Predictors of Extended Intensive Care Unit Utilization After Ovarian Cancer Surgery
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICU | Intensive Care Unit |
| BMI | Body Mass Index |
| ESGO | European Society of Gynecological Oncology |
| SD | Standard Deviation |
| ASA | American Society of Anesthesiologists |
| NACT | NeoAdjuvant ChemoTherapy |
| OR | Odds Ratio |
| CI | Confidence Interval |
| 0 | Enhanced Recovery After Surgery |
| KDIGO | Kidney Disease: Improving Global Outcomes |
References
- PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); Bethesda (MD): Ovarian Epithelial, Fallopian Tube, and Primary Peritoneal Cancer Treatment (PDQ®): Health Professional Version. Available online: https://www.ncbi.nlm.nih.gov/books/NBK66007/ (accessed on 14 March 2024).[Green Version]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef]
- Chan, J.K.; Urban, R.; Cheung, M.K.; Osann, K.; Husain, A.; Teng, N.N.; Kapp, D.S.; Berek, J.S.; Leiserowitz, G.S. Ovarian cancer in younger vs older women: A population-based analysis. Br. J. Cancer 2006, 95, 1314–1320. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2001. Available online: http://www.seer.cancer.gov/csr/1975_2001/ (accessed on 14 March 2024).
- Arora, T.; Mullangi, S.; Lekkala, M.R. Ovarian Cancer. [Updated 2023 Jun 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567760/ (accessed on 20 July 2024).
- Coleridge, S.L.; Bryant, A.; Kehoe, S.; Morrison, J. Neoadjuvant chemotherapy before surgery versus surgery followed by chemotherapy for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2021, 7, CD005343. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Planchamp, F.; Chiva, L.; Fotopoulou, C.; Barton, D.; Cibula, D.; Aletti, G.; Carinelli, S.; Creutzberg, C.; Davidson, B.; et al. European Society of Gynaecological Oncology (ESGO) Guidelines for Ovarian Cancer Surgery. Int. J. Gynecol. Cancer 2017, 27, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; DeJong, S.R.; McGree, M.E.; Weaver, A.L.; Cliby, W.A.; Kumar, A. Frailty in ovarian cancer identified the need for increased postoperative care requirements following cytoreductive surgery. Gynecol. Oncol. 2019, 153, 68–73. [Google Scholar] [CrossRef]
- Davidovic-Grigoraki, M.; Thomakos, N.; Haidopoulos, D.; Vlahos, G.; Rodolakis, A. Do critical care units play a role in the management of gynaecological oncology patients? The contribution of gynaecologic oncologist in running critical care units. Eur. J. Cancer Care 2016, 26, e12438. [Google Scholar] [CrossRef]
- Ross, M.S.; Burriss, M.E.; Winger, D.G.; Edwards, R.P.; Courtney-Brooks, M.; Boisen, M.M. Unplanned postoperative intensive care unit admission for ovarian cancer cytoreduction is associated with significant decrease in overall survival. Gynecol. Oncol. 2018, 150, 306–310. [Google Scholar] [CrossRef]
- Díaz-Montes, T.P.; Zahurak, M.L.; Bristow, R.E. Predictors of extended intensive care unit resource utilization following surgery for ovarian cancer. Gynecol. Oncol. 2007, 107, 464–468. [Google Scholar] [CrossRef]
- Hamaguchi, R.; Ito, T.; Narui, R.; Morikawa, H.; Uemoto, S.; Wada, H. Postoperative Admission in Critical Care Units Following Gynecologic Oncology Surgery: Outcomes Based on a Systematic Review and Authors’ Recommendations. Vivo 2020, 34, 2201–2208. [Google Scholar] [CrossRef]
- Amir, M.; Shabot, M.; Karlan, B.Y. Surgical intensive care unit care after ovarian cancer surgery: An analysis of indications. Am. J. Obstet. Gynecol. 1997, 176, 1389–1393. [Google Scholar] [CrossRef]
- Collins, A.; Spooner, S.; Horne, J.; Chainrai, M.; Runau, F.; Bourne, T.; Moss, E.L.; Davies, Q.; Chattopadhyay, S.; Bharathan, R. Peri-operative Variables Associated with Prolonged Intensive Care Stay Following Cytoreductive Surgery for Ovarian Cancer. Anticancer. Res. 2021, 41, 3059–3065. [Google Scholar] [CrossRef]
- Brooks, S.E.; Ahn, J.; Mullins, C.D.; Baquet, C.R. Resources and use of the intensive care unit in patients who undergo surgery for ovarian carcinoma. Cancer 2002, 95, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Ruskin, R.; Urban, R.R.; Sherman, A.E.; Chen, L.-L.; Powell, C.B.; Burkhardt, D.H. Predictors of Intensive Care Unit Utilization in Gynecologic Oncology Surgery. Int. J. Gynecol. Cancer 2011, 21, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Li, K.; Li, G. Impact of Obesity on Major Surgical Outcomes in Ovarian Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 841306. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Lopes, A.; Das, N.; Kumar, A.; Cliby, W.; Smits, E.; Bekkers, R.; Massuger, L.; Galaal, K. Surgical morbidity and clinical outcomes in ovarian cancer—the role of obesity. BJOG Int. J. Obstet. Gynaecol. 2015, 123, 300–308. [Google Scholar] [CrossRef]
- Wolfberg, A.J.; Montz, F.J.; E Bristow, R. Role of obesity in the surgical management of advanced-stage ovarian cancer. J. Reprod. Med. 2004, 49, 473–476. [Google Scholar]
- Sobol, J.B.; Wunsch, H. Triage of high-risk surgical patients for intensive care. Crit. Care 2011, 15, 217. [Google Scholar] [CrossRef]
- Pedrosa, E.; Silva, M.; Lobo, A.; Barbosa, J.; Mourao, J. Is the ASA Classification Universal? Turk. J. Anaesthesiol. Reanim. 2021, 49, 298–303. [Google Scholar] [CrossRef]
- Kumar, A.; Langstraat, C.L.; DeJong, S.R.; McGree, M.E.; Bakkum-Gamez, J.N.; Weaver, A.L.; LeBrasseur, N.K.; Cliby, W.A. Functional not chronologic age: Frailty index predicts outcomes in advanced ovarian cancer. Gynecol. Oncol. 2017, 147, 104–109. [Google Scholar] [CrossRef]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research Agenda for Frailty in Older Adults: Toward a Better Understanding of Physiology and Etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef]
- Shen, H.; Pang, Q.; Gao, Y.; Liu, H. Effects of epidural anesthesia on the prognosis of ovarian cancer—a systematic review and meta-analysis. BMC Anesthesiol. 2023, 23, 390. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.H.; Cowan, R.A.; Afonso, A.M.; Zhou, Q.; Iasonos, A.; Ali, N.; Thompson, E.; Sonoda, Y.; O’CEarbhaill, R.E.; Chi, D.S.; et al. Perioperative epidural use and survival outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer. Gynecol. Oncol. 2018, 151, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Moslemi-Kebria, M.; El-Nashar, S.A.M.; Aletti, G.D.; Cliby, W.A. Intraoperative Hypothermia During Cytoreductive Surgery for Ovarian Cancer and Perioperative Morbidity. Obstet. Gynecol. 2012, 119, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kurz, A.; Sessler, D.I.; Plattner, O.; Christensen, R.; Dechert, M.; Ikeda, T. Isoflurane Produces Marked and Nonlinear Decreases in the Vasoconstriction and Shivering Thresholds. Anesthesiology 1996, 85, 240–245. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative thermoregulation and heat balance. Lancet 2016, 387, 2655–2664. [Google Scholar] [CrossRef]
- Kaufner, L.; Niggemann, P.; Baum, T.; Casu, S.; Sehouli, J.; Bietenbeck, A.; Boschmann, M.; Spies, C.D.; Henkelmann, A.; von Heymann, C. Impact of brief prewarming on anesthesia-related core-temperature drop, hemodynamics, microperfusion and postoperative ventilation in cytoreductive surgery of ovarian cancer: A randomized trial. BMC Anesthesiol. 2019, 19, 161. [Google Scholar] [CrossRef]
- Long, K.C.; Tanner, E.J.; Frey, M.; Leitao, M.M.; Levine, D.A.; Gardner, G.J.; Sonoda, Y.; Abu-Rustum, N.R.; Barakat, R.R.; Chi, D.S. Intraoperative hypothermia during primary surgical cytoreduction for advanced ovarian cancer: Risk factors and associations with postoperative morbidity. Gynecol. Oncol. 2013, 131, 525–530. [Google Scholar] [CrossRef]
- Alletti, S.G.; Capozzi, V.A.; Rosati, A.; De Blasis, I.; Cianci, S.; Vizzielli, G.; Uccella, S.; Gallotta, V.; Fanfani, F.; Fagotti, A.; et al. Laparoscopy vs. laparotomy for advanced ovarian cancer: A systematic review of the literature. Minerva Medica 2019, 110, 341–357. [Google Scholar] [CrossRef]
- Tortorella, L.; Vizzielli, G.; Fusco, D.; Cho, W.C.; Bernabei, R.; Scambia, G.; Colloca, G. Ovarian Cancer Management in the Oldest Old: Improving Outcomes and Tailoring Treatments. Aging Dis. 2017, 8, 677–684. [Google Scholar] [CrossRef]
| Patient characteristics | n = 74 (%) | <48 h n = 47 (63.5%) | ≥48 h n = 27 (36.5%) |
|---|---|---|---|
| Stage, n (%) | |||
| I | 1 (1.4%) | 1 (2.1%) | 0 (0.0%) |
| III | 50 (67.6%) | 30 (63.8%) | 20 (74.1%) |
| IV | 22 (29.7%) | 16 (34.0%) | 6 (22.2%) |
| V | 1 (1.4%) | 0 (0.0%) | 1 (3.7%) |
| Tumor grade, n (%) | |||
| Grade 1 | 19 (25.7%) | 12 (25.5%) | 7 (25.9%) |
| Grade 2 | 28 (37.8%) | 16 (34.0%) | 12 (44.4%) |
| Grade 3 | 27 (36.5%) | 19 (40.4%) | 8 (29.6%) |
| Cause of icu admission | |||
| Hemodynamic support | 34 (45.9%) | 25 (53.2%) | 9 (33.3%) |
| Mechanical Ventilation | 39 (52.7%) | 22 (46.8%) | 17 (63.0%) |
| Renal replacement therapy | 1 (1.4%) | 0 (0.0%) | 1 (3.7%) |
| Perioperative/Intraoperative Variables | <48 h n = 47 (63.5%) | ≥48 h n = 27 (36.5%) | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Preoperative | ||||
| Age (years) | 60.79 (10.77) 60.00 (IQR: 14) | 56.82 (11.02) 58.00 (IQR: 15) | 3.97 (−1.26, 9.20) | 0.134 |
| ΒΜΙ | 29.29 (6.88) 28.30 (IQR: 13) | 31.77 (6.91) 33.70 (IQR: 13) | −2.48 (−5.80, 0.84) | 0.140 |
| Ascites > 500cc | 0.091 | |||
| No | 27 (73.0%) | 10 (27.0%) | - | |
| Yes | 20 (54.1%) | 17 (45.9%) | - | |
| Presurgery Hb (g/dL) | 11.93 (1.43) 12.00 (IQR: 2) | 11.97 (1.59) 12.00 (IQR: 2.3) | −0.04 (−0.75, 0.68) | 0.928 |
| Presurgery Albumin (g/dL) | 3.93 (0.57) 3.90 (IQR: 0.7) | 3.84 (0.63) 4.00 (IQR: 0.5) | 0.09 (−0.20, 0.39) | 0.517 |
| Presurgery Proteins (g/dL) | 6.81 (0.64) 6.90 (IQR: 0.8) | 6.74 (1.08) 6.94 (IQR: 0.9) | 0.07 (−0.34, 0.48) | 0.734 |
| Number of Chemotherapies | 4.67 (1.13) 4.50 (IQR: 2) | 3.73 (0.91) 4.00 (IQR: 1) | 0.939 (0.15, 1.73) | 0.021 |
| Pathological type | 0.086 | |||
| Non-serous | 5 (41.7%) | 7 (58.3%) | - | |
| Serous | 42 (67.7%) | 20 (32.3%) | - | |
| Grade | 0.595 | |||
| 1 | 12 (63.2%) | 7 (36.8%) | - | |
| 2 | 16 (57.1%) | 12 (42.9%) | - | |
| 3 | 19 (70.4%) | 8 (29.6%) | - | |
| ASA Score | 0.624 | |||
| 1 | 16 (69.6%) | 7 (30.4%) | - | |
| 2 | 19 (57.6%) | 14 (42.4%) | - | |
| 3 | 12 (66.7%) | 6 (33.3%) | - | |
| Intraoperative | ||||
| Time of surgery (min) | 312.13 (77.09) 300.00 (IQR: 120) | 363.15 (91.35) 400.00 (IQR: 150) | −51.02 (−90.75, −11.29) | 0.013 |
| Blood loss (cc) | 757.96 (626.91) 500.00 (IQR: 600) | 969.23 (561.26) 750.00 (IQR: 550) | −211.27 (−509.22, 86.67) | 0.162 |
| Ascites volume (cc) | 2368.42 (1498.17) 2000.00 (IQR: 1000) | 2558.82 (1796.20) 2000.00 (IQR: 2750) | −190.40 (−1306.58, 925.77) | 0.731 |
| Treatment option | 0.166 | |||
| Primary Debulking | 20 (55.6%) | 16 (44.4%) | - | |
| Interval/Late Debulking | 27 (71.1%) | 11 (28.9%) | - | |
| Pleural effusion | 0.142 | |||
| No | 37 (68.5%) | 17 (31.5%) | - | |
| Yes | 10 (50.0%) | 10 (50.0%) | - | |
| Lymphadenectomy | 0.338 | |||
| No | 33 (67.3%) | 16 (32.7%) | - | |
| Yes | 14 (56.0%) | 11 (44.0%) | - | |
| Bowel Resection | 0.742 | |||
| No | 26 (61.9%) | 16 (38.1%) | - | |
| Yes | 21 (65.6%) | 11 (34.4%) | - | |
| Residual disease after surgery | 0.378 | |||
| R0 | 34 (60.7%) | 22 (39.3%) | - | |
| R1/R5 | 13 (72.2%) | 5 (27.8%) | - | |
| Epidural | 0.166 | |||
| No | 20 (55.6%) | 16 (44.4%) | - | |
| Yes | 27 (71.1%) | 11 (28.9%) | - | |
| Intra-op. complications | 0.212 | |||
| No | 30 (58.8%) | 21 (41.2%) | - | |
| Yes | 17 (73.9%) | 6 (26.1%) | - | |
| Postoperative | ||||
| Postsurgery Hb (lowest) (g/dL) | 8.64 (1.13) 8.60 (IQR: 1.5) | 8.42 (1.81) 7.80 (IQR: 1.8) | 0.22 (−0.56, 1.00) | 0.572 |
| Postsurgery Albumin (g/dL) | 2.16 (0.62) 2.20 (IQR: 1) | 2.06 (0.63) 2.27 (IQR: 0.9) | 0.10 (−0.21, 0.42) | 0.522 |
| Postsurgery Proteins (g/dL) | 3.77 (1.14) 3.86 (IQR: 1.4) | 3.58 (1.16) 3.60 (IQR: 1.3) | 0.19 (−0.39, 0.77) | 0.511 |
| Postsurgery body temperature (*Celcius) | 35.87 (0.59) 35.80 (IQR: 1) | 35.06 (0.94) 34.95 (IQR: 1.2) | 0.81 (0.38, 1.23) | <0.001 |
| Cause of icu admission | 0.131 | |||
| Hemodynamic support | 25 (73.5%) | 9 (26.5%) | - | |
| Mechanical Ventilation | 22 (56.4%) | 17 (43.6%) | - | |
| Renal replacement therapy | 0 (0.0%) | 1 (100.0%) | - | |
| Perioperative | ||||
| Blood Transfusion | 0.89 (1.71) 0.00 (IQR: 1) | 0.44 (1.01) 0.00 (IQR: 0) | 0.45 (−0.27, 1.17) | 0.218 |
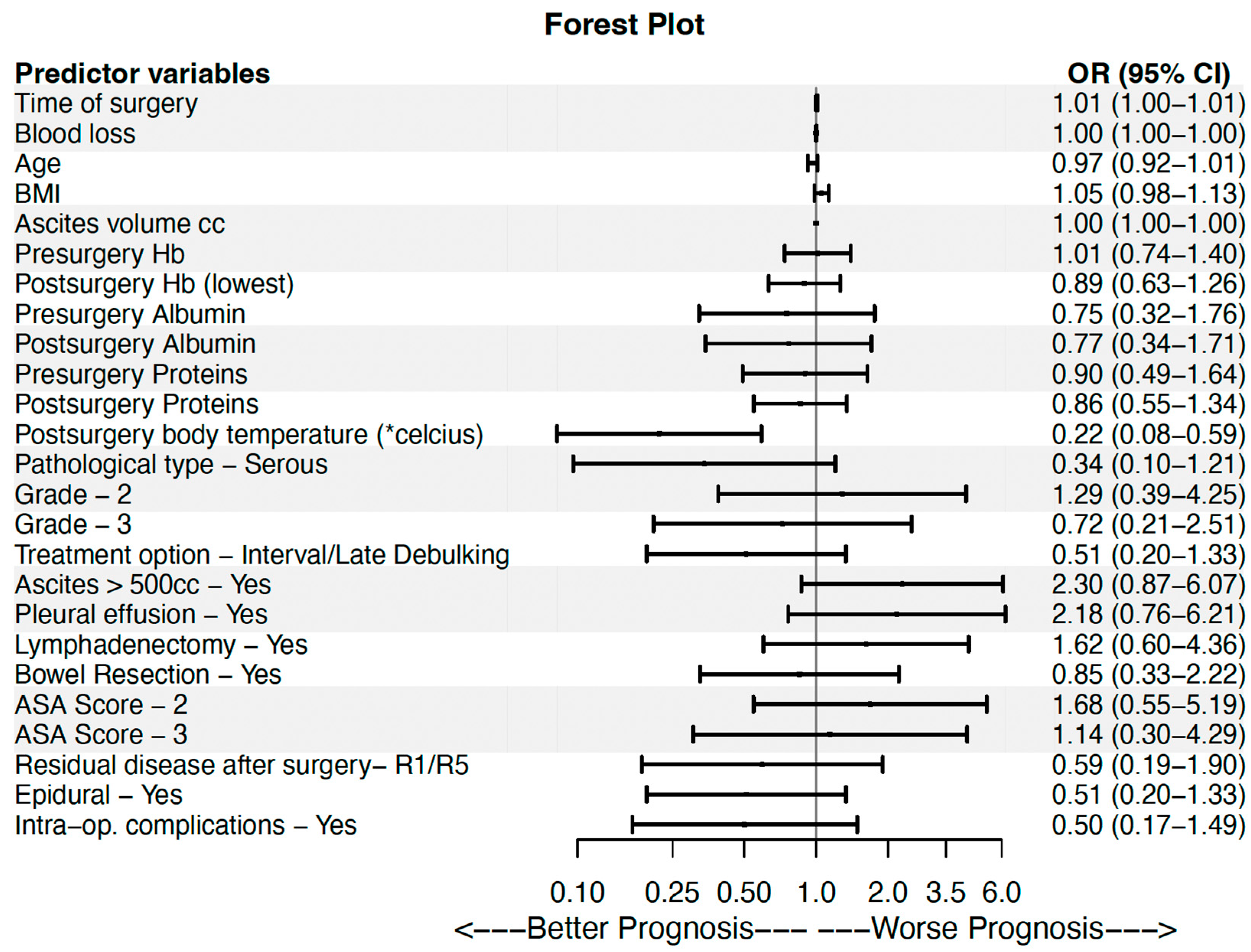
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Predictor Variables | OR | OR 95% CI | p-Value | OR | OR 95% CI | p-Value |
| Time of surgery (min) | 1.007 | (1.001–1.013) | 0.016 | 1.017 | (1.002–1.033) | 0.025 |
| Blood loss (cc) | 1.001 | (1.000–1.001) | 0.171 | 1.000 | (0.998–1.002) | 0.893 |
| Age (years) | 0.966 | (0.923–1.011) | 0.138 | 0.946 | (0.849–1.054) | 0.311 |
| ΒΜΙ | 1.054 | (0.983–1.131) | 0.140 | 1.215 | (1.021–1.446) | 0.028 |
| Ascites volume (cc) | 1.000 | (1.000–1.000) | 0.722 | |||
| Presurgery Hb (g/dL) | 1.015 | (0.736–1.401) | 0.927 | |||
| Postsurgery Hb (lowest) (g/dL) | 0.892 | (0.632–1.260) | 0.517 | |||
| Presurgery Albumin (g/dL) | 0.754 | (0.323–1.758) | 0.513 | |||
| Postsurgery Albumin (g/dL) | 0.767 | (0.344–1.708) | 0.516 | |||
| Presurgery Proteins (g/dL) | 0.899 | (0.492–1.643) | 0.730 | |||
| Postsurgery Proteins (g/dL) | 0.859 | (0.548–1.344) | 0.505 | |||
| Postsurgery body temperature (*celcius) | 0.220 | (0.082–0.589) | 0.003 | 0.082 | (0.013–0.507) | 0.007 |
| Pathological type—Serous (ref. Other types) | 0.340 | (0.096–1.205) | 0.095 | 0.459 | (0.060–3.531) | 0.454 |
| Grade | 0.597 | |||||
| 2 (ref. 1) | 1.286 | (0.389–4.249) | 0.680 | |||
| 3 (ref. 1) | 0.722 | (0.208–2.508) | 0.608 | |||
| Treatment option—Interval/Late Debulking (ref. Primary Debulking) | 0.509 | (0.195–1.331) | 0.169 | 0.293 | (0.033–2.629) | 0.273 |
| Ascites > 500cc—Yes | 2.295 | (0.868–6.065) | 0.094 | 2.481 | (0.234–26.327) | 0.451 |
| Pleural effusion—Yes | 2.176 | (0.763–6.207) | 0.146 | 2.350 | (0.146–37.863) | 0.547 |
| Lymphadenectomy—Yes | 1.621 | (0.602–4.361) | 0.339 | |||
| Bowel Resection—Yes | 0.851 | (0.326–2.221) | 0.742 | |||
| ASA Score | 0.626 | |||||
| 2 (ref. 1) | 1.684 | (0.547–5.187) | 0.364 | |||
| 3 (ref. 1) | 1.143 | (0.305–4.289) | 0.843 | |||
| Residual disease after surgery- R1/R5 (ref. R0) | 0.594 | (0.186–1.901) | 0.380 | |||
| Epidural—Yes | 0.509 | (0.195–1.331) | 0.169 | 1. 064 | (0.112–10.079) | 0.957 |
| Intra-op. complications—Yes | 0.500 | (0.170–1.490) | 0.216 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoulidis, V.; Kissoudi, K.; Chatzistamatiou, K.; Tzitzis, P.; Zouzoulas, D.; Theodoulidis, I.; Anthoulakis, C.; Sifaki, F.; Moysiadis, T.; Koraki, E.; et al. Predictors of Extended Intensive Care Unit Utilization After Ovarian Cancer Surgery. Cancers 2025, 17, 3203. https://doi.org/10.3390/cancers17193203
Theodoulidis V, Kissoudi K, Chatzistamatiou K, Tzitzis P, Zouzoulas D, Theodoulidis I, Anthoulakis C, Sifaki F, Moysiadis T, Koraki E, et al. Predictors of Extended Intensive Care Unit Utilization After Ovarian Cancer Surgery. Cancers. 2025; 17(19):3203. https://doi.org/10.3390/cancers17193203
Chicago/Turabian StyleTheodoulidis, Vasilis, Kalliopi Kissoudi, Kimonas Chatzistamatiou, Panagiotis Tzitzis, Dimitris Zouzoulas, Iakovos Theodoulidis, Christos Anthoulakis, Freideriki Sifaki, Theodoros Moysiadis, Eleni Koraki, and et al. 2025. "Predictors of Extended Intensive Care Unit Utilization After Ovarian Cancer Surgery" Cancers 17, no. 19: 3203. https://doi.org/10.3390/cancers17193203
APA StyleTheodoulidis, V., Kissoudi, K., Chatzistamatiou, K., Tzitzis, P., Zouzoulas, D., Theodoulidis, I., Anthoulakis, C., Sifaki, F., Moysiadis, T., Koraki, E., Grimbizis, G., & Tsolakidis, D. (2025). Predictors of Extended Intensive Care Unit Utilization After Ovarian Cancer Surgery. Cancers, 17(19), 3203. https://doi.org/10.3390/cancers17193203






