Beyond the Skin: Atopic Dermatitis and Increased Gastric Cancer Risk in Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics, Data Source, and Study Design
2.2. Definition of Gastric Cancer (Outcome)
2.3. Definition of Atopic Dermatitis (Exposure)
2.4. Participant Selection and Matching
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Relationship Between Gastric Cancer and Atopic Dermatitis
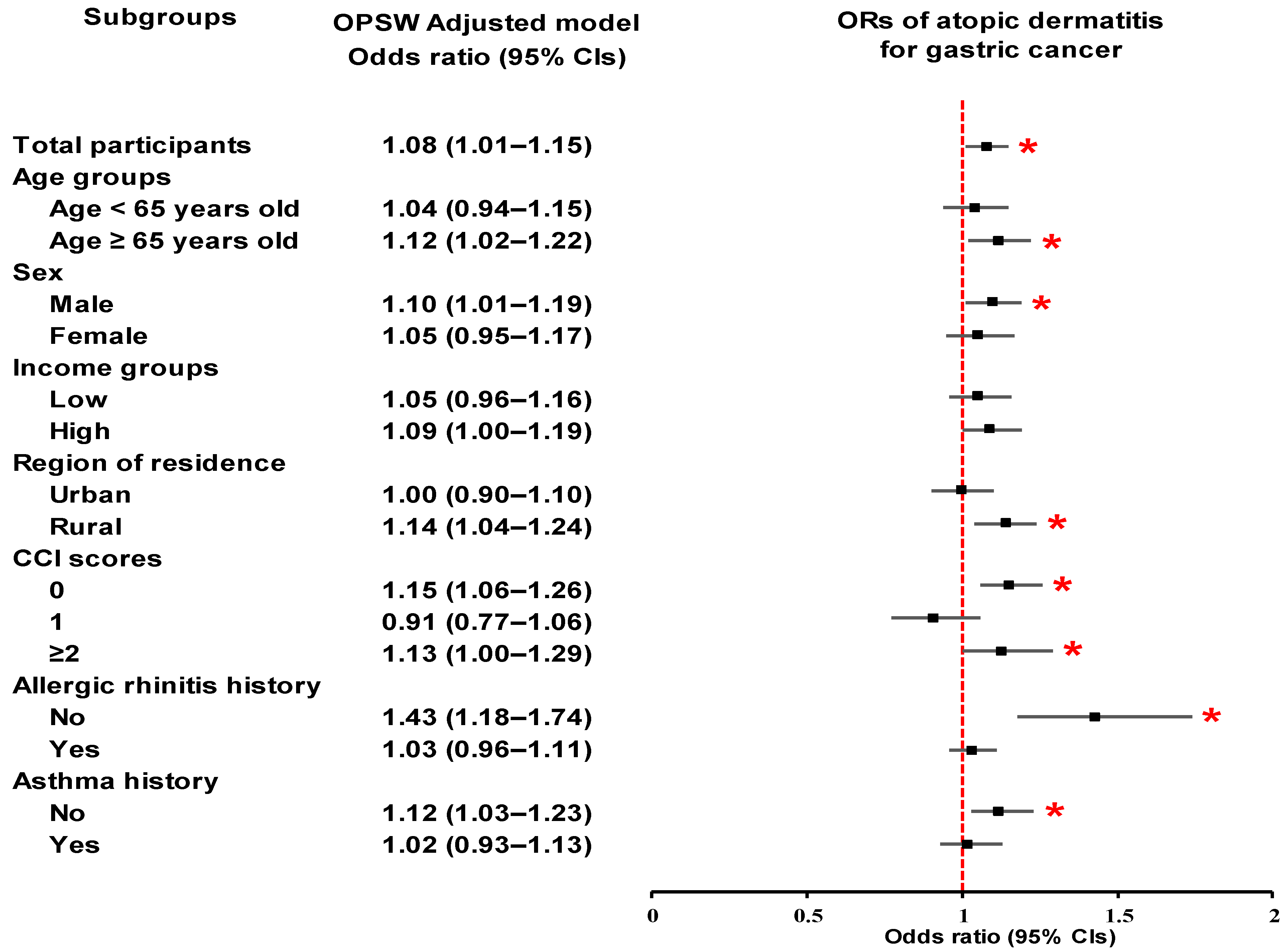
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luk, D.; Hon, K.L.E.; Dizon, M.V.C.; Leong, K.F.; Tay, Y.K.; Koh, M.J.; Chandran, N.S.; Wananukul, S.; Chatproedprai, S.; Luger, T. Practical Recommendations for the Topical Treatment of Atopic Dermatitis in South and East Asia. Dermatol. Ther. 2021, 11, 275–291. [Google Scholar] [CrossRef]
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143. [Google Scholar] [CrossRef]
- Deckers, I.A.; McLean, S.; Linssen, S.; Mommers, M.; van Schayck, C.P.; Sheikh, A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: A systematic review of epidemiological studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef]
- Kahn, D.; Iturriaga, C.; Bertran, K.; Fernandez, I.; Perez-Mateluna, G.; Borzutzky, A.; Brockmann, P.E. Sleep quality in children with atopic dermatitis during flares and after treatment. Sleep Sci. 2020, 13, 172–175. [Google Scholar] [CrossRef]
- Zysk, W.; Mesjasz, A.; Trzeciak, M.; Horvath, A.; Plata-Nazar, K. Gastrointestinal Comorbidities Associated with Atopic Dermatitis—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1194. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Halling, A.S.; Schmid-Grendelmeier, P.; Guttman-Yassky, E.; Silverberg, J.I. Comorbidities of atopic dermatitis-what does the evidence say? J. Allergy Clin. Immunol. 2023, 151, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Arana, A.; Wentworth, C.E.; Fernández-Vidaurre, C.; Schlienger, R.G.; Conde, E.; Arellano, F.M. Incidence of cancer in the general population and in patients with or without atopic dermatitis in the U.K. Br. J. Dermatol. 2010, 163, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Hagströmer, L.; Ye, W.; Nyrén, O.; Emtestam, L. Incidence of Cancer Among Patients With Atopic Dermatitis. Arch. Dermatol. 2005, 141, 1123–1127. [Google Scholar] [CrossRef]
- Olesen, A.B.; Engholm, G.; Storm, H.H.; Thestrup-Pedersen, K. The risk of cancer among patients previously hospitalized for atopic dermatitis. J. Investig. Dermatol. 2005, 125, 445–449. [Google Scholar] [CrossRef]
- Mansfield, K.E.; Schmidt, S.A.J.; Darvalics, B.; Mulick, A.; Abuabara, K.; Wong, A.Y.S.; Sørensen, H.T.; Smeeth, L.; Bhaskaran, K.; Dos Santos Silva, I.; et al. Association Between Atopic Eczema and Cancer in England and Denmark. JAMA Dermatol. 2020, 156, 1086–1097. [Google Scholar] [CrossRef]
- Wang, L.; Bierbrier, R.; Drucker, A.M.; Chan, A.W. Noncutaneous and Cutaneous Cancer Risk in Patients With Atopic Dermatitis: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 158–171. [Google Scholar] [CrossRef]
- Wang, H.; Diepgen, T.L. Atopic dermatitis and cancer risk. Br. J. Dermatol. 2006, 154, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bharti, A. Correlations Between Atopic Dermatitis and the Danger of Stomach Cancer. Stud. J. Health Res. Afr. 2023, 4, 7. [Google Scholar] [CrossRef]
- Shin, W.S.; Xie, F.; Chen, B.; Yu, P.; Yu, J.; To, K.F.; Kang, W. Updated Epidemiology of Gastric Cancer in Asia: Decreased Incidence but Still a Big Challenge. Cancers 2023, 15, 2639. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Hong, S.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2021. Cancer Res. Treat. 2021, 53, 316–322. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Jun, J.K.; Choi, K.S.; Lee, H.Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e7. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.J.; Lee, H.; Min, Y.W.; Min, B.H.; Lee, J.H.; Son, H.J.; Rhee, P.L.; Baek, S.Y.; Kim, S.W.; et al. Associations between Atopic Dermatitis and Risk of Gastric Cancer: A Nationwide Population-based Study. Korean J. Gastroenterol. 2018, 71, 38–44. [Google Scholar] [CrossRef]
- Choi, Y.J.; Han, K.; Jin, E.H.; Lim, J.H.; Shin, C.M.; Lee, D.H. Allergic Diseases and Risk of Malignancy of Gastrointestinal Cancers. Cancers 2023, 15, 3219. [Google Scholar] [CrossRef]
- Kendall, B.E.; Fox, G.A.; Fujiwara, M.; Nogeire, T.M. Demographic heterogeneity, cohort selection, and population growth. Ecology 2011, 92, 1985–1993. [Google Scholar] [CrossRef]
- Cesare, A.D.; Meglio, P.D.; Nestle, F.O. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J. Investig. Dermatol. 2008, 128, 2569–2571. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Sun, S.; Wang, Z. Mapping the relationship between atopic dermatitis and gut microbiota: A bibliometric analysis, 2014–2023. Front. Microbiol. 2024, 15, 1400657. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Kang, H.S.; Kim, J.H.; Kim, J.H.; Kim, S.H.; Kim, N.Y.; Nam, E.S.; Min, K.W.; Choi, H.G. Association between Statin Use and Gastric Cancer: A Nested Case-Control Study Using a National Health Screening Cohort in Korea. Pharmaceuticals 2021, 14, 1283. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Wang, Y.; Wang, X.; Lewis, S.J.; Wang, J. Atopic dermatitis and risk of 14 site-specific cancers: A Mendelian randomization study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2490–2497. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Chen, Y.J.; Lin, M.W.; Chen, T.J.; Chu, S.Y.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; Liu, H.N. Cancer risk in patients with allergic rhinitis, asthma and atopic dermatitis: A nationwide cohort study in Taiwan. Int. J. Cancer 2012, 130, 1160–1167. [Google Scholar] [CrossRef]
- Kang, H.; Kim, D.H. Socioeconomic, health, and social connectedness factors associated with self-rated health of octogenarians and nonagenarians in South Korea: Urban and rural comparison. BMC Public Health 2024, 24, 3477. [Google Scholar] [CrossRef]
- Sato, M.; Matsuo, K.; Susami, Y.; Yamashita, A.; Hayasaka, H.; Hara, Y.; Nishiwaki, K.; Oiso, N.; Kawada, A.; Otsuka, A.; et al. A CCR4 antagonist attenuates atopic dermatitis-like skin inflammation by inhibiting the recruitment and expansion of Th2 cells and Th17 cells. Int. Immunol. 2023, 35, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Chaithongyot, S.; Jantaree, P.; Sokolova, O.; Naumann, M. NF-κB in Gastric Cancer Development and Therapy. Biomedicines 2021, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Lan, T.; Chiari, C.; Ye, X.; Wang, K.; Chen, J. The role of interleukin-17 in inflammation-related cancers. Front. Immunol. 2024, 15, 1479505. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Sun, Y.; Zhu, H.; Liu, Y.; Lin, X.; Shen, H.; Chen, J.; Xu, W.; Xu, H. Th17 cell expansion in gastric cancer may contribute to cancer development and metastasis. Immunol. Res. 2014, 58, 118–124. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Wu, X.; Bian, Z.; Gao, Q. Interleukin 17A promotes gastric cancer invasiveness via NF-κB mediated matrix metalloproteinases 2 and 9 expression. PLoS ONE 2014, 9, e96678. [Google Scholar] [CrossRef]

| Characteristics | Before Overlap PS Weighting Adjustment | After Overlap PS Weighting Adjustment | |||||
|---|---|---|---|---|---|---|---|
| GC | Control | Standardized Difference | GC | Control | Standardized Difference | ||
| Age (n, %) | 0.00 | 0.00 | |||||
| 0–4 | N/A | N/A | N/A | N/A | |||
| 5–9 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 10–14 | 3 (0.03) | 12 (0.03) | 2 (0.02) | 2 (0.02) | |||
| 15–19 | N/A | N/A | N/A | N/A | |||
| 20–24 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 25–29 | 19 (0.19) | 76 (0.19) | 13 (0.17) | 13 (0.17) | |||
| 30–34 | 95 (0.93) | 380 (0.93) | 64 (0.86) | 64 (0.86) | |||
| 35–39 | 205 (2.01) | 820 (2.01) | 143 (1.91) | 143 (1.91) | |||
| 40–44 | 466 (4.58) | 1864 (4.58) | 340 (4.54) | 340 (4.54) | |||
| 45–49 | 711 (6.99) | 2844 (6.99) | 515 (6.88) | 515 (6.88) | |||
| 50–54 | 994 (9.77) | 3976 (9.77) | 716 (9.57) | 716 (9.57) | |||
| 55–59 | 1197 (11.77) | 4788 (11.77) | 872 (11.65) | 872 (11.65) | |||
| 60–64 | 1449 (14.24) | 5796 (14.24) | 1065 (14.23) | 1065 (14.23) | |||
| 65–69 | 1463 (14.38) | 5852 (14.38) | 1081 (14.45) | 1081 (14.45) | |||
| 70–74 | 1490 (14.65) | 5960 (14.65) | 1110 (14.84) | 1110 (14.84) | |||
| 75–79 | 1071 (10.53) | 4284 (10.53) | 802 (10.72) | 802 (10.72) | |||
| 80–84 | 693 (6.81) | 2772 (6.81) | 519 (6.94) | 519 (6.94) | |||
| 85+ | 316 (3.11) | 1264 (3.11) | 239 (3.19) | 239 (3.19) | |||
| Sex (n, %) | 0.00 | 0.00 | |||||
| Male | 6834 (67.17) | 27,336 (67.17) | 5032 (67.26) | 5032 (67.26) | |||
| Female | 3340 (32.83) | 13,360 (32.83) | 2449 (32.74) | 2449 (32.74) | |||
| Income (n, %) | 0.00 | 0.00 | |||||
| 1 (lowest) | 1959 (19.25) | 7836 (19.25) | 1429 (19.10) | 1429 (19.10) | |||
| 2 | 1260 (12.38) | 5040 (12.38) | 918 (12.27) | 918 (12.27) | |||
| 3 | 1621 (15.93) | 6484 (15.93) | 1190 (15.91) | 1190 (15.91) | |||
| 4 | 2144 (21.07) | 8576 (21.07) | 1567 (20.95) | 1567 (20.95) | |||
| 5 (highest) | 3190 (31.35) | 12,760 (31.35) | 2376 (31.76) | 2376 (31.76) | |||
| Region of residence (n, %) | 0.00 | 0.00 | |||||
| Urban | 4310 (42.36) | 17,240 (42.36) | 3168 (42.35) | 3168 (42.35) | |||
| Rural | 5864 (57.64) | 23,456 (57.64) | 4313 (57.65) | 4313 (57.65) | |||
| CCI score (Mean, SD) | 2.40 (2.70) | 1.02 (1.70) | 0.61 | 1.82 (1.98) | 1.82 (1.02) | 0.00 | |
| Allergic rhinitis history (n, %) | 796 (7.82) | 3178 (7.81) | 0.1 | 595 (7.95) | 570 (7.62) | 0.00 | |
| Asthma history (n, %) | 7753 (76.20) | 32,615 (80.14) | 0.09 | 5836 (78.21) | 5836 (78.21) | 0.00 | |
| Atopic dermatitis (n, %) | 3687 (36.24) | 16,482 (40.5) | 0.00 | 2821 (37.80) | 2821 (37.80) | 0.02 | |
| Characteristics | N of GC | N of Control | OR for GC (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| (AD Cases/Total, %) | (AD Cases/Total, %) | Crude | p | Overlap Weighted Model † | p | ||
| Total participants (n = 50,870) | |||||||
| AD | 796/10,174 (7.8) | 3178/40,696 (7.8) | 1.00 (0.92–1.09) | 0.96 | 1.08 (1.01–1.15) | 0.027 * | |
| Control | 9378/10,174 (92.2) | 37,518/40,696 (92.2) | 1 | 1 | |||
| Age < 65 years old (n = 25,705) | |||||||
| AD | 340/5141 (6.6) | 1435/20,564 (7.0) | 0.94 (0.84–1.07) | 0.356 | 1.04 (0.94–1.15) | 0.441 | |
| Control | 4801/5141 (93.4) | 19,129/20,564 (93.0) | 1 | 1 | |||
| Age ≥ 65 years old (n = 25,165) | |||||||
| AD | 456/5033 (9.1) | 1743/20,132 (8.7) | 1.05 (0.94–1.17) | 0.366 | 1.12 (1.02–1.22) | 0.015 * | |
| Control | 4577/5033 (90.9) | 18,389/20,132 (91.3) | 1 | 1 | |||
| Male (n = 34,170) | |||||||
| AD | 509/6834 (7.4) | 1996/27,336 (7.3) | 1.02 (0.92–1.13) | 0.676 | 1.10 (1.01–1.19) | 0.028 * | |
| Control | 6325/6834 (92.6) | 25,340/27,336 (92.7) | 1 | 1 | |||
| Female (n = 16,700) | |||||||
| AD | 287/3340 (8.6) | 1182/13,360 (8.8) | 0.97 (0.85–1.11) | 0.645 | 1.05 (0.95–1.17) | 0.347 | |
| Control | 3053/3340 (91.4) | 12,178/13,360 (91.2) | 1 | 1 | |||
| Low income group (n = 24,200) | |||||||
| AD | 362/4840 (7.5) | 1445/19,360 (7.5) | 1.00 (0.89–1.13) | 0.971 | 1.05 (0.96–1.16) | 0.279 | |
| Control | 4478/4840 (92.5) | 17,915/19,360 (92.5) | 1 | 1 | |||
| High income group (n = 26,670) | |||||||
| AD | 434/5334 (8.1) | 1733/21,336 (8.1) | 1.00 (0.90–1.12) | 0.973 | 1.09 (1.00–1.19) | 0.055 | |
| Control | 4900/5334 (91.9) | 19,603/21,336 (91.9) | 1 | 1 | |||
| Urban resident (n = 21,550) | |||||||
| AD | 333/4310 (7.7) | 1437/17,240 (8.3) | 0.92 (0.81–1.04) | 0.193 | 1.00 (0.90–1.10) | 0.974 | |
| Control | 3977/4310 (92.3) | 15,803/17,240 (91.7) | 1 | 1 | |||
| Rural resident (n = 29,320) | |||||||
| AD | 463/5864 (7.9) | 1741/23,456 (7.4) | 1.07 (0.96–1.19) | 0.219 | 1.14 (1.04–1.24) | 0.003 * | |
| Control | 5401/5864 (92.1) | 21,715/23,456 (92.6) | 1 | 1 | |||
| CCI scores = 0 (n = 27,284) | |||||||
| AD | 307/3480 (8.8) | 1849/23,804 (7.8) | 1.15 (1.01–1.30) | 0.031 * | 1.15 (1.06–1.26) | 0.001 * | |
| Control | 3173/3480 (91.2) | 21,955/23,804 (92.2) | 1 | 1 | |||
| CCI scores = 1 (n = 8937) | |||||||
| AD | 142/1990 (7.1) | 577/6947 (8.3) | 0.85 (0.70–1.03) | 0.091 | 0.91 (0.77–1.06) | 0.222 | |
| Control | 1848/1990 (92.9) | 6370/6947 (91.7) | 1 | 1 | |||
| CCI scores ≥ 2 (n = 14,649) | |||||||
| AD | 347/4704 (7.4) | 752/9945 (7.6) | 0.97 (0.85–1.11) | 0.693 | 1.13 (1.00–1.29) | 0.049 * | |
| Control | 4357/4704 (92.6) | 9193/9945 (92.4) | 1 | 1 | |||
| Non-allergic rhinitis history (n = 10,502) | |||||||
| AD | 114/2421 (4.7) | 293/8081 (3.6) | 1.31 (1.05–1.64) | 0.016 * | 1.43 (1.18–1.74) | <0.001 * | |
| Control | 2307/2421 (95.3) | 7788/8081 (96.4) | 1 | 1 | |||
| Allergic rhinitis history (n = 40,368) | |||||||
| AD | 682/7753 (8.8) | 2885/32,615 (8.8) | 0.99 (0.91–1.08) | 0.891 | 1.03 (0.96–1.11) | 0.354 | |
| Control | 7071/7753 (91.2) | 29,730/32,615 (91.2) | 1 | 1 | |||
| Non-asthma history (n = 30,701) | |||||||
| AD | 442/6487 (6.8) | 1568/24,214 (6.5) | 1.06 (0.95–1.18) | 0.328 | 1.12 (1.03–1.23) | 0.011 * | |
| Control | 6045/6487 (93.2) | 22,646/24,214 (93.5) | 1 | 1 | |||
| Asthma history (n = 20,169) | |||||||
| AD | 354/3687 (9.6) | 1610/16,482 (9.8) | 0.98 (0.87–1.11) | 0.758 | 1.02 (0.93–1.13) | 0.63 | |
| Control | 3333/3687 (90.4) | 14,872/16,482 (90.2) | 1 | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.S.; Han, K.M.; Kim, J.-H.; Kim, J.H.; Choi, H.G.; Yoo, D.M.; Park, H.Y.; Kim, N.Y.; Kwon, M.J. Beyond the Skin: Atopic Dermatitis and Increased Gastric Cancer Risk in Korea. Cancers 2025, 17, 3214. https://doi.org/10.3390/cancers17193214
Kang HS, Han KM, Kim J-H, Kim JH, Choi HG, Yoo DM, Park HY, Kim NY, Kwon MJ. Beyond the Skin: Atopic Dermatitis and Increased Gastric Cancer Risk in Korea. Cancers. 2025; 17(19):3214. https://doi.org/10.3390/cancers17193214
Chicago/Turabian StyleKang, Ho Suk, Kyeong Min Han, Joo-Hee Kim, Ji Hee Kim, Hyo Geun Choi, Dae Myoung Yoo, Ha Young Park, Nan Young Kim, and Mi Jung Kwon. 2025. "Beyond the Skin: Atopic Dermatitis and Increased Gastric Cancer Risk in Korea" Cancers 17, no. 19: 3214. https://doi.org/10.3390/cancers17193214
APA StyleKang, H. S., Han, K. M., Kim, J.-H., Kim, J. H., Choi, H. G., Yoo, D. M., Park, H. Y., Kim, N. Y., & Kwon, M. J. (2025). Beyond the Skin: Atopic Dermatitis and Increased Gastric Cancer Risk in Korea. Cancers, 17(19), 3214. https://doi.org/10.3390/cancers17193214







