Real-World Effectiveness of Cisplatin, 5-Fluorouracil, and Pembrolizumab Combination Therapy for Unresectable or Recurrent Esophageal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection Items
2.3. Treatment Protocol
2.4. Evaluation Criteria
2.5. Statistical Analyess
3. Results
3.1. Characteristics of the Patients
3.2. Dose Intensity of Cisplatin and 5-Fluorouracil and Modification of the Treatment Interval
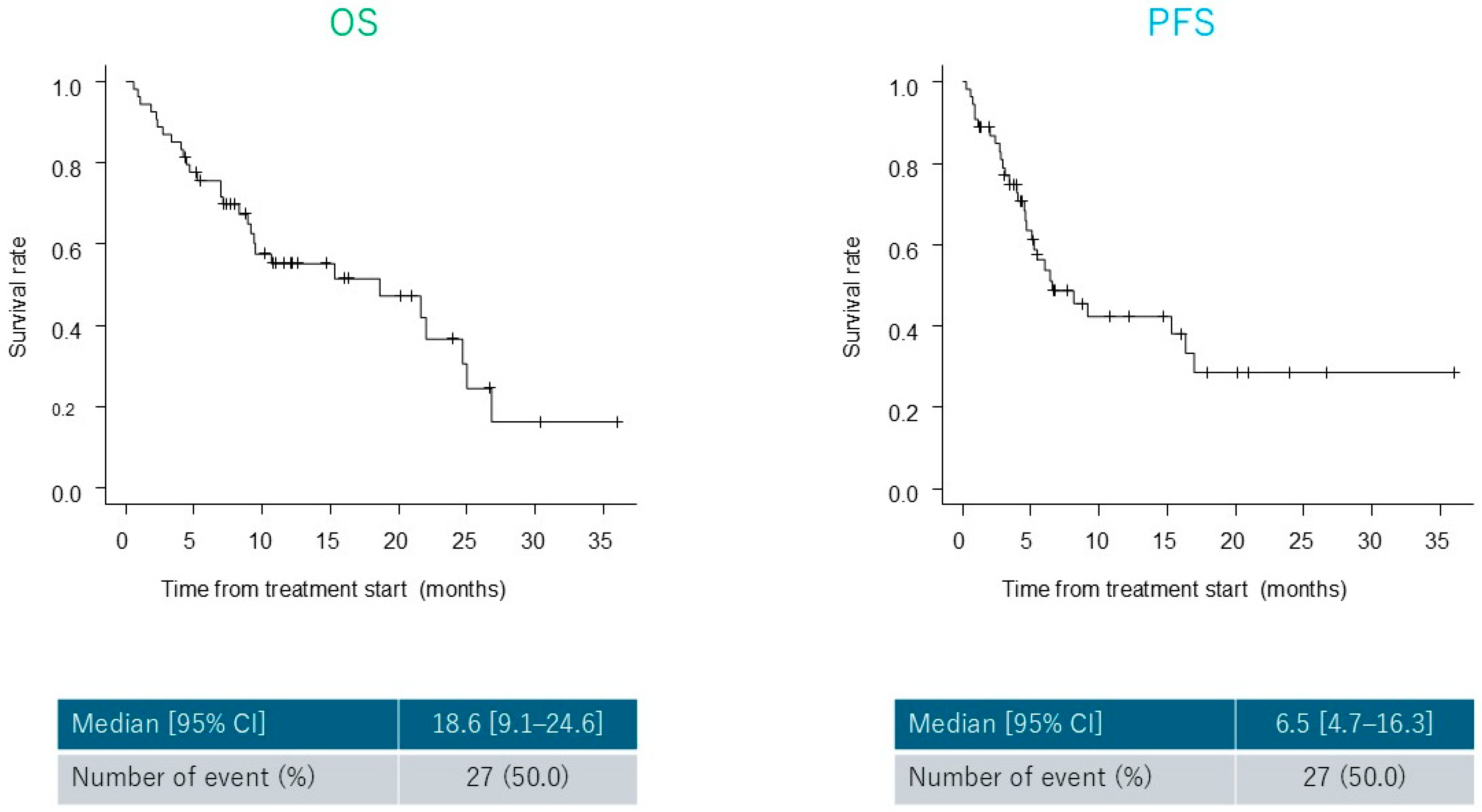
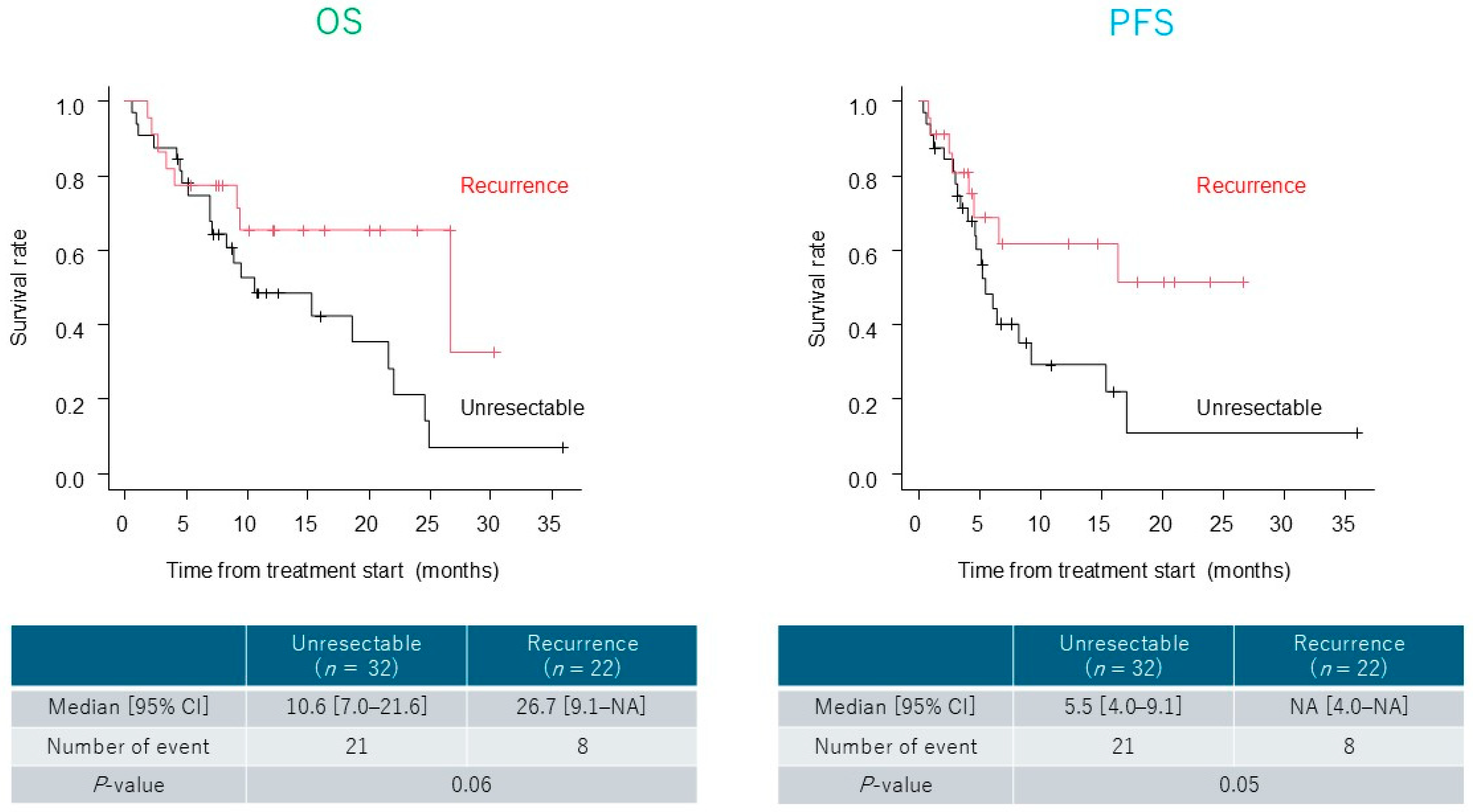
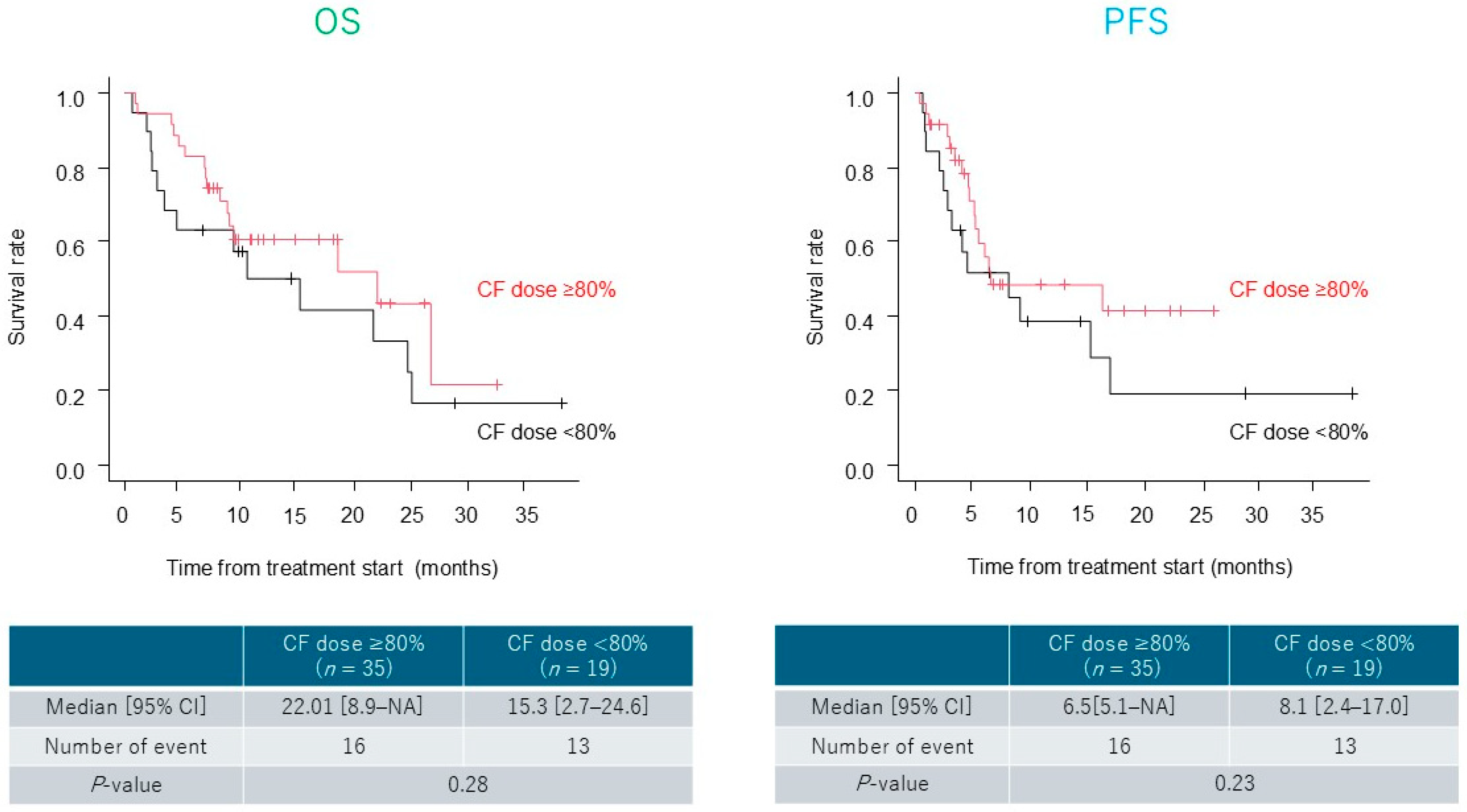
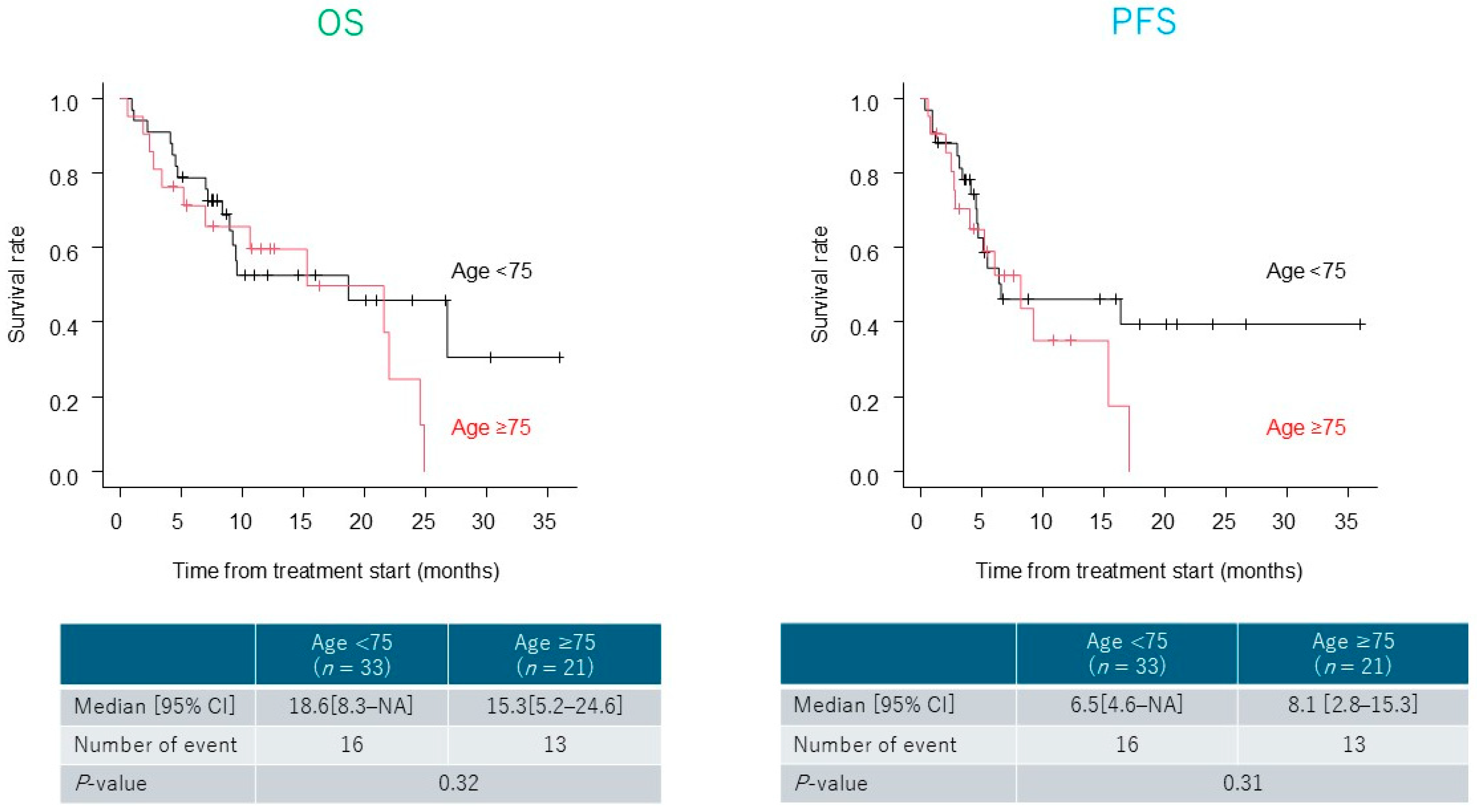
3.3. Treatment Outcomes
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global cancer observatory. In Cancer Today; International Agency for Research on Cancer: Lyon, France, 2022; Available online: https://gco.iarc.who.int/today (accessed on 30 August 2025).
- National Cancer Institute. Japan. Cancer Information Service: Cancer Statistics. Available online: https://ganjoho.jp/reg_stat/statistics/stat/summary.html (accessed on 30 August 2025).
- Guo, D.; Jin, J.; Li, D.; He, Y.; Lin, Y. Analysis of the incidence and mortality trends of esophageal cancer in cancer registry areas of China and Japan. Int. J. Cancer 2024, 155, 1376–1386. [Google Scholar] [CrossRef]
- Kato, H.; Nakajima, M. Treatments for esophageal cancer: A review. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 330–335. [Google Scholar] [CrossRef]
- Bleiberg, H.; Conroy, T.; Paillot, B.; Lacave, A.J.; Blijham, G.; Jacob, J.H.; Bedenne, L.; Namer, M.; De Besi, P.; Gay, F.; et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur. J. Cancer 1997, 33, 1216–1220. [Google Scholar] [CrossRef]
- Iizuka, T.; Kakegawa, T.; Ide, H.; Ando, N.; Watanabe, H.; Tanaka, O.; Takagi, I.; Isono, K.; Ishida, K.; Arimori, M.; et al. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: A Japanese Esophageal Oncology Group Trial. Jpn. J. Clin. Oncol. 1992, 22, 172–176. [Google Scholar] [PubMed]
- Hayashi, K.; Ando, N.; Watanabe, H.; Ide, H.; Nagai, K.; Aoyama, N.; Takiyama, W.; Ishida, K.; Isono, K.; Makuuchi, H.; et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: A Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407). Jpn. J. Clin. Oncol. 2001, 31, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S.; Schuster, T.; Porschen, R.; Al-Batran, S.E.; Hofheinz, R.; Thuss-Patience, P.; Moehler, M.; Grabowski, P.; Arnold, D.; Greten, T.; et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: A randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann. Oncol. 2009, 20, 1667–1673. [Google Scholar] [CrossRef]
- Moehler, M.; Maderer, A.; Thuss-Patience, P.C.; Brenner, B.; Meiler, J.; Ettrich, T.J.; Hofheinz, R.-D.; Al-Batran, S.E.; Vogel, A.; Mueller, L.; et al. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell carcinoma: A prospective, open-label, randomized phase III AIO/EORTC trial. Ann. Oncol. 2020, 31, 228–235. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.H.; Doi, T.; Moriwaki, T.; Kim, S.B.; Lee, S.H.; et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Kato, K.; Kim, S.B.; Ajani, J.A.; Zhao, K.; He, Z.; Yu, X.; Shu, Y.; Luo, Q.; Wang, J.; et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): A randomized phase III study. J. Clin. Oncol. 2022, 40, 3065–3076. [Google Scholar] [CrossRef]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomized, placebo-controlled phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.-L.; Kim, H.; Soto-Perez-de-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: A randomized clinical trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef] [PubMed]
- Bennis, Y.; Savry, A.; Rocca, M.; Gauthier-Villano, L.; Pisano, P.; Pourroy, B. Cisplatin dose adjustment in patients with renal impairment, which recommendations should we follow? Int. J. Clin. Pharm. 2014, 36, 420–429. [Google Scholar] [CrossRef]
- Sato, S.; Ssuzuki, T.; Chinen, T.; Yamaguchi, H.; Suzuki, Y.; Hokamura, N.; Saze, Z.; Kono, K.; Takahashi, K.; Yano, F.; et al. The real-world data of immune-checkpoint inhibitor combination therapy for unresectable or metastatic esophageal cancer: A multi-institutional cohort study. Int. J. Clin. Oncol. 2024, 29, 994–1001. [Google Scholar] [CrossRef]
- Takeno, A.; Hirao, M.; Hamakawa, T.; Yamamoto, M.; Matsui, Y.; Tokuyama, S.; Toshiyama, R.; Kawai, K.; Takahashi, Y.; Sakai, K.; et al. The use of platinum based chemotherapy for esophageal cancer patients with impaired renal function. Gan To Kagaku Ryoho 2023, 50, 1783–1785. [Google Scholar] [PubMed]
- Hamamoto, Y.; Murakami, K.; Kato, K.; Kitagawa, Y. Management of elderly patients with esophageal squamous cell cancer. Jpn. J. Clin. Oncol. 2022, 52, 816–824. [Google Scholar] [CrossRef]
- Won, E.; Ilson, D.H. Management of localized esophageal cancer in the older patient. Oncologist 2014, 19, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Won, E. Issues in the management of esophageal cancer and geriatric patients. Chin. Clin. Oncol. 2017, 6, 51. [Google Scholar] [CrossRef]
- Kawamoto, T.; Shikama, N.; Mine, S.; Tsurumaru, M.; Sasai, K. Clinical impact of baseline renal function on the safety of radiotherapy with concurrent docetaxel for esophageal cancer in elderly patients. Esophagus 2020, 17, 425–432. [Google Scholar] [CrossRef]
- Nakajima, M.; Muroi, H.; Kikuchi, M.; Kubo, T.; Takise, S.; Inoue, N.; Ihara, K.; Nakagawa, M.; Morita, S.; Nakamura, T.; et al. Therapeutic outcome of chemoradiotherapy with dose-reduced nedaplatin and 5-fluorouracil in older esophageal cancer patients aged 80 or more. Dokkyo Med. J. 2024, 3, 206–214. [Google Scholar]
- Rodriguez, J.E.; Naigeon, M.; Goldschmidt, V.; Roulleaux Dugage, M.; Seknazi, L.; Danlos, F.X.; Champiat, S.; Marabelle, A.; Michot, J.M.; Massard, C.; et al. Immunosenescence, inflammaging, and cancer immunotherapy efficacy. Expert Rev. Anticancer Ther. 2022, 22, 915–926. [Google Scholar] [CrossRef]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Theocharopoulos, C.; Aravantinou, K.; Boukouris, A.E.; Stefanou, D.; Anastasopoulou, A.; Lialios, P.P.; Lyrarakis, G.; Gogas, H. Clinical benefit of immune checkpoint inhibitors in elderly cancer patients: Current evidence from immunosenescence pathophysiology to clinical trial results. Crit. Rev. Oncol. Hematol. 2025, 208, 104635. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Y.; Li, C.; Leong, T.L.; Provencio, M.; Oh, I.J.; Zhang, Z.; Su, C. Differential organ-specific tumor response to first-line immune checkpoint inhibitor therapy in non-small cell lung cancer-a retrospective cohort study. Transl. Lung Cancer Res. 2023, 12, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Y.; Zhu, L.; Li, W.; Zhao, L.; Pan, X.; Lin, J.; Guo, T. Pembrolizumab combined with paclitaxel and platinum as induction therapy for locally advanced esophageal squamous cell carcinoma: A retrospective, single-center, three-arm study. J. Gastrointest. Oncol. 2022, 13, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hou, X.; Cai, B.; Yu, W.; Chen, J.; Huang, X.; Li, Y.; Zeng, M.; Ren, Z.; Gabriel, E.; et al. Efficacy and safety of combined treatment with pembrolizumab in patients with locally advanced or metastatic esophageal squamous cell carcinoma in the real world. Ann. Transl. Med. 2022, 10, 12. [Google Scholar] [CrossRef]
| Variable | Value |
|---|---|
| Age (median) | 72.5 (44–91) |
| Sex (male/female) | 40/14 |
| Performance status (0–1) | 41 (75.9%) |
| Renal function | |
| eGFR < 60 | 15 (27.3%) |
| Ccr < 60 | 26 (48.1%) |
| Ccr < 50 | 15 (27.8%) |
| With comorbidities | 37 (68.5%) |
| Cardiovascular disorders | 30 (55.6%) |
| Respiratory disorders | 5 (9.3%) |
| Renal disorders | 8 (14.8%) |
| Hepatic disorders | 5 (9.3%) |
| Endocrine disorders | 14 (25.9%) |
| Reason for treatment (unresectable/recurrent disease) | 32/22 |
| Tumor characteristics | |
| Location (Ut/Mt/Lt/Jz) | 13/28/10/3 |
| Histology (SCC/AD) | 53/1 |
| Macroscopic type (0/1/2/3/4) | 4/5/14/26/5 |
| T (1/2/3/4) | 4/0/28/22 |
| N (0/1/2/3) | 7/19/15/13 |
| M (0/1) | 30/24 |
| Stage (I/II/IIIA/IIIB/IVA/IVB) * | 3/3/11/3/10/24 |
| Metastatic sites | |
| Distant lymph nodes | 15 |
| Lung | 7 |
| Liver | 7 |
| Others | 6 |
| CPS | |
| ≥10 | 16(69.6% **) |
| <10 | 7(30.4% **) |
| Not tested | 31 |
| Variable | Value |
|---|---|
| % Dose of Pembrolizumab (median) | 100% (100) |
| % Dose of CF (median) | 80% (50–100) |
| 100% | 24 (44.4%) |
| 90% | 2 (3.7%) |
| 80% | 9 (16.7%) |
| <80% | 19 (35.2%) |
| Reason for reduction of CF | |
| Renal dysfunction | 24 (44.4%) |
| Age | 14 (26.0%) |
| PS ≥ 2 | 13 (24.1%) |
| Other | 6 (11.1%) |
| Treatment cycle (3 weeks/4 weeks) | 22 (40.7%)/27 (50.0%) * |
| Number of treatment course (median) | 4 [1–23] |
| Post-treatment | 30 (55.6%) |
| 2nd-line chemotherapy | 15 |
| CRT | 9 |
| CS | 5 |
| RFA | 1 |
| Therapeutic Effect | Overall (n = 54) | Dose-Reduced (n = 19) | Age ≥ 75 (n = 21) |
|---|---|---|---|
| CR | 5 | 2 | 3 |
| PR | 25 | 10 | 10 |
| SD | 14 | 5 | 6 |
| PD | 10 | 2 | 2 |
| ORR | 55.6% | 63.2% | 61.9% |
| DCR | 81.5% | 89.5% | 90.5% |
| Adverse Events | Grade (CTCAE ver.5.0) | n (%) * |
|---|---|---|
| Anorexia | 1/2/3 | 3/23/1 (50.0%) |
| Nausea | 2 | 1 (1.9%) |
| Oral mucositis | 2/3 | 2/1 (5.6%) |
| Creatinine increase | 1/2/4 | 2/2/1 (9.3%) |
| Pneumonitis | 2 | 1 (1.9%) |
| Leukopenia | 2/3 | 2/3 (9.3%) |
| Neutropenia | 2/3 | 2/6 (14.8%) |
| Febrile Neutropenia | 3 | 1 (1.9%) |
| Platelet cell decrease | 2 | 1 (1.9%) |
| Cerebral infarction | 2 | 1 (1.9%) |
| Hyponatremia (irAE) | 3 | 1 (1.9%) |
| Hypocalcemia (irAE) | 2 | 1 (1.9%) |
| Hypomagnesemia (irAE) | 2 | 1 (1.9%) |
| Adrenal insufficiency (irAE) | 2 | 3 (5.6%) |
| Hypothyroidism (irAE) | 2 | 2 (3.7%) |
| Autoimmune encephalitis (irAE) | 2 | 1 (1.9%) |
| Interstitial pneumonia (irAE) | 3 | 1 (1.9%) |
| Adverse Events | ≥80% (n = 35) | <80% Dose (n = 19) | p-Value |
|---|---|---|---|
| Anorexia | 17 (48.6%) | 10 (52.6%) | 1 |
| Nausea | 1 (2.9%) | 0 | 1 |
| Oral mucositis | 2 (5.7%) | 1 (5.3%) | 1 |
| Creatinine increase | 5 (14.3%) | 0 | 0.149 |
| Pneumonitis | 1 (2.9%) | 0 | 1 |
| Leukopenia | 3 (8.6%) | 2 (10.5%) | 1 |
| Neutropenia | 6 (17.1%) | 2 (10.5%) | 0.698 |
| Febrile Neutropenia | 1 (2.9%) | 0 | 1 |
| Platelet cell decrease | 1 (2.9%) | 0 | 1 |
| Cerebral infarction | 0 | 1 (5.3%) | 0.352 |
| Hyponatremia (irAE) | 0 | 1 (5.3%) | 0.352 |
| Hypocalcemia (irAE) | 0 | 1 (5.3%) | 0.352 |
| Hypomagnesemia (irAE) | 0 | 1 (5.3%) | 0.352 |
| Adrenal insufficiency (irAE) | 1 (2.9%) | 2 (10.5%) | 0.28 |
| Hypothyroidism (irAE) | 2 (5.7%) | 0 | 0.535 |
| Autoimmune encephalitis (irAE) | 1 (2.9%) | 0 | 1 |
| Interstitial pneumonia (irAE) | 1 (2.9%) | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueta, Y.; Nakajima, M.; Yoshimatsu, M.; Ochiai, T.; Takise, S.; Fujita, J.; Nakagawa, M.; Morita, S.; Kojima, K. Real-World Effectiveness of Cisplatin, 5-Fluorouracil, and Pembrolizumab Combination Therapy for Unresectable or Recurrent Esophageal Cancer. Cancers 2025, 17, 3202. https://doi.org/10.3390/cancers17193202
Ueta Y, Nakajima M, Yoshimatsu M, Ochiai T, Takise S, Fujita J, Nakagawa M, Morita S, Kojima K. Real-World Effectiveness of Cisplatin, 5-Fluorouracil, and Pembrolizumab Combination Therapy for Unresectable or Recurrent Esophageal Cancer. Cancers. 2025; 17(19):3202. https://doi.org/10.3390/cancers17193202
Chicago/Turabian StyleUeta, Yu, Masanobu Nakajima, Masaki Yoshimatsu, Takahiro Ochiai, Shuhei Takise, Junki Fujita, Masatoshi Nakagawa, Shinji Morita, and Kazuyuki Kojima. 2025. "Real-World Effectiveness of Cisplatin, 5-Fluorouracil, and Pembrolizumab Combination Therapy for Unresectable or Recurrent Esophageal Cancer" Cancers 17, no. 19: 3202. https://doi.org/10.3390/cancers17193202
APA StyleUeta, Y., Nakajima, M., Yoshimatsu, M., Ochiai, T., Takise, S., Fujita, J., Nakagawa, M., Morita, S., & Kojima, K. (2025). Real-World Effectiveness of Cisplatin, 5-Fluorouracil, and Pembrolizumab Combination Therapy for Unresectable or Recurrent Esophageal Cancer. Cancers, 17(19), 3202. https://doi.org/10.3390/cancers17193202





