Co-Stimulatory and Immune Checkpoint Molecule Expression on Peripheral Immune Cells Differs Age Dependently Between Healthy Donors and Patients with Head and Neck Squamous Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Case Selection
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
2.3. Flow Cytometry
2.4. Gating Strategy
2.5. Analyses and Statistics
3. Results
3.1. Patient Characteristics
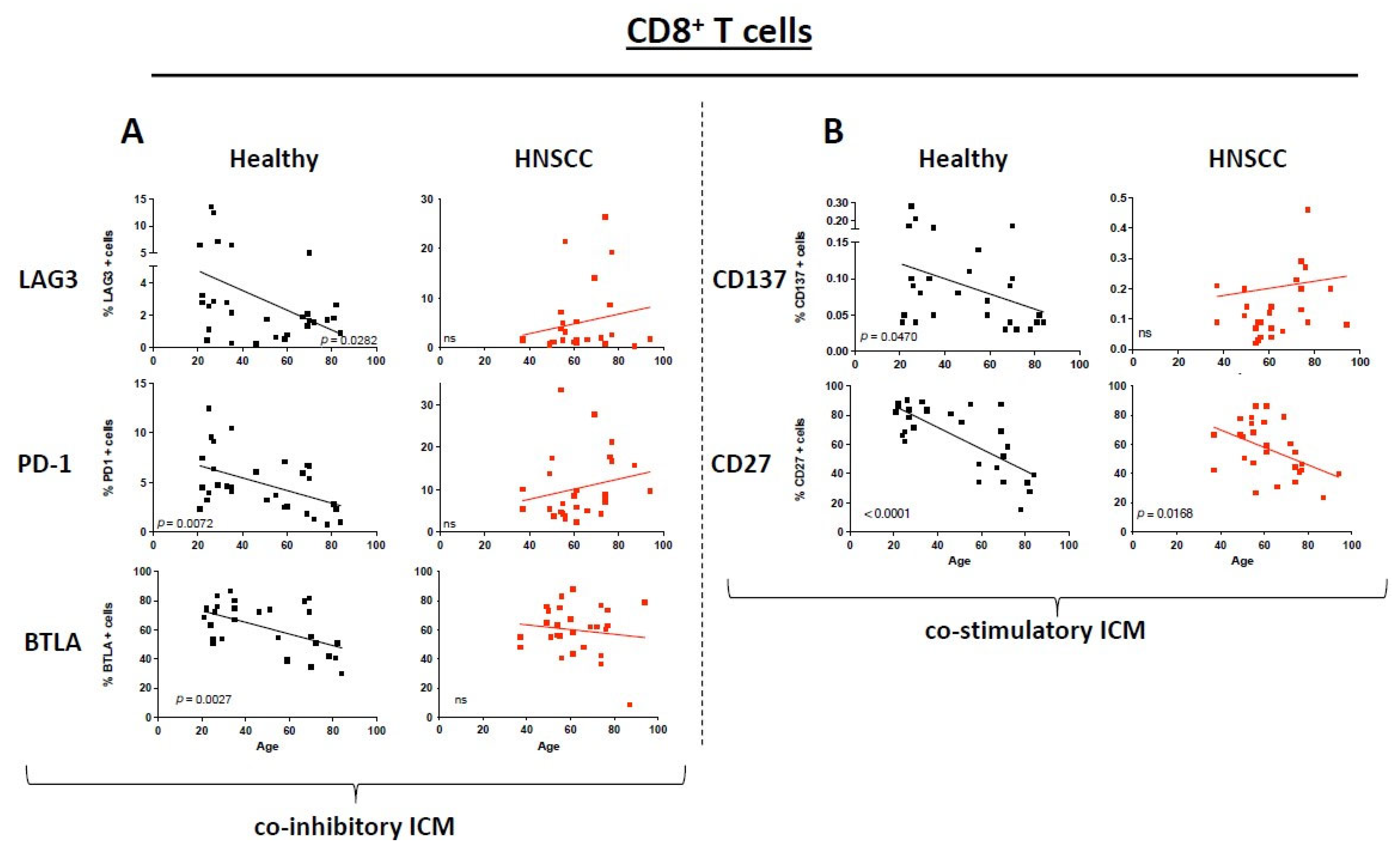
3.2. ICM and Co-Stimulatory Molecule Expression on CD8+ T Cells Differs Between Healthy Donors and HNSCC Patients Depending on Their Age
3.3. ICM and Co-Stimulatory Molecule Expression on CD4+ T Cells Varies Between Healthy Donors and HNSCC Patients Depending on Their Age
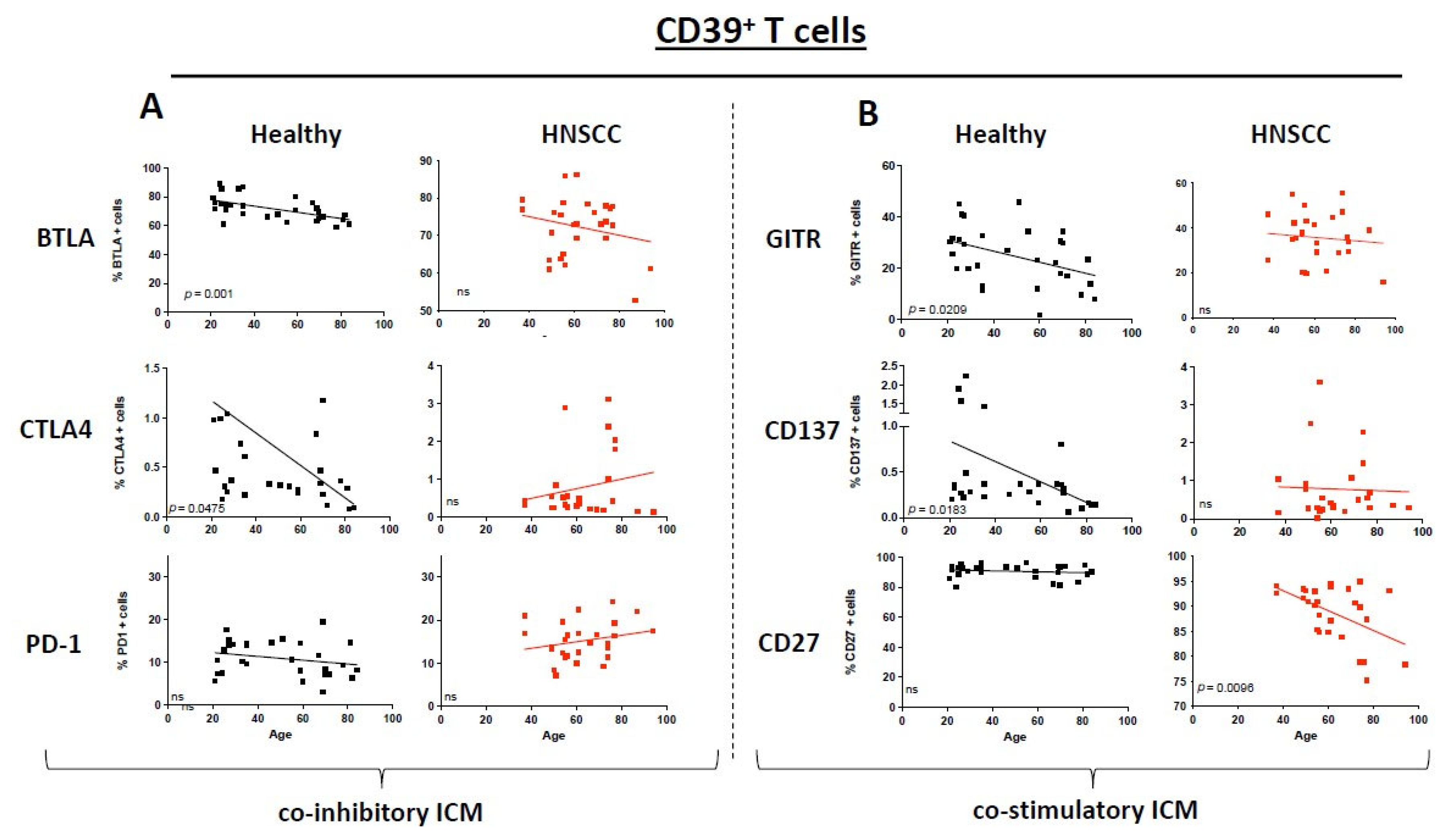
3.4. Immunosuppressive CD39+ T Cells Exhibit a Different ICM and Co-Stimulatory MOLECULE Expression Pattern
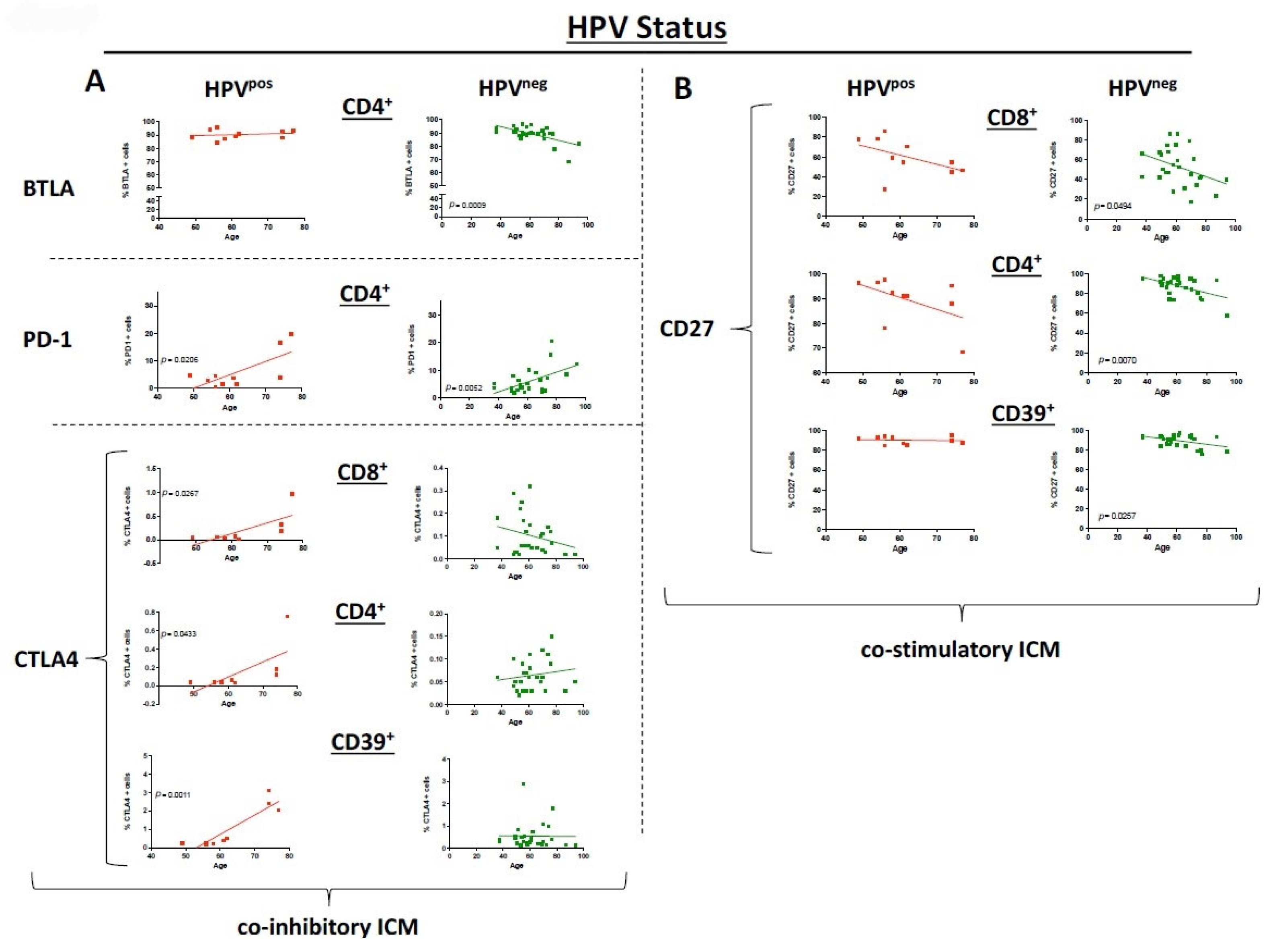
3.5. HPV Status Has a Significant Influence on ICM and Co-Stimulatory Molecule Expression
3.6. ICM and Co-Stimulatory Molecule Expression Differs Between the Sexes
4. Discussion
4.1. Age-Related Differences in Co-Stimulatory Molecules/ICM Expression and Implications
4.2. HPV Status and Immune Landscape Differences
4.3. Sex Differences in Immune Aging
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Syrjanen, S.; Waterboer, T.; Kero, K.; Rautava, J.; Syrjanen, K.; Grenman, S.; Pawlita, M. Oral human papillomavirus infection in men might contribute to HPV serology. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 237–245. [Google Scholar] [CrossRef]
- Andre, K.; Schraub, S.; Mercier, M.; Bontemps, P. Role of alcohol and tobacco in the aetiology of head and neck cancer: A case-control study in the Doubs region of France. Eur. J. Cancer B Oral Oncol. 1995, 31B, 301–309. [Google Scholar] [CrossRef]
- von Witzleben, A.; Currall, E.; Wood, O.; Chudley, L.; Akinyegun, O.; Thomas, J.; Bendjama, K.; Thomas, G.J.; Friedmann, P.S.; King, E.V.; et al. Correlation of HPV16 Gene Status and Gene Expression with Antibody Seropositivity and TIL Status in OPSCC. Front. Oncol. 2020, 10, 591063. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Soulieres, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- von Witzleben, A.; Fehn, A.; Grages, A.; Ezić, J.; Jeske, S.S.; Puntigam, L.K.; Brunner, C.; Kraus, J.M.; Kestler, H.A.; Doescher, J.; et al. Prospective longitudinal study of immune checkpoint molecule (ICM) expression in immune cell subsets during curative conventional therapy of head and neck squamous cell carcinoma (HNSCC). Int. J. Cancer 2021, 148, 2023–2035. [Google Scholar] [CrossRef]
- Sadighi Akha, A.A. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef]
- Vahl, J.M.; Wigand, M.C.; Denkinger, M.; Dallmeier, D.; Steiger, C.; Welke, C.; Kuhn, P.; Idel, C.; Doescher, J.; von Witzleben, A.; et al. Increasing Mean Age of Head and Neck Cancer Patients at a German Tertiary Referral Center. Cancers 2021, 13, 832. [Google Scholar] [CrossRef]
- Puntigam, L.K.; Jeske, S.S.; Götz, M.; Greiner, J.; Laban, S.; Theodoraki, M.N.; Doescher, J.; Weissinger, S.E.; Brunner, C.; Hoffmann, T.K.; et al. Immune Checkpoint Expression on Immune Cells of HNSCC Patients and Modulation by Chemo- and Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5181. [Google Scholar] [CrossRef]
- Schuler, P.J.; Harasymczuk, M.; Schilling, B.; Lang, S.; Whiteside, T.L. Separation of human CD4+CD39+ T cells by magnetic beads reveals two phenotypically and functionally different subsets. J. Immunol. Methods 2011, 369, 59–68. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Paderi, A.; Fancelli, S.; Caliman, E.; Pillozzi, S.; Gambale, E.; Mela, M.M.; Doni, L.; Mazzoni, F.; Antonuzzo, L. Safety of Immune Checkpoint Inhibitors in Elderly Patients: An Observational Study. Curr. Oncol. 2021, 28, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.S.; Schuler, P.J.; Doescher, J.; Theodoraki, M.N.; Laban, S.; Brunner, C.; Hoffmann, T.K.; Wigand, M.C. Age-related changes in T lymphocytes of patients with head and neck squamous cell carcinoma. Immun. Ageing 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Kawczak, P.; Feszak, I.J.; Bączek, T. Comparative Mechanistic Insights and Therapeutic Potential of Pembrolizumab, Durvalumab, and Ipilimumab as Immune Checkpoint Inhibitors in the Targeted Management of Oral and Head and Neck Squamous Cell Carcinoma. Cancers 2025, 17, 2805. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Moreira, A.; Gross, S.; Kirchberger, M.C.; Erdmann, M.; Schuler, G.; Heinzerling, L. Senescence markers: Predictive for response to checkpoint inhibitors. Int. J. Cancer 2019, 144, 1147–1150. [Google Scholar] [CrossRef]
- Demerlé, C.; Gorvel, L.; Olive, D. BTLA-HVEM Couple in Health and Diseases: Insights for Immunotherapy in Lung Cancer. Front. Oncol. 2021, 11, 682007. [Google Scholar] [CrossRef]
- Voruganti, T.; Soulos, P.R.; Mamtani, R.; Presley, C.J.; Gross, C.P. Association Between Age and Survival Trends in Advanced Non–Small Cell Lung Cancer After Adoption of Immunotherapy. JAMA Oncol. 2023, 9, 334–341. [Google Scholar] [CrossRef]
- Kugel, C.H., III; Douglass, S.M.; Webster, M.R.; Kaur, A.; Liu, Q.; Yin, X.; Weiss, S.A.; Darvishian, F.; Al-Rohil, R.N.; Ndoye, A.; et al. Age Correlates with Response to Anti-PD1, Reflecting Age-Related Differences in Intratumoral Effector and Regulatory T-Cell Populations. Clin. Cancer Res. 2018, 24, 5347–5356. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Song, Y.; Fu, Y.; Jun, W.; Tang, J.; Zhang, Z.; Song, Q.; Zhang, B. The efficacy of immune checkpoint inhibitors is limited in elderly NSCLC: A retrospective efficacy study and meta-analysis. Aging 2023, 15, 15025–15049. [Google Scholar] [CrossRef] [PubMed]
- von Witzleben, A.; Grages, A.; Thomas, J.; Ezić, J.; Brunner, C.; Schuler, P.J.; Kraus, J.M.; Kestler, H.A.; Vahl, J.M.; Doescher, J.; et al. Immune checkpoint expression on tumor-infiltrating lymphocytes (TIL) is dependent on HPV status in oropharyngeal carcinoma (OPSCC)—A single-cell RNA sequencing analysis. Oral Oncol. 2024, 159, 107107. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef]
- Solomon, B.; Young, R.J.; Bressel, M.; Urban, D.; Hendry, S.; Thai, A.; Angel, C.; Haddad, A.; Kowanetz, M.; Fua, T.; et al. Prognostic Significance of PD-L1+ and CD8+ Immune Cells in HPV+ Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol. Res. 2018, 6, 295–304. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Zeng, Q.; Guo, X.-J.; Wang, H.; Liu, H.-H.; Dong, Z.-Y. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 2019, 9, 13404. [Google Scholar] [CrossRef]
- Lassen, P.; Alsner, J.; Primdahl, H.; Plaschke, C.C.; Maare, C.; Johansen, J.; Andersen, M.; Farhadi, M.; Overgaard, J. Subsite variation of HPV-related p16-expression in oropharynx cancer: Incidence and prognostic impact in a population-based DAHANCA cohort 1986–2020. Acta Oncol. 2025, 64, 1071–1078. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Cook-Wiens, G.; Yoshida, E.; Shiao, S.L.; Lee, N.Y.; Mita, A.; Jeon, C.; Goodman, M.T.; Ho, A.S. Incidence of Oropharyngeal Cancer Among Elderly Patients in the United States. JAMA Oncol. 2016, 2, 1617–1623. [Google Scholar] [CrossRef]
- Hirokawa, K.; Utsuyama, M.; Hayashi, Y.; Kitagawa, M.; Makinodan, T.; Fulop, T. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing 2013, 10, 19. [Google Scholar] [CrossRef]


| Control Group | HNSCC Patients | |
|---|---|---|
| N (female/male) | 30 (18/12) | 37 (12/25) |
| Age (±SD) range (y) | 49 ± 22 (21–84) | 62 ± 12 (37–94) |
| Stage | n= | |
| T1 | 8 | |
| T2 | 14 | |
| T3 | 7 | |
| T4 | 8 | |
| Nodal status | n= | |
| N0 | 10 | |
| N1 | 12 | |
| N2 | 6 | |
| N3 | 9 | |
| Location | n= | |
| Oral cavity | 11 | |
| Oropharynx | 15 | |
| Hypopharynx | 5 | |
| Larynx | 6 | |
| HPV status (of OPSCC cases) | n= | |
| Positive | 10 | |
| Negative | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puntigam, L.K.; Grages, A.; Vahl, J.M.; Oliveri, F.; Brand, M.; Laban, S.; Greve, J.; Theodoraki, M.N.; Brunner, C.; Hoffmann, T.K.; et al. Co-Stimulatory and Immune Checkpoint Molecule Expression on Peripheral Immune Cells Differs Age Dependently Between Healthy Donors and Patients with Head and Neck Squamous Cell Carcinoma. Cancers 2025, 17, 3215. https://doi.org/10.3390/cancers17193215
Puntigam LK, Grages A, Vahl JM, Oliveri F, Brand M, Laban S, Greve J, Theodoraki MN, Brunner C, Hoffmann TK, et al. Co-Stimulatory and Immune Checkpoint Molecule Expression on Peripheral Immune Cells Differs Age Dependently Between Healthy Donors and Patients with Head and Neck Squamous Cell Carcinoma. Cancers. 2025; 17(19):3215. https://doi.org/10.3390/cancers17193215
Chicago/Turabian StylePuntigam, Lisa K., Ayla Grages, Julius M. Vahl, Franziska Oliveri, Matthias Brand, Simon Laban, Jens Greve, Marie N. Theodoraki, Cornelia Brunner, Thomas K. Hoffmann, and et al. 2025. "Co-Stimulatory and Immune Checkpoint Molecule Expression on Peripheral Immune Cells Differs Age Dependently Between Healthy Donors and Patients with Head and Neck Squamous Cell Carcinoma" Cancers 17, no. 19: 3215. https://doi.org/10.3390/cancers17193215
APA StylePuntigam, L. K., Grages, A., Vahl, J. M., Oliveri, F., Brand, M., Laban, S., Greve, J., Theodoraki, M. N., Brunner, C., Hoffmann, T. K., Schuler, P. J., & Von Witzleben, A. (2025). Co-Stimulatory and Immune Checkpoint Molecule Expression on Peripheral Immune Cells Differs Age Dependently Between Healthy Donors and Patients with Head and Neck Squamous Cell Carcinoma. Cancers, 17(19), 3215. https://doi.org/10.3390/cancers17193215







