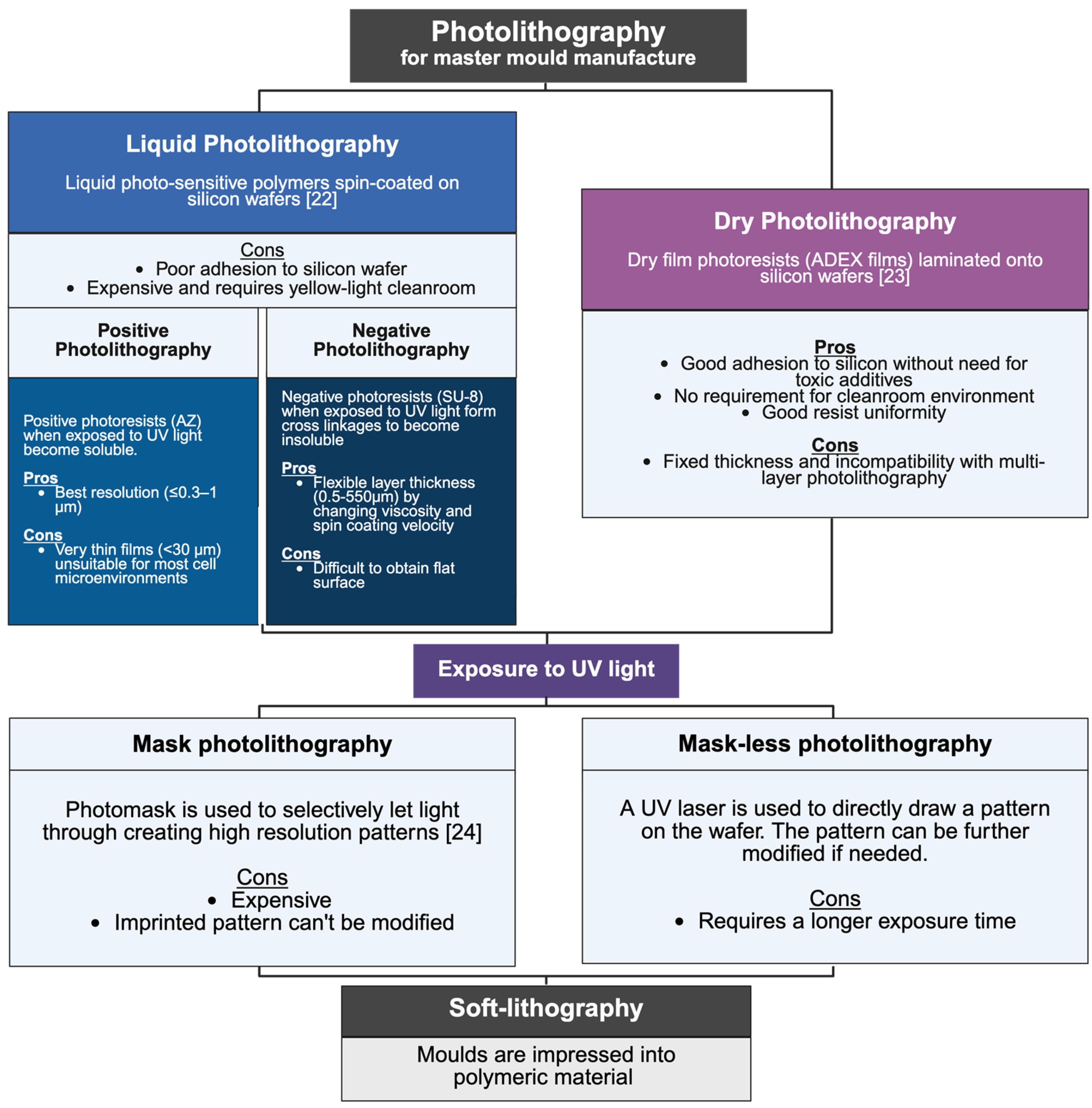
3.1. Review of CoC Manufacturing
3.2. Review of CoC Design Approaches Used in Cancer Research
3.2.1. Bottom-Up Approach
3.2.2. Top-Down Approach
3.2.3. Media Perifusion
3.2.4. Drug Screening Platforms
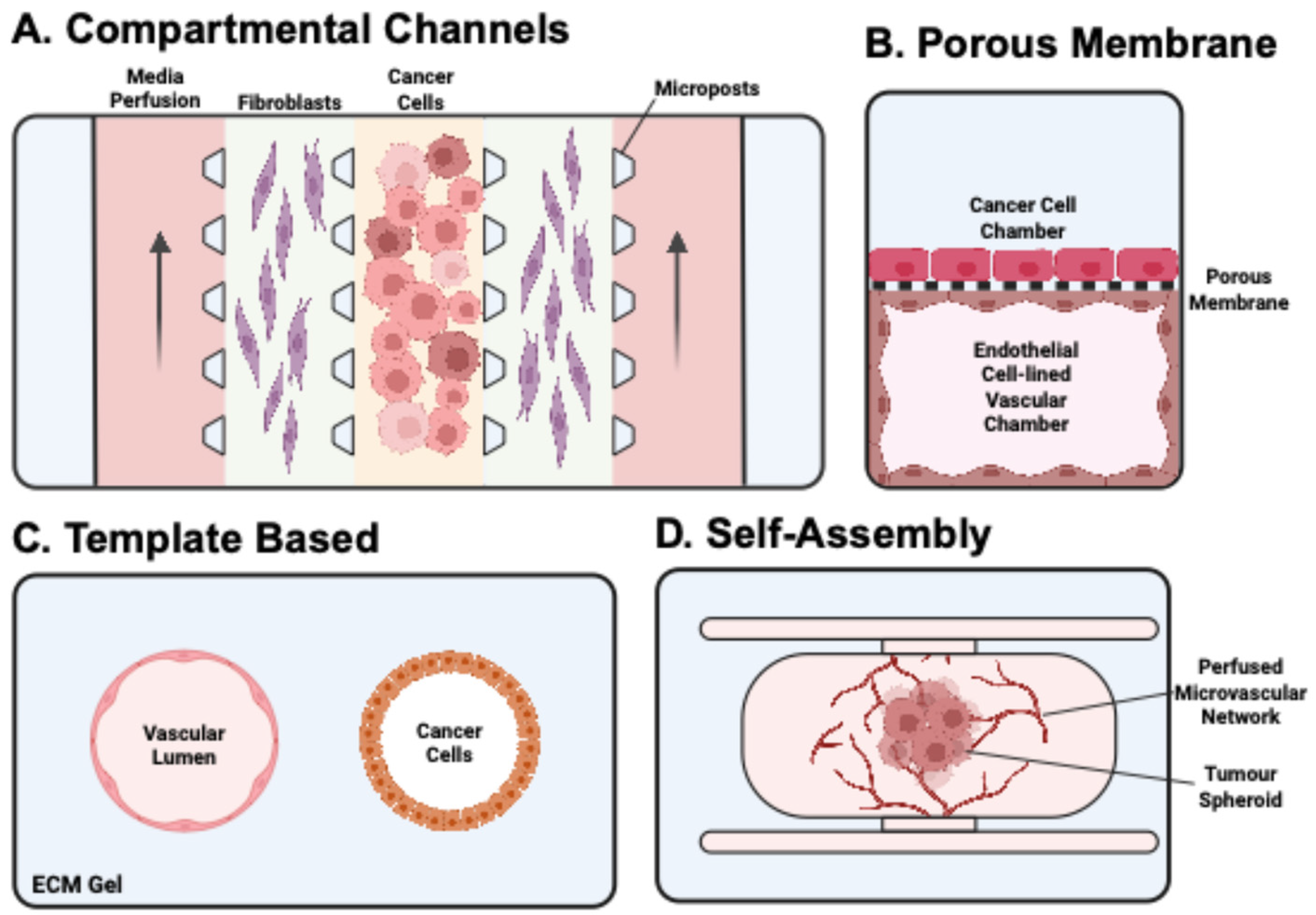
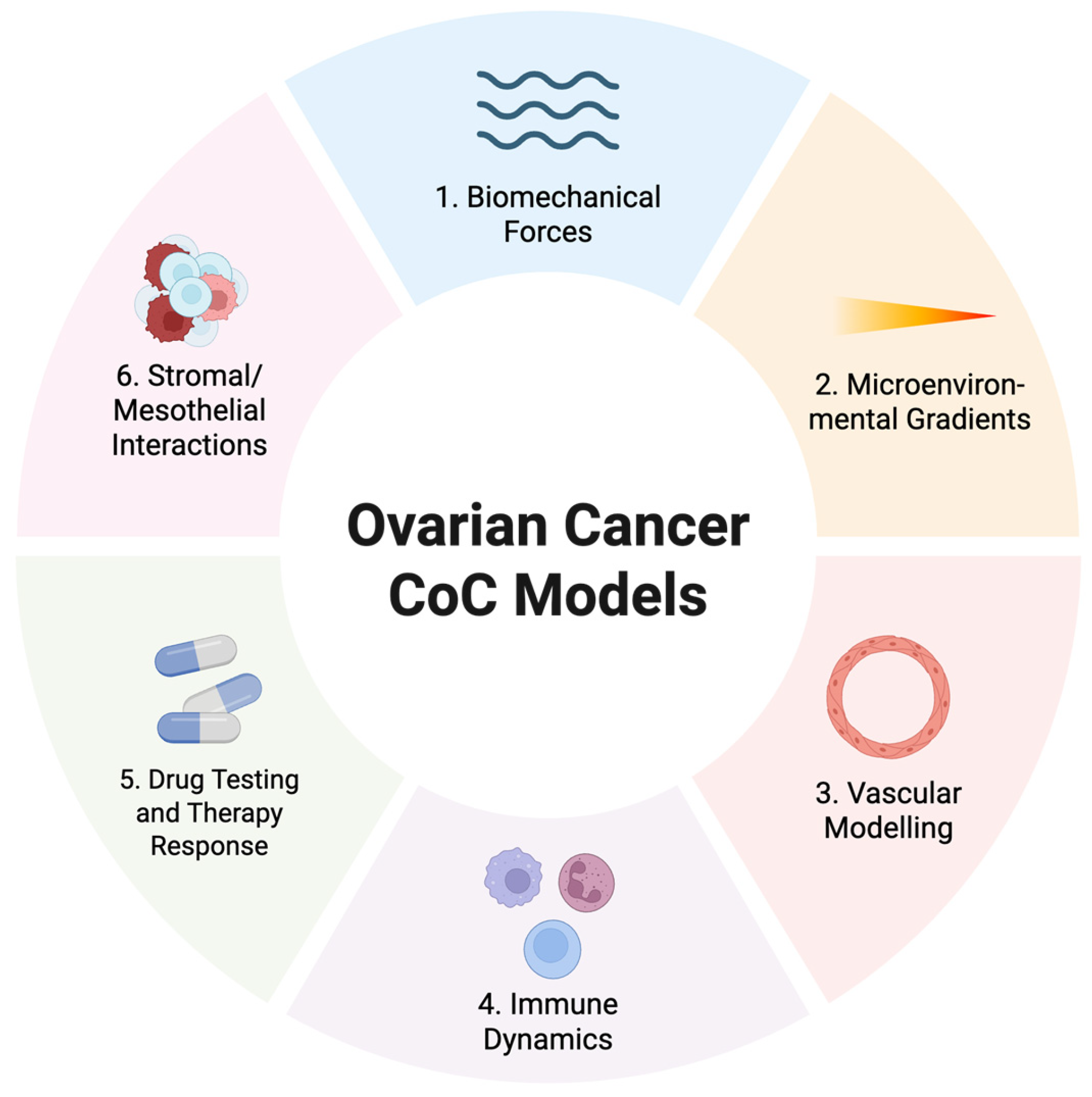
3.3. Review of CoC Models Used in Ovarian Cancer Research
3.3.1. Simple Designs to Introduce Biomechanical Forces
3.3.2. Compartmentalised Designs
3.3.3. Complex Multi-Compartment Designs
3.3.4. Porous Membrane Designs
3.4. Extracellular Matrix–Specific Effects on Ovarian Cancer Biology
3.5. Points to Be Considered When Approaching New Designs for CoC Models
4. Discussion
4.1. Biological Challenges
4.2. Technical Limitations
4.3. Lack of Standardisation
4.4. Future Prospects
4.5. Clinical Translation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Auersperg, N.; Wong, A.S.T.; Choi, K.-C.; Kang, S.K.; Leung, P.C.K. Ovarian Surface Epithelium: Biology, Endocrinology, and Pathology. Endocr. Rev. 2001, 22, 255–288. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; deFazio, A.; et al. Driver Mutations in TP53 Are Ubiquitous in High Grade Serous Carcinoma of the Ovary. J. Pathol. 2010, 221, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.-P.; Calcagno, A.M.; Varma, S.; Marino, M.; Green, L.J.; Vora, M.I.; Patel, C.; Orina, J.N.; Eliseeva, T.A.; Singal, V.; et al. Redefining the Relevance of Established Cancer Cell Lines to the Study of Mechanisms of Clinical Anti-Cancer Drug Resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 18708–18713. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating Cell Lines as Tumour Models by Comparison of Genomic Profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Ciucci, A.; Buttarelli, M.; Fagotti, A.; Scambia, G.; Gallo, D. Preclinical Models of Epithelial Ovarian Cancer: Practical Considerations and Challenges for a Meaningful Application. Cell. Mol. Life Sci. 2022, 79, 364. [Google Scholar] [CrossRef]
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In Vitro Three-dimensional (3D) Models in Cancer Research: An Update. Mol. Carcinog. 2013, 52, 167–182. [Google Scholar] [CrossRef]
- Burleson, K.M.; Casey, R.C.; Skubitz, K.M.; Pambuccian, S.E.; Oegema, T.R.; Skubitz, A.P.N. Ovarian Carcinoma Ascites Spheroids Adhere to Extracellular Matrix Components and Mesothelial Cell Monolayers. Gynecol. Oncol. 2004, 93, 170–181. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; De Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.; Fosse, V.; Kleinmanns, K.; Bjørge, L.; McCormack, E. Xenograft Models of Ovarian Cancer for Therapy Evaluation. In Ovarian Cancer; Kreeger, P.K., Ed.; Springer: New York, NY, USA, 2022; Volume 2424, pp. 275–293. ISBN 9781071619551. [Google Scholar]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a Syngeneic Mouse Model for Events Related to Ovarian Cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Howell, V.M. Genetically Engineered Mouse Models for Epithelial Ovarian Cancer: Are We There Yet? Semin. Cell Dev. Biol. 2014, 27, 106–117. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic Organs-on-Chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Liu, X.; Fang, J.; Huang, S.; Wu, X.; Xie, X.; Wang, J.; Liu, F.; Zhang, M.; Peng, Z.; Hu, N. Tumor-on-a-Chip: From Bioinspired Design to Biomedical Application. Microsyst. Nanoeng. 2021, 7, 50. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, X.; He, W.; Zhao, Y.; Hao, S.; Wu, Q.; Li, S.; Zhang, S.; Shi, M. Fluid Shear Stress and Tumor Metastasis. Am. J. Cancer Res. 2018, 8, 763–777. [Google Scholar]
- Garcia-Garcia, F.C.; Candarlioglu, P.L.; Porter, J.D.; Davies, D.E.; Swindle, E.J.; Morgan, H. Microfluidic technologies for ex vivo tissue biopsies: A review. Organs-on-a-Chip 2022, 4, 100020. [Google Scholar] [CrossRef]
- Bouquerel, C.; Dubrova, A.; Hofer, I.; Phan, D.T.T.; Bernheim, M.; Ladaigue, S.; Cavaniol, C.; Maddalo, D.; Cabel, L.; Mechta-Grigoriou, F.; et al. Bridging the Gap between Tumor-on-Chip and Clinics: A Systematic Review of 15 Years of Studies. Lab. Chip 2023, 23, 3906–3935. [Google Scholar] [CrossRef]
- Ferrari, E.; Nebuloni, F.; Rasponi, M.; Occhetta, P. Photo and Soft Lithography for Organ-on-Chip Applications. Methods Mol Biol. 2022, 2373, 1–19. [Google Scholar] [CrossRef]
- Winter, P.M.; Lanza, G.M.; Wickline, S.A.; Madou, M.; Wang, C.; Deotare, P.B.; Loncar, M.; Yap, Y.K.; Rose, J.; Auffan, M.; et al. Photolithography. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 2051–2060. ISBN 9789048197507. [Google Scholar]
- Mukherjee, P.; Nebuloni, F.; Gao, H.; Zhou, J.; Papautsky, I. Rapid Prototyping of Soft Lithography Masters for Microfluidic Devices Using Dry Film Photoresist in a Non-Cleanroom Setting. Micromachines 2019, 10, 192. [Google Scholar] [CrossRef]
- Rizvi, S. (Ed.) Handbook of Photomask Manufacturing Technology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Torino, S.; Corrado, B.; Iodice, M.; Coppola, G. PDMS-Based Microfluidic Devices for Cell Culture. Inventions 2018, 3, 65. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, B.J.; De Vries, H.; Firth, K.S.A.; Van Weerd, J.; Tertoolen, L.G.J.; Karperien, H.B.J.; Jonkheijm, P.; Denning, C.; IJzerman, A.P.; Mummery, C.L. Small Molecule Absorption by PDMS in the Context of Drug Response Bioassays. Biochem. Biophys. Res. Commun. 2017, 482, 323–328. [Google Scholar] [CrossRef]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.-E.; Ahn, S.; Kang, J.H. Robust Chemical Bonding of PMMA Microfluidic Devices to Porous PETE Membranes for Reliable Cytotoxicity Testing of Drugs. Lab Chip 2019, 19, 3706–3713. [Google Scholar] [CrossRef]
- Wu, X.; Shi, W.; Liu, X.; Gu, Z. Recent Advances in 3D-Printing-Based Organ-on-a-Chip. EngMedicine 2024, 1, 100003. [Google Scholar] [CrossRef]
- Alifui-Segbaya, F.; Varma, S.; Lieschke, G.J.; George, R. Biocompatibility of Photopolymers in 3D Printing. 3D Print. Addit. Manuf. 2017, 4, 185–191. [Google Scholar] [CrossRef]
- Guttridge, C.; Shannon, A.; O’Sullivan, A.; O’Sullivan, K.J.; O’Sullivan, L.W. Biocompatible 3D Printing Resins for Medical Applications: A Review of Marketed Intended Use, Biocompatibility Certification, and Post-Processing Guidance. Ann. 3D Print. Med. 2022, 5, 100044. [Google Scholar] [CrossRef]
- Venzac, B.; Deng, S.; Mahmoud, Z.; Lenferink, A.; Costa, A.; Bray, F.; Otto, C.; Rolando, C.; Le Gac, S. PDMS Curing Inhibition on 3D-Printed Molds: Why? Also, How to Avoid It? Anal. Chem. 2021, 93, 7180–7187. [Google Scholar] [CrossRef]
- Fruncillo, S.; Su, X.; Liu, H.; Wong, L.S. Lithographic processes for the scalable fabrication of micro- and nanostructures for biochips and biosensors. ACS Sens. 2021, 6, 2002–2024. [Google Scholar] [CrossRef] [PubMed]
- Jouybar, M.; De Winde, C.M.; Wolf, K.; Friedl, P.; Mebius, R.E.; Den Toonder, J.M.J. Cancer-on-Chip Models for Metastasis: Importance of the Tumor Microenvironment. Trends Biotechnol. 2024, 42, 431–448. [Google Scholar] [CrossRef]
- Kim, J.; Chung, M.; Kim, S.; Jo, D.H.; Kim, J.H.; Jeon, N.L. Engineering of a Biomimetic Pericyte-Covered 3D Microvascular Network. PLoS ONE 2015, 10, e0133880. [Google Scholar] [CrossRef] [PubMed]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized Cancer on a Chip: The Effect of Perfusion on Growth and Drug Delivery of Tumor Spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, J.; Li, R.; Ma, A.; Kwan, V.; Luong, K.; Sohn, L.L. Hydrophobic Patterning-Based 3D Microfluidic Cell Culture Assay. Adv. Healthc. Mater. 2018, 7, 1800122. [Google Scholar] [CrossRef]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-Dimensional Microfluidic Model for Tumor Cell Intravasation and Endothelial Barrier Function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef]
- Choi, Y.; Hyun, E.; Seo, J.; Blundell, C.; Kim, H.C.; Lee, E.; Lee, S.H.; Moon, A.; Moon, W.K.; Huh, D. A Microengineered Pathophysiological Model of Early-Stage Breast Cancer. Lab Chip 2015, 15, 3350–3357. [Google Scholar] [CrossRef]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef]
- Bischel, L.L.; Lee, S.-H.; Beebe, D.J. A Practical Method for Patterning Lumens through ECM Hydrogels via Viscous Finger Patterning. SLAS Technol. 2012, 17, 96–103. [Google Scholar] [CrossRef]
- Moon, H.; Ospina-Muñoz, N.; Noe-Kim, V.; Yang, Y.; Elzey, B.D.; Konieczny, S.F.; Han, B. Subtype-Specific Characterization of Breast Cancer Invasion Using a Microfluidic Tumor Platform. PLoS ONE 2020, 15, e0234012. [Google Scholar] [CrossRef]
- Nguyen, D.-H.T.; Lee, E.; Alimperti, S.; Norgard, R.J.; Wong, A.; Lee, J.J.-K.; Eyckmans, J.; Stanger, B.Z.; Chen, C.S. A Biomimetic Pancreatic Cancer On-Chip Reveals Endothelial Ablation via ALK7 Signaling. Sci. Adv. 2019, 5, eaav6789. [Google Scholar] [CrossRef]
- Boussommier-Calleja, A.; Atiyas, Y.; Haase, K.; Headley, M.; Lewis, C.; Kamm, R.D. The Effects of Monocytes on Tumor Cell Extravasation in a 3D Vascularized Microfluidic Model. Biomaterials 2019, 198, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Auger, F.A.; Gibot, L.; Lacroix, D. The Pivotal Role of Vascularization in Tissue Engineering. Annu. Rev. Biomed. Eng. 2013, 15, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.; Khong, Y.-M.; Chang, S.; Ni, J.; Sun, W.; Yu, H. Perfusion Culture Improves the Maintenance of Cultured Liver Tissue Slices. Tissue Eng. 2007, 13, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Dorrigiv, D.; Simeone, K.; Communal, L.; Kendall-Dupont, J.; St-Georges-Robillard, A.; Péant, B.; Carmona, E.; Mes-Masson, A.-M.; Gervais, T. Microdissected Tissue vs. Tissue Slices—A Comparative Study of Tumor Explant Models Cultured On-Chip and Off-Chip. Cancers 2021, 13, 4208. [Google Scholar] [CrossRef]
- Van Midwoud, P.M.; Groothuis, G.M.M.; Merema, M.T.; Verpoorte, E. Microfluidic Biochip for the Perifusion of Precision-cut Rat Liver Slices for Metabolism and Toxicology Studies. Biotech Bioeng. 2010, 105, 184–194. [Google Scholar] [CrossRef]
- Kennedy, R.; Kuvshinov, D.; Sdrolia, A.; Kuvshinova, E.; Hilton, K.; Crank, S.; Beavis, A.W.; Green, V.; Greenman, J. A Patient Tumour-on-a-Chip System for Personalised Investigation of Radiotherapy Based Treatment Regimens. Sci. Rep. 2019, 9, 6327. [Google Scholar] [CrossRef]
- Eslami Amirabadi, H.; Donkers, J.M.; Wierenga, E.; Ingenhut, B.; Pieters, L.; Stevens, L.; Donkers, T.; Westerhout, J.; Masereeuw, R.; Bobeldijk-Pastorova, I.; et al. Intestinal Explant Barrier Chip: Long-Term Intestinal Absorption Screening in a Novel Microphysiological System Using Tissue Explants. Lab Chip 2022, 22, 326–342. [Google Scholar] [CrossRef]
- Horowitz, L.F.; Rodriguez, A.D.; Dereli-Korkut, Z.; Lin, R.; Castro, K.; Mikheev, A.M.; Monnat, R.J.; Folch, A.; Rostomily, R.C. Multiplexed Drug Testing of Tumor Slices Using a Microfluidic Platform. npj Precis. Oncol. 2020, 4, 12. [Google Scholar] [CrossRef]
- Rizvi, I.; Gurkan, U.A.; Tasoglu, S.; Alagic, N.; Celli, J.P.; Mensah, L.B.; Mai, Z.; Demirci, U.; Hasan, T. Flow Induces Epithelial-Mesenchymal Transition, Cellular Heterogeneity and Biomarker Modulation in 3D Ovarian Cancer Nodules. Proc. Natl. Acad. Sci. USA 2013, 110, E1974–E1983. [Google Scholar] [CrossRef]
- Li, S.-S.; Ip, C.K.M.; Tang, M.Y.H.; Sy, S.K.H.; Yung, S.; Chan, T.-M.; Yang, M.; Shum, H.C.; Wong, A.S.T. Modeling Ovarian Cancer Multicellular Spheroid Behavior in a Dynamic 3D Peritoneal Microdevice. JoVE 2017, 55337. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, E.-K.; Park, J.; Suh, P.-G.; Cho, Y.-K. RhoA and Rac1 Play Independent Roles in Lysophosphatidic Acid-Induced Ovarian Cancer Chemotaxis. Integr. Biol. 2014, 6, 267–276. [Google Scholar] [CrossRef]
- Scott, A.L.; Jazwinska, D.E.; Kulawiec, D.G.; Zervantonakis, I.K. Paracrine Ovarian Cancer Cell-Derived CSF1 Signaling Regulates Macrophage Migration Dynamics in a 3D Microfluidic Model That Recapitulates In Vivo Infiltration Patterns in Patient-Derived Xenografts. Adv. Healthc. Mater. 2024, 13, 2401719. [Google Scholar] [CrossRef] [PubMed]
- Wimalachandra, D.C.; Li, Y.; Liu, J.; Shikha, S.; Zhang, J.; Lim, Y.-C.; Zhang, Y. Microfluidic-Based Immunomodulation of Immune Cells Using Upconversion Nanoparticles in Simulated Blood Vessel–Tumor System. ACS Appl. Mater. Interfaces 2019, 11, 37513–37523. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.; Kramer, M.; Johnson, B.; Huskin, G.; Berry, J.; Sewell-Loftin, M.K. Mechanical Activation and Expression of HSP27 in Epithelial Ovarian Cancer. Sci. Rep. 2024, 14, 2856. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.D.; Malacrida, B.; Laforêts, F.; Kotantaki, P.; Maniati, E.; Manchanda, R.; Annibaldi, A.; Hopkins, S.; Garrobo-Calleja, I.; Gautrot, J.; et al. Human 3D Ovarian Cancer Models Reveal Malignant Cell–Intrinsic and –Extrinsic Factors That Influence CAR T-Cell Activity. Cancer Res. 2024, 84, 2432–2449. [Google Scholar] [CrossRef]
- Plesselova, S.; Calar, K.; Axemaker, H.; Sahly, E.; Bhagia, A.; Faragher, J.L.; Fink, D.M.; De La Puente, P. Multicompartmentalized Microvascularized Tumor-on-a-Chip to Study Tumor-Stroma Interactions and Drug Resistance in Ovarian Cancer. Cell. Mol. Bioeng. 2024, 17, 345–367. [Google Scholar] [CrossRef]
- Ibrahim, L.I.; Hajal, C.; Offeddu, G.S.; Gillrie, M.R.; Kamm, R.D. Omentum-on-a-Chip: A Multicellular, Vascularized Microfluidic Model of the Human Peritoneum for the Study of Ovarian Cancer Metastases. Biomaterials 2022, 288, 121728. [Google Scholar] [CrossRef]
- Surendran, V.; Rutledge, D.; Colmon, R.; Chandrasekaran, A. A Novel Tumor-Immune Microenvironment (TIME)-on-Chip Mimics Three Dimensional Neutrophil-Tumor Dynamics and Neutrophil Extracellular Traps (NETs)-Mediated Collective Tumor Invasion. Biofabrication 2021, 13, 035029. [Google Scholar] [CrossRef]
- Saha, B.; Mathur, T.; Tronolone, J.J.; Chokshi, M.; Lokhande, G.K.; Selahi, A.; Gaharwar, A.K.; Afshar-Kharghan, V.; Sood, A.K.; Bao, G.; et al. Human Tumor Microenvironment Chip Evaluates the Consequences of Platelet Extravasation and Combinatorial Antitumor-Antiplatelet Therapy in Ovarian Cancer. Sci. Adv. 2021, 7, eabg5283. [Google Scholar] [CrossRef]
- Saha, B.; Mathur, T.; Handley, K.F.; Hu, W.; Afshar-Kharghan, V.; Sood, A.K.; Jain, A. OvCa-Chip Microsystem Recreates Vascular Endothelium–Mediated Platelet Extravasation in Ovarian Cancer. Blood Adv. 2020, 4, 3329–3342. [Google Scholar] [CrossRef]
- Flont, M.; Jastrzębska, E.; Brzózka, Z. A Multilayered Cancer-on-a-Chip Model to Analyze the Effectiveness of New-Generation Photosensitizers. Analyst 2020, 145, 6937–6947. [Google Scholar] [CrossRef]
- Avraham-Chakim, L.; Elad, D.; Zaretsky, U.; Kloog, Y.; Jaffa, A.; Grisaru, D. Fluid-Flow Induced Wall Shear Stress and Epithelial Ovarian Cancer Peritoneal Spreading. PLoS ONE 2013, 8, e60965. [Google Scholar] [CrossRef]
- Jeffrey, B.; Udaykumar, H.S.; Schulze, K.S. Flow Fields Generated by Peristaltic Reflex in Isolated Guinea Pig Ileum: Impact of Contraction Depth and Shoulders. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 285, G907–G918. [Google Scholar] [CrossRef]
- Ip, C.K.M.; Li, S.-S.; Tang, M.Y.H.; Sy, S.K.H.; Ren, Y.; Shum, H.C.; Wong, A.S.T. Stemness and Chemoresistance in Epithelial Ovarian Carcinoma Cells under Shear Stress. Sci. Rep. 2016, 6, 26788. [Google Scholar] [CrossRef]
- Neilson, L.J.; Cartwright, D.; Risteli, M.; Jokinen, E.M.; McGarry, L.; Sandvik, T.; Nikolatou, K.; Hodge, K.; Atkinson, S.; Vias, M.; et al. Omentum-Derived Matrix Enables the Study of Metastatic Ovarian Cancer and Stromal Cell Functions in a Physiologically Relevant Environment. Matrix Biol. Plus 2023, 19–20, 100136. [Google Scholar] [CrossRef]
| Device | Objective | Design Approach | TME Cells Used | OC Cells | Matrix | Output Measurements | Main Outcome |
|---|---|---|---|---|---|---|---|
| OC micronodules in microfluidic platform [53] | Investigate the role of fluidic forces on metastasis | Microchannel | N/A | OVCAR-5 | Growth-factor reduced (GFR) matrigel | Fluorescent microscopy, RT-PCR, WB | OC cells under flow had morphological, genetic, and protein profiles associated with increased epithelial–mesenchymal transition |
| Multicellular Spheroid in Peritoneal Microdevice [54] | OC spheroids perfused over a layer of mesothelial cells | Microchannel | Peritoneal mesothelial cells | SKOV-3 | Fibronectin coating | N/A | OC spheroids were successfully cultured over mesothelial cells under flow |
| Microfluidic Chemotaxis assay [55] | Tracking the chemotaxis of individual cells and response to RhoA/ROCK inhibition | Compartmentalised channel | N/A | SKOV-3 | Collagen gel and agarose | Fluorescent microscopy, image-based cell tracking assay | SKOV-3 migration speed and directional persistence measured, RhoA/ROCK pathway inhibition reduced directional persistence |
| Macrophage Migration Dynamics in a 3D Microfluidic Model [56] | Modelling macrophage infiltration towards OC cells embedded in ECM matrix | Compartmentalised channel | Macrophages | ID8, DF83 and DF216 patient-derived xenografts | Collagen-1 gel | Fluorescent microscopy, enzyme-linked immunosorbent assay (ELISA) | Macrophage recruited in response to both direct and paracrine interactions with OC cells, CSF1 signalling important for macrophage-rich OC TIME |
| Simulated Blood Vessel–Tumor System [57] | Testing for folic acid-conjugated nanoparticle’s ability to target tumour cells and recruit dendritic and T cells within a vasculature–tumor interface | Compartmentalised channel | Endothelial, Jurkat, dendritic cells | OVCAR-3 | Fibrinogen/thrombin gel, fibronectin coated channel | Fluorescent microscopy | Nanoparticle uptake across the endothelial barrier was verified and T and dendritic cells were recruited. |
| Vascularised TME model [58] | Observing changes to mechanically stimulated cells when they are cultured in a new environment | Compartmentalised channel | Endothelial cells | SKOV-3, SKOV-3 taxol resistant, OVCAR-8 | Fibrinogen and collagen-1 gel | Immunofluorescence, Western blot (WB) | When strained cells were incorporated into the chip, they demonstrated mechanical memory by maintaining their heat shock protein (HSP) expression |
| Vascularised spheroid microfluidic chip [59] | Modelling CAR T-cell activity in a vascularised spheroid chip | Compartmentalised channel | Endothelial cells, fibroblasts | G164, G33 | Fibrin and collagen gel | Fluorescent microscopy, immunofluorescence, chip effluent collected for cytokine assay | CAR T-cells extravasated vascular network, penetrated ECM gel, and induced apoptosis in OC cells |
| Multi-vascularised multi-niche tumour-on-chip [60] | Generating nutrient and oxygen gradients, stromal interactions, drug uptake | Compartmentalised channel | Normal fibroblasts, CAFs, endothelial cells | KURAMOCHI, SKOV-3 | Human plasma matrix based on fibrinogen crosslinking | Fluorescent microscopy, immunofluorescence, imaging flow cytometry | Tumour-CAF interactions, invasion into stromal compartments, CAF induced ECM remodelling, hypoxia, drug resistance |
| Omentum-on-a-chip [61] | Modelling the omental TME and ascites formation | Compartmentalised channel | Adipocytes, endothelial and mesothelial cells | SKOV-3, OV90, OCVAR3 | Fibrin hydrogel | Permeability assays using fluorescent tracers, fluorescent microscopy | Adipocytes increase mesothelium and vessel permeability, ECM composition, and mesothelium vulnerability to tumour attachment |
| Tumor-immune microenvironment (TIME)-on-Chip [62] | Modelling neutrophil recruitment and activation and OC invasion | Porous membrane | Neutrophils | OVCAR-3 | Collagen gel and hydrogel microwells | Immunostaining | Neutrophils respond to tumour spheroid by chemotaxis and neutrophil extracellular traps. |
| OvCa/OTME-chip [63,64] | OC interaction with platelets, metastasis | Porous membrane | Endothelial cells | A2780 | Collagen–fibronectin-coated porous PDMS membrane, collagen-1 hydrogel | Fluorescent microscopy, chip effluent collected for cytokine assay, flow cytometry, RNAseq | OC cells induced vascular dysfunction, demonstrated platelet extravasation and tumour interactions |
| Mutlilayered cancer-on-a-chip model [65] | Multilayered co-culture to study effectiveness of photodynamic therapy | Microchamber | Fibroblasts | A2780 | N/A | Fluorescent intensity quantification using plate-reader, fluorescence microscopy. | Novel photosensitisers’ effectiveness was verified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.Y.; Aboelnasr, L.S.; El-Bahrawy, M. Tumour-on-Chip Models for the Study of Ovarian Cancer: Current Challenges and Future Prospects. Cancers 2025, 17, 3239. https://doi.org/10.3390/cancers17193239
Lim SY, Aboelnasr LS, El-Bahrawy M. Tumour-on-Chip Models for the Study of Ovarian Cancer: Current Challenges and Future Prospects. Cancers. 2025; 17(19):3239. https://doi.org/10.3390/cancers17193239
Chicago/Turabian StyleLim, Sung Yeon, Lamia Sabry Aboelnasr, and Mona El-Bahrawy. 2025. "Tumour-on-Chip Models for the Study of Ovarian Cancer: Current Challenges and Future Prospects" Cancers 17, no. 19: 3239. https://doi.org/10.3390/cancers17193239
APA StyleLim, S. Y., Aboelnasr, L. S., & El-Bahrawy, M. (2025). Tumour-on-Chip Models for the Study of Ovarian Cancer: Current Challenges and Future Prospects. Cancers, 17(19), 3239. https://doi.org/10.3390/cancers17193239






