Lymphatic Metastasis of Esophageal Squamous Cell Carcinoma: The Role of NRF2 and Therapeutic Strategies
Simple Summary
Abstract
1. Introduction
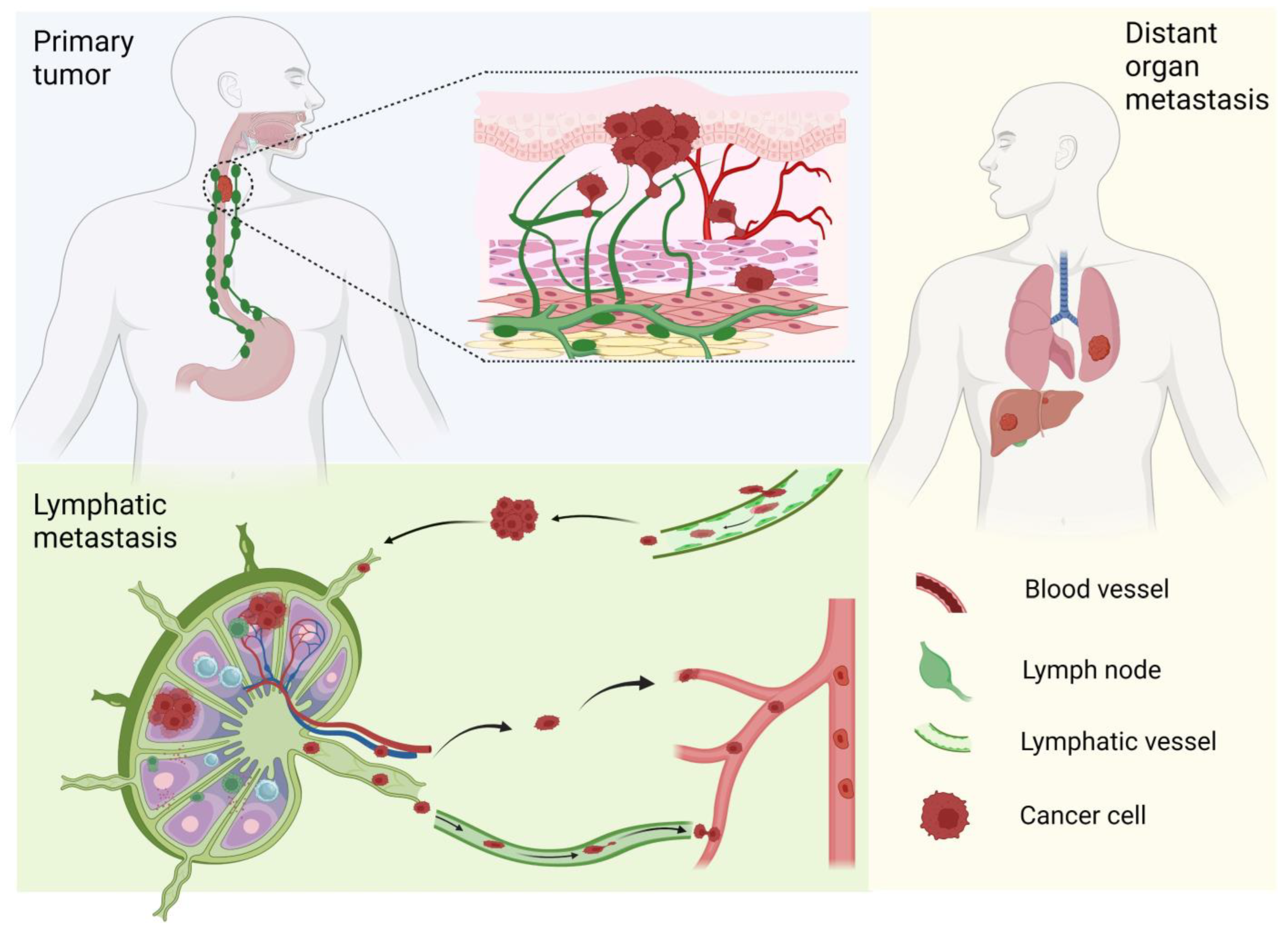
2. ESCC Lymphatic Metastasis
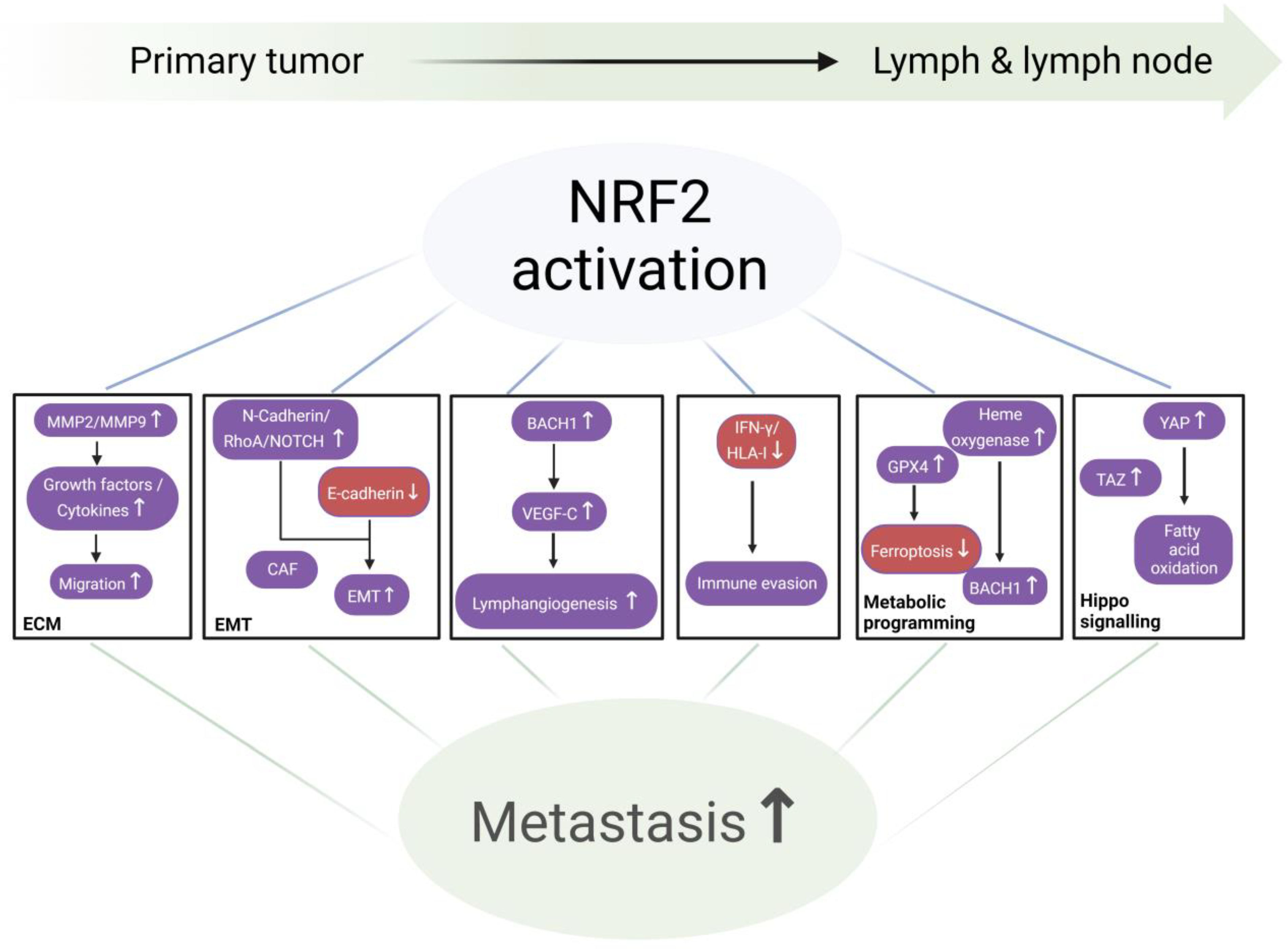
3. NRF2 Signaling Pathway and Metastasis
NRF2 and the Cascade of Lymphatic Metastasis (Figure 3)
4. Current Status of NRF2 Inhibitors
Approaches for Developing Small Molecule NRF2 Inhibitors (Figure 4)
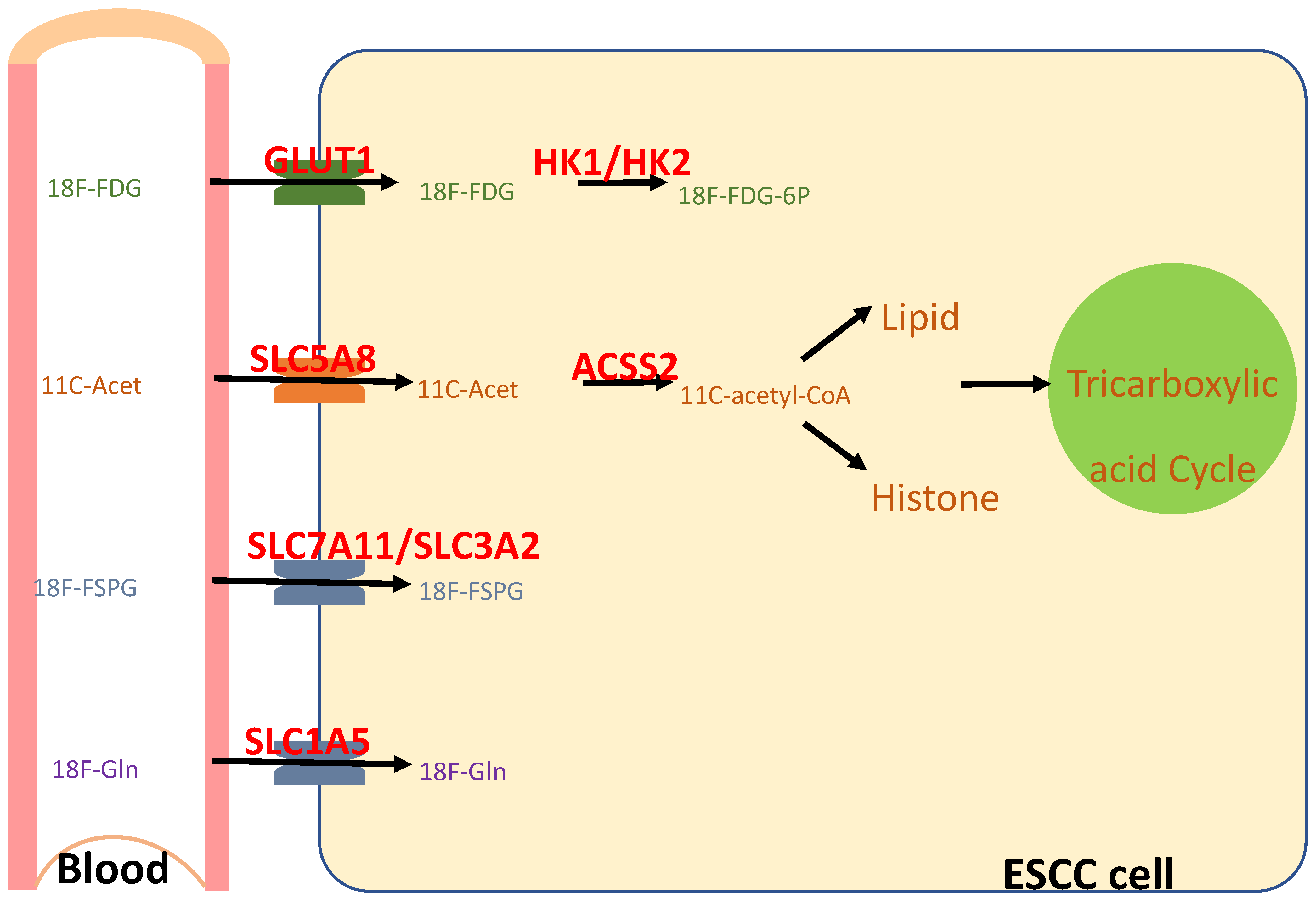
5. Animal Models of ESCC Lymphatic Metastasis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | artificial intelligence; |
| ARE | antioxidant response element; |
| CAF | cancer-associated fibroblast; |
| CUL3 | Cullin 3; |
| DHFR | dihydrofolate reductase; |
| ECM | extracellular matrix; |
| EMT | epithelial–mesenchymal transition; |
| ESCC | esophageal squamous cell carcinoma; |
| 18F-FSPG | (S)-4-(3-18F-fluoropropyl)-L-glutamic acid; |
| 18F-FDG | 2-deoxy-2-[18F]fluoro-D-glucose; |
| 18F-FRPG | (R)-4-(3-18F-fluoropropyl)-L-glutamic acid; |
| 18F-Gln | 2S,4R-4-18F-fluoroglutamine; |
| HDAC | histone deacetylase; |
| HIF1α | hypoxia-inducible factor 1α; |
| HO1 | heme oxygenase 1 |
| KEAP1 | Kelch-like ECH-associated protein 1; |
| LUSC | lung squamous cell carcinoma; |
| NQO1 | NAD(P)H quinone dehydrogenase 1; |
| NRF2/NFE2L2 | nuclear factor erythroid 2-related factor 2; |
| NRF2high | NRF2 hyperactivation; |
| NSCLC | non-small cell lung cancer |
| PET/CT | positron emission tomography/computed tomography; |
| ROS | reactive oxygen species. |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kang, X.; Chen, K.; Li, Y.; Li, J.; D’Amico, T.A.; Chen, X. Personalized targeted therapy for esophageal squamous cell carcinoma. World J. Gastroenterol. 2015, 21, 7648–7658. [Google Scholar] [CrossRef]
- Shah, M.A.; Altorki, N.; Patel, P.; Harrison, S.; Bass, A.; Abrams, J.A. Improving outcomes in patients with oesophageal cancer. Nat. Rev. Clin. Oncol. 2023, 20, 390–407. [Google Scholar] [CrossRef]
- Kayani, B.; Zacharakis, E.; Ahmed, K.; Hanna, G.B. Lymph node metastases and prognosis in oesophageal carcinoma—A systematic review. Eur. J. Surg. Oncol. 2011, 37, 747–753. [Google Scholar] [CrossRef]
- Wu, N.; Chen, Z.; Pang, L.; Ma, Q.; Chen, G. Prognostic significance of lymph node characteristics on survival in esophageal squamous cell carcinomas. Wien. Klin. Wochenschr. 2013, 125, 26–33. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Jian, J.; Chen, H.; Zhu, X.; Xie, J.; Xu, X. The Prognostic Significance of Lymph Node Ratio for Esophageal Cancer: A Meta-Analysis. J. Surg. Res. 2023, 292, 53–64. [Google Scholar] [CrossRef]
- Sun, S.; Yang, W.; Yang, Y.; Fan, M.; Wang, F.; He, L.; Han, B.; Chen, C. Nomogram for predicting survival after lymphatic metastasis in esophageal cancer: A SEER analysis. Medicine 2023, 102, e34189. [Google Scholar] [CrossRef]
- American Cancer Society. Survival Rates for Esophageal Cancer. Available online: https://www.cancer.org/cancer/types/esophagus-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 23 May 2025).
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Uno, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawamura, O.; Kusano, M.; Kuwano, H.; Takeuchi, H.; et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 2. Esophagus 2019, 16, 25–43. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Saeki, H.; Nakashima, Y.; Nakaji, Y.; Kudou, K.; Tsutsumi, R.; Nishimura, S.; Akiyama, S.; Tajiri, H.; Yukaya, T.; et al. Distant lymph node metastases caused by esophageal cancer invasion to the lamina propria: A case report. Surg. Case Rep. 2016, 2, 143. [Google Scholar] [CrossRef][Green Version]
- Jones, D.; Pereira, E.R.; Padera, T.P. Growth and immune evasion of lymph node metastasis. Front. Oncol. 2018, 8, 36. [Google Scholar] [CrossRef]
- Gur-Cohen, S.; Yang, H.; Baksh, S.C.; Miao, Y.; Levorse, J.; Kataru, R.P.; Liu, X.; de la Cruz-Racelis, J.; Mehrara, B.J.; Fuchs, E. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 2019, 366, 1218–1225. [Google Scholar] [CrossRef]
- Ji, X.; Cai, J.; Chen, Y.; Chen, L.Q. Lymphatic spreading and lymphadenectomy for esophageal carcinoma. World J. Gastrointest. Surg. 2016, 8, 90–94. [Google Scholar] [CrossRef]
- Kuge, K.; Murakami, G.; Mizobuchi, S.; Hata, Y.; Aikou, T.; Sasaguri, S. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. J. Thorac. Cardiovasc. Surg. 2003, 125, 1343–1349. [Google Scholar] [CrossRef]
- Tachimori, Y. Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: Efficacy of lymph node dissection according to tumor location. J. Thorac. Dis. 2017, 9, S724–S730. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Xia, W.; Wang, F. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag. Res. 2018, 10, 6295–6303. [Google Scholar] [CrossRef]
- Oshiro, H.; Osaka, Y.; Tachibana, S.; Aoki, T.; Tsuchiya, T.; Nagao, T. Retrograde Lymphatic Spread of Esophageal Cancer: A Case Report. Medicine 2015, 94, e1139. [Google Scholar] [CrossRef]
- Shang, Q.X.; Yang, Y.S.; Xu, L.Y.; Yang, H.; Li, Y.; Li, Y.; Wu, Z.Y.; Fu, J.H.; Yao, X.D.; Xu, X.E.; et al. Prognostic Role of Nodal Skip Metastasis in Thoracic Esophageal Squamous Cell Carcinoma: A Large-Scale Multicenter Study. Ann. Surg. Oncol. 2021, 28, 6341–6352. [Google Scholar] [CrossRef]
- Gockel, I.; Sgourakis, G.; Lyros, O.; Polotzek, U.; Schimanski, C.C.; Lang, H.; Hoppo, T.; Jobe, B.A. Risk of lymph node metastasis in submucosal esophageal cancer: A review of surgically resected patients. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 371–384. [Google Scholar] [CrossRef]
- Moriya, H.; Ohbu, M.; Kobayashi, N.; Tanabe, S.; Katada, N.; Futawatari, N.; Sakuramoto, S.; Kikuchi, S.; Okayasu, I.; Watanabe, M. Lymphatic tumor emboli detected by D2-40 immunostaining can more accurately predict lymph-node metastasis. World J. Surg. 2011, 35, 2031–2037. [Google Scholar] [CrossRef]
- Wang, A.; Lu, L.; Fan, J.; Wang, S.; Chen, X. Lymph node metastatic patterns and its clinical significance for thoracic superficial esophageal squamous cell carcinoma. J. Cardiothorac. Surg. 2020, 15, 262. [Google Scholar] [CrossRef]
- Cho, J.W.; Choi, S.C.; Jang, J.Y.; Shin, S.K.; Choi, K.D.; Lee, J.H.; Kim, S.G.; Sung, J.K.; Jeon, S.W.; Choi, I.J. Lymph node metastases in esophageal carcinoma: An endoscopist’s view. Clin. Endosc. 2014, 47, 523–529. [Google Scholar] [CrossRef]
- Lin, K.; Li, B.; Sun, Y.; Hu, H.; Zhang, Y.; Xiang, J.; Chen, H. Precise pattern of lymphatic spread of esophageal squamous cell carcinoma: Results of 1074 patients with N1 disease. J. Cancer Res. Clin. Oncol. 2023, 149, 15819–15825. [Google Scholar] [CrossRef]
- Nagaraja, V.; Eslick, G.D.; Cox, M.R. Sentinel lymph node in oesophageal cancer-a systematic review and meta-analysis. J. Gastrointest. Oncol. 2014, 5, 127–141. [Google Scholar] [CrossRef]
- Duan, X.; Shang, X.; Yue, J.; Ma, Z.; Chen, C.; Tang, P.; Jiang, H.; Yu, Z. A nomogram to predict lymph node metastasis risk for early esophageal squamous cell carcinoma. BMC Cancer 2021, 21, 431. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Xiang, J.; Zhang, Y.; Kong, Y.; Garfield, D.H.; Li, H. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2013, 146, 1198–1203. [Google Scholar] [CrossRef]
- Shen, W.; Shen, Y.; Tan, L.; Jin, C.; Xi, Y. A nomogram for predicting lymph node metastasis in surgically resected T1 esophageal squamous cell carcinoma. J. Thorac. Dis. 2018, 10, 4178–4185. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.X.; Shen, D.J.; Zhao, Q. A prediction model for lymph node metastasis in T1 esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2018, 155, 1902–1908. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Q.; Xiong, Z.; Paiboonrungruang, C.; Adekoya, T.; Li, Y.; Chen, X. Lymphatic Drainage System and Lymphatic Metastasis of Cancer Cells in the Mouse Esophagus. Dig. Dis. Sci. 2023, 68, 803–812. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Shah, R.; Rosso, K. Sentinel lymph node metastases in cancer: Causes, detection and their role in disease progression. Semin. Cell Dev. Biol. 2015, 38, 106–116. [Google Scholar] [CrossRef]
- Groth, S.S.; Virnig, B.A.; Whitson, B.A.; DeFor, T.E.; Li, Z.Z.; Tuttle, T.M.; Maddaus, M.A. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: Data from the Surveillance Epidemiology and End Results database. J. Thorac. Cardiovasc. Surg. 2010, 139, 612–620. [Google Scholar] [CrossRef]
- Altorki, N.; Mynard, N.; Nasar, A.; Spinelli, C.; Villena-Vargas, J.; Chow, O.; Lee, B.; Harrison, S.; Port, J. Ten-Year Survival and Recurrence Patterns After Three-Field Lymph Node Dissection for Squamous Cell and Adenocarcinoma of the Esophagus. Ann. Surg. 2023, 278, e43–e50. [Google Scholar] [CrossRef]
- Kamel, M.K.; Harrison, S.; Lee, B.; Port, J.L.; Stiles, B.M.; Altorki, N.K. Extended Lymphadenectomy Improves Survival After Induction Chemoradiation for Esophageal Cancer: A Propensity-Matched Analysis of the National Cancer Database. Ann. Surg. 2023, 277, e772–e776. [Google Scholar] [CrossRef]
- Isono, K.; Sato, H.; Nakayama, K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991, 48, 411–420. [Google Scholar] [CrossRef]
- van der Schaaf, M.; Johar, A.; Wijnhoven, B.; Lagergren, P.; Lagergren, J. Extent of lymph node removal during esophageal cancer surgery and survival. J. Natl. Cancer Inst. 2015, 107, djv043. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Miao, L.; Ma, L.; Luo, X.; Zhang, Y.; Ye, T.; Li, H.; Zhang, J.; Li, Y.; et al. Esophagectomy With Three-Field Versus Two-Field Lymphadenectomy for Middle and Lower Thoracic Esophageal Cancer: Long-Term Outcomes of a Randomized Clinical Trial. J. Thorac. Oncol. 2021, 16, 310–317. [Google Scholar] [CrossRef]
- Matsuda, S.; Takeuchi, M.; Kawakubo, H.; Kitagawa, Y. Lymph node metastatic patterns and the development of multidisciplinary treatment for esophageal cancer. Dis. Esophagus 2023, 36, doad006. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Dodson, M.; Shakya, A.; Anandhan, A.; Chen, J.; Garcia, J.G.N.; Zhang, D.D. NRF2 and Diabetes: The Good, the Bad, and the Complex. Diabetes 2022, 71, 2463–2476. [Google Scholar] [CrossRef]
- Paiboonrungruang, C.; Simpson, E.; Xiong, Z.; Huang, C.; Li, J.; Li, Y.; Chen, X. Development of targeted therapy of NRF2high esophageal squamous cell carcinoma. Cell. Signal. 2021, 86, 110105. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef]
- Hirose, W.; Oshikiri, H.; Taguchi, K.; Yamamoto, M. The KEAP1-NRF2 System and Esophageal Cancer. Cancers 2022, 14, 4702. [Google Scholar] [CrossRef]
- Cloer, E.W.; Goldfarb, D.; Schrank, T.P.; Weissman, B.E.; Major, M.B. NRF2 Activation in Cancer: From DNA to Protein. Cancer Res. 2019, 79, 889–898. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Wang, Q.; Yi, Y.; Li, B. Integrated cohort of esophageal squamous cell cancer reveals genomic features underlying clinical characteristics. Nat. Commun. 2022, 13, 5268. [Google Scholar] [CrossRef]
- Iwasaki, T.; Shirota, H.; Sasaki, K.; Ouchi, K.; Nakayama, Y.; Oshikiri, H.; Otsuki, A.; Suzuki, T.; Yamamoto, M.; Ishioka, C. Specific cancer types and prognosis in patients with variations in the KEAP1-NRF2 system: A retrospective cohort study. Cancer Sci. 2024, 115, 4034–4044. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Okumura, H.; Uchikado, Y.; Kita, Y.; Sasaki, K.; Owaki, T.; Ishigami, S.; Natsugoe, S. Nrf2 is useful for predicting the effect of chemoradiation therapy on esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2014, 21, 2347–2352. [Google Scholar] [CrossRef]
- Shibata, T.; Kokubu, A.; Saito, S.; Narisawa-Saito, M.; Sasaki, H.; Aoyagi, K.; Yoshimatsu, Y.; Tachimori, Y.; Kushima, R.; Kiyono, T.; et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia 2011, 13, 864–873. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, X.; Yu, X.; Chen, X.; Hu, X.; Lu, J.; Zhao, H.; Cao, Q.; Gu, Y.; Yang, Y.; et al. High expression of nuclear NRF2 combined with NFE2L2 alterations predicts poor prognosis in esophageal squamous cell carcinoma patients. Mod. Pathol. 2022, 35, 929–937. [Google Scholar] [CrossRef]
- Tamborero, D.; Gonzalez-Perez, A.; Perez-Llamas, C.; Deu-Pons, J.; Kandoth, C.; Reimand, J.; Lawrence, M.S.; Getz, G.; Bader, G.D.; Ding, L.; et al. Comprehensive identification of mutational cancer driver genes across 12 tumor types. Sci. Rep. 2013, 3, 2650. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Paiboonrungruan, C.; Yan, T.; Williams, K.P.; Major, M.B.; Chen, X.L. Targeted therapy of esophageal squamous cell carcinoma: The NRF2 signaling pathway as target. Ann. N. Y. Acad. Sci. 2018, 1434, 164–172. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Kong, P.; Liu, Y.; Huang, J.; Xu, E.; Wei, W.; Li, G.; Cheng, X.; Xue, L.; et al. Integrated multi-omics profiling yields a clinically relevant molecular classification for esophageal squamous cell carcinoma. Cancer Cell 2023, 41, 181–195.e9. [Google Scholar] [CrossRef]
- Lignitto, L.; LeBoeuf, S.E.; Homer, H.; Jiang, S.; Askenazi, M.; Karakousi, T.R.; Pass, H.I.; Bhutkar, A.J.; Tsirigos, A.; Ueberheide, B.; et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell 2019, 178, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 2019, 178, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Long, M.; Huang, Y.; Zhang, L.; Zhang, R.; Zheng, Y.; Liao, X.; Wang, Y.; Liao, Q.; et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016, 8, 334ra51. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.A.; Arslan, E.; Bartels, M.; Michikawa, C.; Lindemann, A.; Tomczak, K.; Yu, W.; Sandulache, V.; Ma, W.; Shen, L.; et al. Dysregulation and Epigenetic Reprogramming of NRF2 Signaling Axis Promote Acquisition of Cisplatin Resistance and Metastasis in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2023, 29, 1344–1359. [Google Scholar] [CrossRef]
- Zhang, H.S.; Zhang, Z.G.; Du, G.Y.; Sun, H.L.; Liu, H.Y.; Zhou, Z.; Gou, X.M.; Wu, X.H.; Yu, X.Y.; Huang, Y.H. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1alpha/Notch1 axis. J. Cell. Mol. Med. 2019, 23, 3451–3463. [Google Scholar] [CrossRef]
- Cheung, E.C.; DeNicola, G.M.; Nixon, C.; Blyth, K.; Labuschagne, C.F.; Tuveson, D.A.; Vousden, K.H. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer. Cancer Cell 2020, 37, 168–182.e4. [Google Scholar] [CrossRef]
- Ueda, Y.; Kiyonaka, S.; Selfors, L.M.; Inoue, K.; Harada, H.; Doura, T.; Onuma, K.; Uchiyama, M.; Kurogi, R.; Yamada, Y.; et al. Intratumour oxidative hotspots provide a niche for cancer cell dissemination. Nat. Cell Biol. 2025, 27, 530–543. [Google Scholar] [CrossRef]
- Satoh, H.; Moriguchi, T.; Taguchi, K.; Takai, J.; Maher, J.M.; Suzuki, T.; Winnard, P.T., Jr.; Raman, V.; Ebina, M.; Nukiwa, T.; et al. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis 2010, 31, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Satoh, H.; Suzuki, T.; Moriguchi, T.; Pi, J.; Shimosegawa, T.; Yamamoto, M. Myeloid lineage-specific deletion of antioxidant system enhances tumor metastasis. Cancer Prev. Res. 2014, 7, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Stott, S.L.; Lee, R.J.; Nagrath, S.; Yu, M.; Miyamoto, D.T.; Ulkus, L.; Inserra, E.J.; Ulman, M.; Springer, S.; Nakamura, Z. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci. Transl. Med. 2010, 2, 25ra23. [Google Scholar] [CrossRef]
- Follain, G.; Herrmann, D.; Harlepp, S.; Hyenne, V.; Osmani, N.; Warren, S.C.; Timpson, P.; Goetz, J.G. Fluids and their mechanics in tumour transit: Shaping metastasis. Nat. Rev. Cancer 2020, 20, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Nazarenko, I.; Thiele, W. Do all roads lead to Rome? Routes to metastasis development. Int. J. Cancer 2011, 128, 2511–2526. [Google Scholar] [CrossRef]
- Hoshida, T.; Isaka, N.; Hagendoorn, J.; di Tomaso, E.; Chen, Y.-L.; Pytowski, B.; Fukumura, D.; Padera, T.P.; Jain, R.K. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: Therapeutic implications. Cancer Res. 2006, 66, 8065–8075. [Google Scholar] [CrossRef]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. VEGF-C–induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007, 109, 1010–1017. [Google Scholar] [CrossRef]
- Kai, F.; Drain, A.P.; Weaver, V.M. The extracellular matrix modulates the metastatic journey. Dev. Cell 2019, 49, 332–346. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Yauch, R.L.; Gould, S.E.; Scales, S.J.; Tang, T.; Tian, H.; Ahn, C.P.; Marshall, D.; Fu, L.; Januario, T.; Kallop, D. A paracrine requirement for hedgehog signalling in cancer. Nature 2008, 455, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.-M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Risson, E.; Nobre, A.R.; Maguer-Satta, V.; Aguirre-Ghiso, J.A. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 2020, 1, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.; Uhrin, P.; Sixt, M. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018, 359, 1408–1411. [Google Scholar] [CrossRef]
- Pereira, E.R.; Kedrin, D.; Seano, G.; Gautier, O.; Meijer, E.F.J.; Jones, D.; Chin, S.M.; Kitahara, S.; Bouta, E.M.; Chang, J.; et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 2018, 359, 1403–1407. [Google Scholar] [CrossRef]
- Dixon, J.B.; Greiner, S.T.; Gashev, A.A.; Cote, G.L.; Moore, J.E., Jr.; Zawieja, D.C. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 2006, 13, 597–610. [Google Scholar] [CrossRef]
- Ubellacker, J.M.; Tasdogan, A.; Ramesh, V.; Shen, B.; Mitchell, E.C.; Martin-Sandoval, M.S.; Gu, Z.; McCormick, M.L.; Durham, A.B.; Spitz, D.R.; et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 2020, 585, 113–118. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Wang, J.; Cai, Y.; Chen, X.; Chen, Y.; Shi, Y.; Chen, G.; Zhu, K. Multi-Region Genomic Landscape Analysis for the Preoperative Prediction of Lymph Node Metastasis in Esophageal Carcinoma. Front. Genet. 2022, 13, 830601. [Google Scholar] [CrossRef]
- Ye, B.; Fan, D.; Xiong, W.; Li, M.; Yuan, J.; Jiang, Q.; Zhao, Y.; Lin, J.; Liu, J.; Lv, Y.; et al. Oncogenic enhancers drive esophageal squamous cell carcinogenesis and metastasis. Nat. Commun. 2021, 12, 4457. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, B.; Zhang, C.; Kwong, D.L.; Chang, Z.; Li, S.; Wang, Z.; Han, H.; Li, J.; Zhong, Y.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Esophageal Squamous Cell Carcinoma. Adv. Sci. 2023, 10, e2204565. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rojo de la Vega, M.; Wen, Q.; Bharara, M.; Jiang, T.; Zhang, R.; Zhou, S.; Wong, P.K.; Wondrak, G.T.; Zheng, H.; et al. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 2016, 65, 780–793. [Google Scholar] [CrossRef]
- Shibata, T.; Saito, S.; Kokubu, A.; Suzuki, T.; Yamamoto, M.; Hirohashi, S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010, 70, 9095–9105. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, X.; Ma, N.; Wei, Z.; Ci, X.; Zhang, S. The promoting effect and mechanism of Nrf2 on cell metastasis in cervical cancer. J. Transl. Med. 2023, 21, 433. [Google Scholar] [CrossRef]
- Bocci, F.; Tripathi, S.C.; Vilchez Mercedes, S.A.; George, J.T.; Casabar, J.P.; Wong, P.K.; Hanash, S.M.; Levine, H.; Onuchic, J.N.; Jolly, M.K. NRF2 activates a partial epithelial-mesenchymal transition and is maximally present in a hybrid epithelial/mesenchymal phenotype. Integr. Biol. 2019, 11, 251–263. [Google Scholar] [CrossRef]
- Vilchez Mercedes, S.A.; Bocci, F.; Ahmed, M.; Eder, I.; Zhu, N.; Levine, H.; Onuchic, J.N.; Jolly, M.K.; Wong, P.K. Nrf2 modulates the hybrid epithelial/mesenchymal phenotype and Notch signaling during collective cancer migration. Front. Mol. Biosci. 2022, 9, 807324. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.J.; Bao, Q.C.; Wang, L.; Guo, T.K.; Chen, W.L.; Xu, L.L.; Zhou, H.S.; Bian, J.L.; Yang, Y.R.; et al. NRF2 promotes breast cancer cell proliferation and metastasis by increasing RhoA/ROCK pathway signal transduction. Oncotarget 2016, 7, 73593–73606. [Google Scholar] [CrossRef]
- Lv, J.; Xie, M.; Zhao, S.; Qiu, W.; Wang, S.; Cao, M. Nestin is essential for cellular redox homeostasis and gastric cancer metastasis through the mediation of the Keap1-Nrf2 axis. Cancer Cell Int. 2021, 21, 603. [Google Scholar] [CrossRef]
- Jin, M.; Wang, J.; Ji, X.; Cao, H.; Zhu, J.; Chen, Y.; Yang, J.; Zhao, Z.; Ren, T.; Xing, J. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 136. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Acar, A.; Eaton, E.N.; Mellody, K.T.; Scheel, C.; Ben-Porath, I.; Onder, T.T.; Wang, Z.C.; Richardson, A.L.; Weinberg, R.A. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 20009–20014. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, D.H.; Sung, K.W.; Shim, S.M.; Cha-Molstad, H.; Soung, N.K.; Lee, K.H.; Hwang, J.; Lee, H.G.; Kwon, Y.T. p62-induced cancer-associated fibroblast activation via the Nrf2-ATF6 pathway promotes lung tumorigenesis. Cancers 2021, 13, 864. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Hung, W.-C. Reprogramming of sentinel lymph node microenvironment during tumor metastasis. J. Biomed. Sci. 2022, 29, 84. [Google Scholar] [CrossRef]
- van der Horst, D.; Carter-Timofte, M.E.; van Grevenynghe, J.; Laguette, N.; Dinkova-Kostova, A.T.; Olagnier, D. Regulation of innate immunity by Nrf2. Curr. Opin. Immunol. 2022, 78, 102247. [Google Scholar] [CrossRef]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef]
- Marzio, A.; Kurz, E.; Sahni, J.M.; Di Feo, G.; Puccini, J.; Jiang, S.; Hirsch, C.A.; Arbini, A.A.; Wu, W.L.; Pass, H.I.; et al. EMSY inhibits homologous recombination repair and the interferon response, promoting lung cancer immune evasion. Cell 2022, 185, 169–183.e19. [Google Scholar] [CrossRef]
- Olagnier, D.; Brandtoft, A.M.; Gunderstofte, C.; Villadsen, N.L.; Krapp, C.; Thielke, A.L.; Laustsen, A.; Peri, S.; Hansen, A.L.; Bonefeld, L.; et al. Nrf2 negatively regulates STING indicating a link between antiviral sensing and metabolic reprogramming. Nat. Commun. 2018, 9, 3506. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Harkonen, J.; Polonen, P.; Deen, A.J.; Selvarajan, I.; Teppo, H.R.; Dimova, E.Y.; Kietzmann, T.; Ahtiainen, M.; Vayrynen, J.P.; Vayrynen, S.A.; et al. A pan-cancer analysis shows immunoevasive characteristics in NRF2 hyperactive squamous malignancies. Redox Biol. 2023, 61, 102644. [Google Scholar] [CrossRef]
- Zhu, B.; Tang, L.; Chen, S.; Yin, C.; Peng, S.; Li, X.; Liu, T.; Liu, W.; Han, C.; Stawski, L.; et al. Targeting the upstream transcriptional activator of PD-L1 as an alternative strategy in melanoma therapy. Oncogene 2018, 37, 4941–4954. [Google Scholar] [CrossRef] [PubMed]
- Harrell, M.I.; Iritani, B.M.; Ruddell, A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007, 170, 774–786. [Google Scholar] [CrossRef]
- Breslin, J.W.; Gaudreault, N.; Watson, K.D.; Reynoso, R.; Yuan, S.Y.; Wu, M.H. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am. J. Physiol.-Heart Circ. Physiol. 2007, 293, H709–H718. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Hur, E.G.; Kang, S.J.; Kim, J.A.; Thapa, D.; Lee, Y.M.; Ku, S.K.; Jung, Y.; Kwak, M.K. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res. 2011, 71, 2260–2275. [Google Scholar] [CrossRef]
- Wei, Y.; Gong, J.; Thimmulappa, R.K.; Kosmider, B.; Biswal, S.; Duh, E.J. Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proc. Natl. Acad. Sci. USA 2013, 110, E3910–E3918. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Shin, S.; Slocum, S.L.; Agoston, E.S.; Wakabayashi, J.; Kwak, M.-K.; Misra, V.; Biswal, S.; Yamamoto, M.; Kensler, T.W. Regulation of notch1 signaling by nrf2: Implications for tissue regeneration. Sci. Signal. 2010, 3, ra52. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W.; et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef]
- Cohen, B.; Tempelhof, H.; Raz, T.; Oren, R.; Nicenboim, J.; Bochner, F.; Even, R.; Jelinski, A.; Eilam, R.; Ben-Dor, S. BACH family members regulate angiogenesis and lymphangiogenesis by modulating VEGFC expression. Life Sci. Alliance 2020, 3, e202000666. [Google Scholar] [CrossRef]
- Wei, Q.; Qian, Y.; Yu, J.; Wong, C.C. Metabolic rewiring in the promotion of cancer metastasis: Mechanisms and therapeutic implications. Oncogene 2020, 39, 6139–6156. [Google Scholar] [CrossRef]
- Kerins, M.J.; Ooi, A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef]
- Xiong, G.; Feng, Y.; Yi, X.; Zhang, X.; Li, X.; Yang, L.; Yi, Z.; Sai, B.; Yang, Z.; Zhang, Q.; et al. NRF2-directed PRPS1 upregulation to promote the progression and metastasis of melanoma. Front. Immunol. 2022, 13, 989263. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, L. The Lymphatic Fluid. Int. Rev. Cell Mol. Biol. 2018, 337, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Zhang, B.; Huang, C.; Fu, Y.; Zhao, Y.; Gong, L.; Tan, Y.; Wang, H.; Chen, W.; Luo, J.; et al. FAO-fueled OXPHOS and NRF2-mediated stress resilience in MICs drive lymph node metastasis. Proc. Natl. Acad. Sci. USA 2025, 122, e2411241122. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Muramatsu, T.; Imoto, I.; Matsui, T.; Kozaki, K.; Haruki, S.; Sudol, M.; Shimada, Y.; Tsuda, H.; Kawano, T.; Inazawa, J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis 2011, 32, 389–398. [Google Scholar] [CrossRef]
- Chai, A.W.Y.; Yee, P.S.; Price, S.; Yee, S.M.; Lee, H.M.; Tiong, V.K.; Goncalves, E.; Behan, F.M.; Bateson, J.; Gilbert, J.; et al. Genome-wide CRISPR screens of oral squamous cell carcinoma reveal fitness genes in the Hippo pathway. eLife 2020, 9, e57761. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Z.; Hu, X.; Su, T.; Su, L.; Pu, H. Clinical significance of YAP1 and TAZ in esophageal squamous cell carcinoma. Medicine 2021, 100, e26597. [Google Scholar] [CrossRef]
- Lee, C.K.; Jeong, S.H.; Jang, C.; Bae, H.; Kim, Y.H.; Park, I.; Kim, S.K.; Koh, G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019, 363, 644–649. [Google Scholar] [CrossRef]
- Kamisako, T.; Tanaka, Y.; Kishino, Y.; Ikeda, T.; Yamamoto, K.; Masuda, S.; Ogawa, H. Role of Nrf2 in the alteration of cholesterol and bile acid metabolism-related gene expression by dietary cholesterol in high fat-fed mice. J. Clin. Biochem. Nutr. 2014, 54, 90–94. [Google Scholar] [CrossRef]
- Escoll, M.; Lastra, D.; Pajares, M.; Robledinos-Anton, N.; Rojo, A.I.; Fernandez-Gines, R.; Mendiola, M.; Martinez-Marin, V.; Esteban, I.; Lopez-Larrubia, P.; et al. Transcription factor NRF2 uses the Hippo pathway effector TAZ to induce tumorigenesis in glioblastomas. Redox Biol. 2020, 30, 101425. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xiong, Z.; Huang, C.; Li, J.; Yang, W.; Han, Y.; Paiboonrungruan, C.; Major, M.B.; Chen, K.N.; Kang, X.; et al. Hyperactivity of the transcription factor Nrf2 causes metabolic reprogramming in mouse esophagus. J. Biol. Chem. 2019, 294, 327–340. [Google Scholar] [CrossRef]
- Ciamporcero, E.; Daga, M.; Pizzimenti, S.; Roetto, A.; Dianzani, C.; Compagnone, A.; Palmieri, A.; Ullio, C.; Cangemi, L.; Pili, R.; et al. Crosstalk between Nrf2 and YAP contributes to maintaining the antioxidant potential and chemoresistance in bladder cancer. Free Radic. Biol. Med. 2018, 115, 447–457. [Google Scholar] [CrossRef]
- Xie, X.; Yu, T.; Li, X.; Zhang, N.; Foster, L.J.; Peng, C.; Huang, W.; He, G. Recent advances in targeting the “undruggable” proteins: From drug discovery to clinical trials. Signal Transduct. Target. Ther. 2023, 8, 335. [Google Scholar] [CrossRef]
- Paiboonrungruang, C.; Xiong, Z.; Lamson, D.; Li, Y.; Bowman, B.; Chembo, J.; Huang, C.; Li, J.; Livingston, E.W.; Frank, J.E.; et al. Small molecule screen identifies pyrimethamine as an inhibitor of NRF2-driven esophageal hyperplasia. Redox Biol. 2023, 67, 102901. [Google Scholar] [CrossRef] [PubMed]
- Chembo, J.; Bowman, B.M.; Lapak, K.; Wilkerson, E.; Paiboonrungruang, C.; Cho, K.; Medcalf, M.R.; Patti, G.J.; Dolle, R.E.; Chen, X.; et al. Pyrimethamine and a potent analogue WCDD115 inhibit NRF2 by suppressing DHFR and one-carbon metabolism. bioRxiv 2025. [Google Scholar] [CrossRef]
- Kawami, M.; Honda, M.; Hara, T.; Yumoto, R.; Takano, M. Role of Nrf2 in Methotrexate-Induced Epithelial-Mesenchymal Transition in Alveolar A549 Cells. Biol. Pharm. Bull. 2022, 45, 1069–1076. [Google Scholar] [CrossRef]
- Lizardo, M.M.; Hughes, C.; Huang, Y.Z.; Shyp, T.; Delaidelli, A.; Zhang, H.F.; Shaool, S.S.; Renner, A.F.; Burwag, F.; Sayles, L.C.; et al. Pharmacologic Inhibition of EIF4A Blocks NRF2 Synthesis to Prevent Osteosarcoma Metastasis. Clin. Cancer Res. 2024, 30, 4464–4481. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, Y.; Ma, J.; Xiao, M.; Cao, R.; Xi, Y.; Li, T.; Huang, T.; Yan, M. Triptolide induces hepatotoxicity by promoting ferroptosis through Nrf2 degradation. Cell Biol. Toxicol. 2024, 40, 94. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Y.; Zhou, Y.; Ruiz-Rodado, V.; Larion, M.; Xu, G.; Yang, C. Triptolide suppresses IDH1-mutated malignancy via Nrf2-driven glutathione metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 9964–9972. [Google Scholar] [CrossRef]
- Hou, Z.; Lockwood, L.; Zhang, D.; Occhiuto, C.J.; Mo, L.; Aldrich, K.E.; Stoub, H.E.; Gallo, K.A.; Liby, K.T.; Odom, A.L. Exploring structural effects in a new class of NRF2 inhibitors. RSC Med. Chem. 2023, 14, 74–84. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, Z.; Aldrich, K.E.; Lockwood, L.; Odom, A.L.; Liby, K.T. A Novel Nrf2 Pathway Inhibitor Sensitizes Keap1-Mutant Lung Cancer Cells to Chemotherapy. Mol. Cancer Ther. 2021, 20, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Wyseure, T.; Lo, I.-C.; Metzger, J.; Esissler, C.L.; Bernard, S.M.; Bok, I.; Snead, A.N.; Parker, A.; Green, J.C.; et al. Suppression of NRF2-dependent cancer growth by a covalent allosteric molecular glue. bioRxiv 2024. [Google Scholar] [CrossRef]
- Aboulkassim, T.; Tian, X.; Liu, Q.; Qiu, D.; Hancock, M.; Wu, J.H.; Batist, G. A NRF2 inhibitor selectively sensitizes KEAP1 mutant tumor cells to cisplatin and gefitinib by restoring NRF2-inhibitory function of KEAP1 mutants. Cell Rep. 2023, 42, 113104. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Ma, S.; Zhu, Y.; Zhao, J.; Tong, Y.; You, Q.; Jiang, Z. ARE-PROTACs Enable Co-degradation of an Nrf2-MafG Heterodimer. J. Med. Chem. 2023, 66, 6070–6081. [Google Scholar] [CrossRef]
- Read, T.J.; Steel, R.; Damon, L.; Yoo, R.; Goliaei, A.; Rimel, J.; Hsu, J.; Simpson, D.; Azofeifa, J. Discovery of a novel molecular glue degrader of Nrf2. In Proceedings of the AACR 2025, Chicago, IL, USA, 25–30 April 2025. [Google Scholar]
- Simov, V.; Altman, M.D.; Bianchi, E.; DelRizzo, S.; DiNunzio, E.N.; Feng, G.; Goldenblatt, P.; Ingenito, R.; Johnson, S.A.; Mansueto, M.S.; et al. Discovery and characterization of novel peptide inhibitors of the NRF2/MAFG/DNA ternary complex for the treatment of cancer. Eur. J. Med. Chem. 2021, 224, 113686. [Google Scholar] [CrossRef]
- Wiedemann, B.; Kamps, D.; Depta, L.; Weisner, J.; Cvetreznik, J.; Tomassi, S.; Gentz, S.; Hoffmann, J.E.; Muller, M.P.; Koch, O.; et al. Design and synthesis of Nrf2-derived hydrocarbon stapled peptides for the disruption of protein-DNA-interactions. PLoS ONE 2022, 17, e0267651. [Google Scholar] [CrossRef]
- Modi, R.; McKee, N.; Zhang, N.; Alwali, A.; Nelson, S.; Lohar, A.; Ostafe, R.; Zhang, D.D.; Parkinson, E.I. Stapled Peptides as Direct Inhibitors of Nrf2-sMAF Transcription Factors. J. Med. Chem. 2023, 66, 6184–6192. [Google Scholar] [CrossRef]
- Zhao, J.; Moore, A.N.; Redell, J.B.; Dash, P.K. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 2007, 27, 10240–10248. [Google Scholar] [CrossRef]
- He, X.; Zhou, Y.; Chen, W.; Zhao, X.; Duan, L.; Zhou, H.; Li, M.; Yu, Y.; Zhao, J.; Guo, Y.; et al. Repurposed pizotifen malate targeting NRF2 exhibits anti-tumor activity through inducing ferroptosis in esophageal squamous cell carcinoma. Oncogene 2023, 42, 1209–1223. [Google Scholar] [CrossRef]
- Mo, X.; Niu, Q.; Ivanov, A.A.; Tsang, Y.H.; Tang, C.; Shu, C.; Li, Q.; Qian, K.; Wahafu, A.; Doyle, S.P.; et al. Systematic discovery of mutation-directed neo-protein-protein interactions in cancer. Cell 2022, 185, 1974–1985.e12. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, E.I.; Hergenrother, P.J. Deoxynyboquinones as NQO1-Activated Cancer Therapeutics. Acc. Chem. Res. 2015, 48, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Guise, C.P.; Abbattista, M.R.; Singleton, R.S.; Holford, S.D.; Connolly, J.; Dachs, G.U.; Fox, S.B.; Pollock, R.; Harvey, J.; Guilford, P.; et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010, 70, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Li, W.F.; Jung, D.; Wang, C.C.; Qi, T.; Shia, C.S.; Hsu, R.Y.; Hsieh, Y.C.; Duan, J. A novel selective AKR1C3-activated prodrug AST-3424/OBI-3424 exhibits broad anti-tumor activity. Am. J. Cancer Res. 2021, 11, 3645–3659. [Google Scholar]
- Pan, D.; Yang, W.; Zeng, Y.; Qin, H.; Xu, Y.; Gui, Y.; Fan, X.; Tian, G.; Wu, Y.; Sun, H.; et al. AKR1C3 regulated by NRF2/MAFG complex promotes proliferation via stabilizing PARP1 in hepatocellular carcinoma. Oncogene 2022, 41, 3846–3858. [Google Scholar] [CrossRef]
- Ding, H.; Chen, Z.; Wu, K.; Huang, S.M.; Wu, W.L.; LeBoeuf, S.E.; Pillai, R.G.; Rabinowitz, J.D.; Papagiannakopoulos, T. Activation of the NRF2 antioxidant program sensitizes tumors to G6PD inhibition. Sci. Adv. 2021, 7, eabk1023. [Google Scholar] [CrossRef]
- Sayin, V.I.; LeBoeuf, S.E.; Singh, S.X.; Davidson, S.M.; Biancur, D.; Guzelhan, B.S.; Alvarez, S.W.; Wu, W.L.; Karakousi, T.R.; Zavitsanou, A.M.; et al. Activation of the NRF2 antioxidant program generates an imbalance in central carbon metabolism in cancer. eLife 2017, 6, e28083. [Google Scholar] [CrossRef]
- Rais, R.; Lemberg, K.M.; Tenora, L.; Arwood, M.L.; Pal, A.; Alt, J.; Wu, Y.; Lam, J.; Aguilar, J.M.H.; Zhao, L.; et al. Discovery of DRP-104, a tumor-targeted metabolic inhibitor prodrug. Sci. Adv. 2022, 8, eabq5925. [Google Scholar] [CrossRef]
- Paik, P.K.; Fan, P.D.; Qeriqi, B.; Namakydoust, A.; Daly, B.; Ahn, L.; Kim, R.; Plodkowski, A.; Ni, A.; Chang, J.; et al. Targeting NFE2L2/KEAP1 Mutations in Advanced NSCLC With the TORC1/2 Inhibitor TAK-228. J. Thorac. Oncol. 2023, 18, 516–526. [Google Scholar] [CrossRef]
- Duong, H.Q.; Yi, Y.W.; Kang, H.J.; Hong, Y.B.; Tang, W.; Wang, A.; Seong, Y.S.; Bae, I. Inhibition of NRF2 by PIK-75 augments sensitivity of pancreatic cancer cells to gemcitabine. Int. J. Oncol. 2014, 44, 959–969. [Google Scholar] [CrossRef]
- Karagiannis, D.; Wu, W.; Li, A.; Hayashi, M.; Chen, X.; Yip, M.; Mangipudy, V.; Xu, X.; Sanchez-Rivera, F.J.; Soto-Feliciano, Y.M.; et al. Metabolic reprogramming by histone deacetylase inhibition preferentially targets NRF2-activated tumors. Cell Rep. 2024, 43, 113629. [Google Scholar] [CrossRef] [PubMed]
- Afjei, R.; Sadeghipour, N.; Kumar, S.U.; Pandrala, M.; Kumar, V.; Malhotra, S.V.; Massoud, T.F.; Paulmurugan, R. A New Nrf2 Inhibitor Enhances Chemotherapeutic Effects in Glioblastoma Cells Carrying p53 Mutations. Cancers 2022, 14, 6120. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.S.; Byun, W.S.; Ock, C.W.; Kim, W.K.; Park, H.J.; Lee, S.K. Periplocin exerts antitumor activity by regulating Nrf2-mediated signaling pathway in gemcitabine-resistant pancreatic cancer cells. Biomed. Pharmacother. 2023, 157, 114039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, L.; Ye, Q.; Zhang, S.; Kung, H.; Jiang, F.; Jiang, G.; Miao, J.; Zhao, B. Discovery of a novel Nrf2 inhibitor that induces apoptosis of human acute myeloid leukemia cells. Oncotarget 2017, 8, 7625–7636. [Google Scholar] [CrossRef]
- Du, L.; Zhu, X.; Jiang, Z.; Wang, W.; Liu, P.; Zhu, L.; Zhang, F. Resveratrol inhibits ferroptosis in the lung tissues of heat stroke-induced rats via the Nrf2 pathway. BMC Pharmacol. Toxicol. 2024, 25, 88. [Google Scholar] [CrossRef]
- Evison, B.J.; Sleebs, B.E.; Watson, K.G.; Phillips, D.R.; Cutts, S.M. Mitoxantrone, More than Just Another Topoisomerase II Poison. Med. Res. Rev. 2016, 36, 248–299. [Google Scholar] [CrossRef]
- Chan, K.; Lu, R.; Chang, J.C.; Kan, Y.W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. USA 1996, 93, 13943–13948. [Google Scholar] [CrossRef]
- Choi, E.J.; Jung, B.J.; Lee, S.H.; Yoo, H.S.; Shin, E.A.; Ko, H.J.; Chang, S.; Kim, S.Y.; Jeon, S.M. A clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancer. Oncogene 2017, 36, 5285–5295. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Lee, A.G.; Briones-Martin-Del-Campo, M.; Conn, C.S.; Simpson, D.R.; Scott, A.I.; Le, A.; Cowan, T.M.; Ruggero, D.; Sweet-Cordero, E.A. Oncogenic KRAS Regulates Amino Acid Homeostasis and Asparagine Biosynthesis via ATF4 and Alters Sensitivity to L-Asparaginase. Cancer Cell 2018, 33, 91–107.e6. [Google Scholar] [CrossRef]
- Islam, S.; Jin, H.; Liu, D.; Lu, D.; Zhang, Y.; Chang, R.; Austin, J.; Beer, T.; Tang, H.; Huang, L.; et al. Combinatorial use of VHL and KEAP1-based PROTACs reveals unexpected synergy and hook effect relief. In Proceedings of the AACR 2025, Chicago, IL, USA, 25–30 April 2025. [Google Scholar]
- Zhang, B.; Gao, S.; Wu, T.; Ma, Y.; Fang, S.; Rong, M.; Jia, W.; Zhang, S.; Hou, H.; Wang, X.; et al. Rational Design of Dual Degraders by Incorporating Molecular Glue Structural Features into PROTAC Degraders. J. Med. Chem. 2025, 68, 10268–10298. [Google Scholar] [CrossRef] [PubMed]
- Casas, G.; Perche, F.; Midoux, P.; Pichon, C.; Malinge, J.M. DNA minicircles as novel STAT3 decoy oligodeoxynucleotides endowed with anticancer activity in triple-negative breast cancer. Mol. Ther. Nucleic Acids 2022, 29, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Baird, L.; Yamamoto, M. The clinical-grade CBP/p300 inhibitor CCS1477 represses the global NRF2-dependent cytoprotective transcription program and re-sensitizes cancer cells to chemotherapeutic drugs. Free Radic. Biol. Med. 2025, 233, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.C.; Yoon, H.Y.; Lim, D.K.; Kim, K. Recent Trends in In Situ Enzyme-Activatable Prodrugs for Targeted Cancer Therapy. Bioconjug. Chem. 2020, 31, 1012–1024. [Google Scholar] [CrossRef]
- Baird, L.; Suzuki, T.; Takahashi, Y.; Hishinuma, E.; Saigusa, D.; Yamamoto, M. Geldanamycin-Derived HSP90 Inhibitors Are Synthetic Lethal with NRF2. Mol. Cell. Biol. 2020, 40, e00377-20. [Google Scholar] [CrossRef]
- Oshikiri, H.; Taguchi, K.; Hirose, W.; Taniyama, Y.; Kamei, T.; Siegel, D.; Ross, D.; Kitson, R.R.A.; Baird, L.; Yamamoto, M. Anticancer Effect of C19-Position Substituted Geldanamycin Derivatives Targeting NRF2-NQO1-activated Esophageal Squamous Cell Carcinoma. Mol. Cell. Biol. 2024, 45, 79–97. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. NRF2-Dependent Bioactivation of Mitomycin C as a Novel Strategy To Target KEAP1-NRF2 Pathway Activation in Human Cancer. Mol. Cell. Biol. 2021, 41, e00473-20. [Google Scholar] [CrossRef]
- Huang, X.; Dong, Y.; Bey, E.A.; Kilgore, J.A.; Bair, J.S.; Li, L.S.; Patel, M.; Parkinson, E.I.; Wang, Y.; Williams, N.S.; et al. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res. 2012, 72, 3038–3047. [Google Scholar] [CrossRef]
- Baird, L.; Kensler, T.W.; Yamamoto, M. Novel NRF2-activated cancer treatments utilizing synthetic lethality. IUBMB Life 2022, 74, 1209–1231. [Google Scholar] [CrossRef]
- Weiss-Sadan, T.; Ge, M.; Hayashi, M.; Gohar, M.; Yao, C.H.; de Groot, A.; Harry, S.; Carlin, A.; Fischer, H.; Shi, L.; et al. NRF2 activation induces NADH-reductive stress, providing a metabolic vulnerability in lung cancer. Cell Metab. 2023, 35, 722. [Google Scholar] [CrossRef]
- Ge, M.; Papagiannakopoulos, T.; Bar-Peled, L. Reductive stress in cancer: Coming out of the shadows. Trends Cancer 2024, 10, 103–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Lu, W.; Li, J.; Yu, S.; Brown, E.J.; Stanger, B.Z.; Rabinowitz, J.D.; Yang, X. G6PD-mediated increase in de novo NADP(+) biosynthesis promotes antioxidant defense and tumor metastasis. Sci. Adv. 2022, 8, eabo0404. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Sayin, V.I.; Davidson, S.M.; Bauer, M.R.; Singh, S.X.; LeBoeuf, S.E.; Karakousi, T.R.; Ellis, D.C.; Bhutkar, A.; Sanchez-Rivera, F.J.; et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017, 23, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; LeBoeuf, S.E.; Hao, Y.; New, C.; Blum, J.L.E.; Rashidfarrokhi, A.; Huang, S.M.; Bahamon, C.; Wu, W.L.; Karadal-Ferrena, B.; et al. Glutamine antagonist DRP-104 suppresses tumor growth and enhances response to checkpoint blockade in KEAP1 mutant lung cancer. Sci. Adv. 2024, 10, eadm9859. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Wang, S.; Moghaddam, S.J.; Ooi, A.; Chapman, E.; Wong, P.K.; Zhang, D.D. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 2014, 74, 7430–7441. [Google Scholar] [CrossRef]
- Lien, E.C.; Lyssiotis, C.A.; Juvekar, A.; Hu, H.; Asara, J.M.; Cantley, L.C.; Toker, A. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat. Cell Biol. 2016, 18, 572–578. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Jiang, Y.; Li, C.Q.; Zhang, Y.; Dakle, P.; Kaur, H.; Deng, J.W.; Lin, R.Y.; Han, L.; Xie, J.J.; et al. TP63, SOX2, and KLF5 Establish a Core Regulatory Circuitry That Controls Epigenetic and Transcription Patterns in Esophageal Squamous Cell Carcinoma Cell Lines. Gastroenterology 2020, 159, 1311–1327.e19. [Google Scholar] [CrossRef]
- Gao, W.; Li, Y.; Lin, X.; Deng, K.; Long, X.; Li, D.; Huang, M.; Wang, X.; Xu, Y.; She, X.; et al. Procyanidin B1 Promotes PSMC3-NRF2 Ubiquitination to Induce Ferroptosis in Glioblastoma. Phytother. Res. 2024, 38, 5583–5597. [Google Scholar] [CrossRef]
- Bollong, M.J.; Yun, H.; Sherwood, L.; Woods, A.K.; Lairson, L.L.; Schultz, P.G. A Small Molecule Inhibits Deregulated NRF2 Transcriptional Activity in Cancer. ACS Chem. Biol. 2015, 10, 2193–2198. [Google Scholar] [CrossRef]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S.; et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Farley, K.; Bhattacharya, S.; Cleland, J.; Chandran, P.; Wu, J. The targeted protein degradation landscape. Nat. Rev. Drug Discov. 2025, 24, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Dewey, J.A.; Delalande, C.; Azizi, S.A.; Lu, V.; Antonopoulos, D.; Babnigg, G. Molecular Glue Discovery: Current and Future Approaches. J. Med. Chem. 2023, 66, 9278–9296. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Tenchov, R.; Wang, D.; Johnson, L.S.; Wang, X.; Zhou, Q.A. Molecular Glues: The Adhesive Connecting Targeted Protein Degradation to the Clinic. Biochemistry 2023, 62, 601–623. [Google Scholar] [CrossRef]
- Oleinikovas, V.; Gainza, P.; Ryckmans, T.; Fasching, B.; Thoma, N.H. From Thalidomide to Rational Molecular Glue Design for Targeted Protein Degradation. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 291–312. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Polykovskiy, D.; Bezrukov, D.; Zagribelnyy, B.; Aladinskiy, V.; Kamya, P.; Aliper, A.; Ren, F.; Zhavoronkov, A. Chemistry42: An AI-Driven Platform for Molecular Design and Optimization. J. Chem. Inf. Model. 2023, 63, 695–701. [Google Scholar] [CrossRef]
- Ren, F.; Aliper, A.; Chen, J.; Zhao, H.; Rao, S.; Kuppe, C.; Ozerov, I.V.; Zhang, M.; Witte, K.; Kruse, C.; et al. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nat. Biotechnol. 2025, 43, 63–75. [Google Scholar] [CrossRef]
- Xiong, Z.; Ladd, Z.; Bui-Linh, C.; Spitz, F.; Wang, H.; Chen, X. AI-assisted Design of Novel NRF2Mut Inhibitors. In Proceedings of the AACR Special Conference in Cancer Research: Artificial Intelligence and Machine Learning, Montreal, QC, Canada, 10–12 July 2025. [Google Scholar]
- Wamsley, N.T.; Wilkerson, E.M.; Guan, L.; LaPak, K.M.; Schrank, T.P.; Holmes, B.J.; Sprung, R.W.; Gilmore, P.E.; Gerndt, S.P.; Jackson, R.S.; et al. Targeted Proteomic Quantitation of NRF2 Signaling and Predictive Biomarkers in HNSCC. Mol. Cell. Proteom. 2023, 22, 100647. [Google Scholar] [CrossRef]
- Levings, D.C.; Wang, X.; Kohlhase, D.; Bell, D.A.; Slattery, M. A distinct class of antioxidant response elements is consistently activated in tumors with NRF2 mutations. Redox Biol. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Schwenck, J.; Sonanini, D.; Cotton, J.M.; Rammensee, H.G.; la Fougere, C.; Zender, L.; Pichler, B.J. Advances in PET imaging of cancer. Nat. Rev. Cancer 2023, 23, 474–490. [Google Scholar] [CrossRef]
- Greenwood, H.E.; McCormick, P.N.; Gendron, T.; Glaser, M.; Pereira, R.; Maddocks, O.D.K.; Sander, K.; Zhang, T.; Koglin, N.; Lythgoe, M.F.; et al. Measurement of Tumor Antioxidant Capacity and Prediction of Chemotherapy Resistance in Preclinical Models of Ovarian Cancer by Positron Emission Tomography. Clin. Cancer Res. 2019, 25, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- McCormick, P.N.; Greenwood, H.E.; Glaser, M.; Maddocks, O.D.K.; Gendron, T.; Sander, K.; Gowrishankar, G.; Hoehne, A.; Zhang, T.; Shuhendler, A.J.; et al. Assessment of Tumor Redox Status through (S)-4-(3-[(18)F]fluoropropyl)-L-Glutamic Acid PET Imaging of System x(c) (-) Activity. Cancer Res. 2019, 79, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Timmermand, O.V.; Witney, T.H. Imaging the Tumor Antioxidant Response with [(18)F]FSPG PET. Methods Mol. Biol. 2024, 2729, 233–249. [Google Scholar] [CrossRef]
- Greenwood, H.E.; Edwards, R.; Koglin, N.; Berndt, M.; Baark, F.; Kim, J.; Firth, G.; Khalil, E.; Mueller, A.; Witney, T.H. Radiotracer stereochemistry affects substrate affinity and kinetics for improved imaging of system x(C)(-) in tumors. Theranostics 2022, 12, 1921–1936. [Google Scholar] [CrossRef]
- Habib, E.; Linher-Melville, K.; Lin, H.X.; Singh, G. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef]
- Greenwood, H.E.; Barber, A.R.; Edwards, R.S.; Tyrrell, W.E.; George, M.E.; Dos Santos, S.N.; Baark, F.; Tanc, M.; Khalil, E.; Falzone, A.; et al. Imaging NRF2 activation in non-small cell lung cancer with positron emission tomography. Nat. Commun. 2024, 15, 10484. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cui, J.; Wang, W.; Hu, Q.; Xue, Y.; Liu, X.; Gong, T.; Lu, Y.; Ma, H.; Yang, X.; et al. PPIA dictates NRF2 stability to promote lung cancer progression. Nat. Commun. 2024, 15, 4703. [Google Scholar] [CrossRef]
- Lister, A.; Bourgeois, S.; Imenez Silva, P.H.; Rubio-Aliaga, I.; Marbet, P.; Walsh, J.; Shelton, L.M.; Keller, B.; Verrey, F.; Devuyst, O.; et al. NRF2 regulates the glutamine transporter Slc38a3 (SNAT3) in kidney in response to metabolic acidosis. Sci. Rep. 2018, 8, 5629. [Google Scholar] [CrossRef]
- Viswanath, V.; Zhou, R.; Lee, H.; Li, S.; Cragin, A.; Doot, R.K.; Mankoff, D.A.; Pantel, A.R. Kinetic Modeling of (18)F-(2S,4R)4-Fluoroglutamine in Mouse Models of Breast Cancer to Estimate Glutamine Pool Size as an Indicator of Tumor Glutamine Metabolism. J. Nucl. Med. 2021, 62, 1154–1162. [Google Scholar] [CrossRef]
- Dunphy, M.P.S.; Harding, J.J.; Venneti, S.; Zhang, H.; Burnazi, E.M.; Bromberg, J.; Omuro, A.M.; Hsieh, J.J.; Mellinghoff, I.K.; Staton, K.; et al. In Vivo PET Assay of Tumor Glutamine Flux and Metabolism: In-Human Trial of (18)F-(2S,4R)-4-Fluoroglutamine. Radiology 2018, 287, 667–675. [Google Scholar] [CrossRef]
- Zhou, R.; Pantel, A.R.; Li, S.; Lieberman, B.P.; Ploessl, K.; Choi, H.; Blankemeyer, E.; Lee, H.; Kung, H.F.; Mach, R.H.; et al. [(18)F](2S,4R)4-Fluoroglutamine PET Detects Glutamine Pool Size Changes in Triple-Negative Breast Cancer in Response to Glutaminase Inhibition. Cancer Res. 2017, 77, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, C.; Liu, G.; Bi, C.; Wang, X.; Zhou, Q.; Jin, H. SLC7A11/xCT in cancer: Biological functions and therapeutic implications. Am. J. Cancer Res. 2020, 10, 3106–3126. [Google Scholar] [PubMed]
- Bae, S.W.; Wang, J.; Georgiou, D.K.; Wen, X.; Cohen, A.S.; Geng, L.; Tantawy, M.N.; Manning, H.C. Feasibility of [(18)F]FSPG PET for Early Response Assessment to Combined Blockade of EGFR and Glutamine Metabolism in Wild-Type KRAS Colorectal Cancer. Tomography 2023, 9, 497–508. [Google Scholar] [CrossRef]
- Tétreault, M.-P. Esophageal cancer: Insights from mouse models. Cancer Growth Metastasis 2015, 8, 37–46. [Google Scholar] [CrossRef]
- Mahmoudian, R.A.; Farshchian, M.; Golyan, F.F.; Mahmoudian, P.; Alasti, A.; Moghimi, V.; Maftooh, M.; Khazaei, M.; Hassanian, S.M.; Ferns, G.A.; et al. Preclinical tumor mouse models for studying esophageal cancer. Crit. Rev. Oncol. Hematol. 2023, 189, 104068. [Google Scholar] [CrossRef]
- Opitz, O.G.; Harada, H.; Suliman, Y.; Rhoades, B.; Sharpless, N.E.; Kent, R.; Kopelovich, L.; Nakagawa, H.; Rustgi, A.K. A mouse model of human oral-esophageal cancer. J. Clin. Investig. 2002, 110, 761–769. [Google Scholar] [CrossRef]
- Bibby, M. Orthotopic models of cancer for preclinical drug evaluation: Advantages and disadvantages. Eur. J. Cancer 2004, 40, 852–857. [Google Scholar] [CrossRef]
- Hoffman, R.M. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: A bridge to the clinic. Investig. New Drugs 1999, 17, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.C.; Ko, J.M.; Yu, V.Z.; Chan, K.W.; Lam, A.K.; Law, S.; Tong, D.K.; Lung, M.L. A versatile orthotopic nude mouse model for study of esophageal squamous cell carcinoma. BioMed Res. Int. 2015, 2015, 910715. [Google Scholar] [CrossRef][Green Version]
- Kuroda, S.; Kubota, T.; Aoyama, K.; Kikuchi, S.; Tazawa, H.; Nishizaki, M.; Kagawa, S.; Fujiwara, T. Establishment of a Non-Invasive Semi-Quantitative Bioluminescent Imaging Method for Monitoring of an Orthotopic Esophageal Cancer Mouse Model. PLoS ONE 2014, 9, e114562. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D.; Li, X.; Lv, J.; Xie, L.; Zheng, L.; Gavine, P.R.; Hu, Q.; Shi, Y.; Tan, L. Establishment and characterization of esophageal squamous cell carcinoma patient-derived xenograft mouse models for preclinical drug discovery. Lab. Investig. 2014, 94, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Guan, L.; Nambiar, D.K.; Cao, H.; Viswanathan, V.; Kwok, S.; Hui, A.B.; Hou, Y.; Hildebrand, R.; von Eyben, R.; Holmes, B.J.; et al. NFE2L2 Mutations Enhance Radioresistance in Head and Neck Cancer by Modulating Intratumoral Myeloid Cells. Cancer Res. 2023, 83, 861–874. [Google Scholar] [CrossRef]
- Huang, T.; Yang, J.; Liu, B.; Fu, L. A new mouse esophageal cancer cell line (mEC25)-derived pre-clinical syngeneic tumor model for immunotherapy. Cancer Commun. 2020, 40, 316–320. [Google Scholar] [CrossRef]
- Kono, M.; Saito, S.; Egloff, A.M.; Allen, C.T.; Uppaluri, R. The mouse oral carcinoma (MOC) model: A 10-year retrospective on model development and head and neck cancer investigations. Oral Oncol. 2022, 132, 106012. [Google Scholar] [CrossRef] [PubMed]
- Predina, J.D.; Judy, B.; Aliperti, L.A.; Fridlender, Z.G.; Blouin, A.; Kapoor, V.; Laguna, B.; Nakagawa, H.; Rustgi, A.K.; Aguilar, L.; et al. Neoadjuvant in situ gene-mediated cytotoxic immunotherapy improves postoperative outcomes in novel syngeneic esophageal carcinoma models. Cancer Gene Ther. 2011, 18, 871–883. [Google Scholar] [CrossRef]
- Thomas, G.R.; Chen, Z.; Oechsli, M.N.; Hendler, F.J.; Van Waes, C. Decreased expression of CD80 is a marker for increased tumorigenicity in a new murine model of oral squamous-cell carcinoma. Int. J. Cancer 1999, 82, 377–384. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Oren, Y.; Tsabar, M.; Cuoco, M.S.; Amir-Zilberstein, L.; Cabanos, H.F.; Hutter, J.C.; Hu, B.; Thakore, P.I.; Tabaka, M.; Fulco, C.P.; et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature 2021, 596, 576–582. [Google Scholar] [CrossRef]
- Muller, N.; Lorenz, C.; Ostendorp, J.; Heisel, F.S.; Friese, U.P.; Cartolano, M.; Plenker, D.; Tumbrink, H.; Heimsoeth, A.; Baedeker, P.; et al. Characterizing Evolutionary Dynamics Reveals Strategies to Exhaust the Spectrum of Subclonal Resistance in EGFR-Mutant Lung Cancer. Cancer Res. 2023, 83, 2471–2479. [Google Scholar] [CrossRef]
- Nandi, I.; Ji, L.; Smith, H.W.; Avizonis, D.; Papavasiliou, V.; Lavoie, C.; Pacis, A.; Attalla, S.; Sanguin-Gendreau, V.; Muller, W.J. Targeting fatty acid oxidation enhances response to HER2-targeted therapy. Nat. Commun. 2024, 15, 6587. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Chen, M.; Mariella, E.; Peng, H.; Rehman, S.K.; Sancho, E.; Sogari, A.; Toh, T.S.; Balaban, N.Q.; Batlle, E.; et al. Cancer drug-tolerant persister cells: From biological questions to clinical opportunities. Nat. Rev. Cancer 2024, 24, 694–717. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, Y.; Huang, C.; Wei, X. Redox signaling in drug-tolerant persister cells as an emerging therapeutic target. EBioMedicine 2023, 89, 104483. [Google Scholar] [CrossRef]
- Franca, G.S.; Baron, M.; King, B.R.; Bossowski, J.P.; Bjornberg, A.; Pour, M.; Rao, A.; Patel, A.S.; Misirlioglu, S.; Barkley, D.; et al. Cellular adaptation to cancer therapy along a resistance continuum. Nature 2024, 631, 876–883. [Google Scholar] [CrossRef]
- Kerins, M.J.; Ooi, A. A catalogue of somatic NRF2 gain-of-function mutations in cancer. Sci. Rep. 2018, 8, 12846. [Google Scholar] [CrossRef]
- Cantor, J.R. The Rise of Physiologic Media. Trends Cell Biol. 2019, 29, 854–861. [Google Scholar] [CrossRef]
- Rubin, H.; Nomura, T. Use of lymph in cell culture to model hormonal and nutritional constraints on tumor growth in vivo. Cancer Res. 1987, 47, 4924–4931. [Google Scholar] [PubMed]
- Doh, S.J.; Yamakawa, M.; Santosa, S.M.; Montana, M.; Guo, K.; Sauer, J.R.; Curran, N.; Han, K.Y.; Yu, C.; Ema, M.; et al. Fluorescent reporter transgenic mice for in vivo live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 2018, 21, 677–698. [Google Scholar] [CrossRef]
- Quinn, J.J.; Jones, M.G.; Okimoto, R.A.; Nanjo, S.; Chan, M.M.; Yosef, N.; Bivona, T.G.; Weissman, J.S. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts. Science 2021, 371, eabc1944. [Google Scholar] [CrossRef]
- Xu, W.; Harris, N.R.; Caron, K.M. Lymphatic Vasculature: An Emerging Therapeutic Target and Drug Delivery Route. Annu. Rev. Med. 2021, 72, 167–182. [Google Scholar] [CrossRef]
- Kodama, T.; Matsuki, D.; Tada, A.; Takeda, K.; Mori, S. New concept for the prevention and treatment of metastatic lymph nodes using chemotherapy administered via the lymphatic network. Sci. Rep. 2016, 6, 32506. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.O.; Oluwafemi, O.S.; Songca, S.P.; Sukhbaatar, A.; Mori, S.; Okajima, J.; Komiya, A.; Maruyama, S.; Kodama, T. A novel treatment for metastatic lymph nodes using lymphatic delivery and photothermal therapy. Sci. Rep. 2017, 7, 45459. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, N.L.; Kaminskas, L.M.; Porter, C.J. From sewer to saviour—Targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 2015, 14, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Yao, Q.; Ling, R.; Li, K.; Wang, H. Drug concentrations in axillary lymph nodes after lymphatic chemotherapy on patients with breast cancer. Breast Cancer Res. 2004, 6, R474–R477. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef]


| Category | Compound | Mechanisms of Action | Note |
| I. Inhibition of NRF2 transcription or translation | Mitoxantrone [126] | Interference of mRNA translation by intercalating with the GC-rich region of NFE2L2 mRNA | - |
| Pyrimethamine [126,127] | DHFR inhibition suppresses one-carbon metabolism | NCT05678348 (Washington University in St. Louis; recruiting) | |
| Methotrexate [127,128] | DHFR inhibition suppresses one-carbon metabolism | - | |
| MGY825 | KRAS inhibitor | NCT05275868 (Novartis; recruiting) | |
| CR-1-31B and zotatifin [129] | EIF4A1 inhibitor | - | |
| II. Increase in NRF2 proteasomal degradation | Pyrimethamine [126] | Unclear | NCT05678348 (Washington University in St. Louis; recruiting) |
| Triptolide [130,131] | Unclear | - | |
| MSU38225 and its derivatives [132,133] | Unclear | - | |
| VVD-130037 | Unclear | NCT05954312 (Vividion; recruiting) | |
| VVD-065 [134] | Increase KEAP1 activity by stabilizing a KEAP1 conformation that favors CUL3 binding | - | |
| R16 [135] | Binds KEAP1Mut and restores its NRF2-inhibitory function | - | |
| C2 [136] | PROTAC consisting of an NRF2-binding element and a CRBN ligand, which degrades the NRF2-MafG heterodimer | - | |
| NRF2 degrader 1 | PROTAC degrader of NRF2 | WIPO WO2024006742A2 | |
| ARP-4922 [137] | β-TrCP-dependent degrader of NRF2 | ||
| III. Inhibition of NRF2 transcriptional activity | Peptide 18 [138] | A peptide which inhibits NRF2/sMAF binding to ARE | - |
| Peptide 4 [139] | A stapled peptide that binds ARE | - | |
| N1S [140] | A stapled peptide that inhibits NRF2/sMAF heterodimerization | - | |
| ARE-containing decoy nucleotide [141] | Sequestering NRF2 | - | |
| Pizotifen malate [142] | Binding with the Neh1 domain of NRF2 and thus inhibiting the NRF2-ARE binding | - | |
| IV. Synthetic lethality through NRF2 target genes | Deoxynyboquinone [143] | Metabolic activation by NRF2-regulated NQO1 [144] | |
| PR-104A [145], AST-3424 [146] | Metabolic activation by NRF2-regulated AKR1C3 [147] | ||
| V. Inhibition of metabolic pathways or kinases critical for NRF2high cells | G6PDi-1 [148] | Glucose-6-phosphate dehydrogenase inhibitor | - |
| CB-839 [149] | Glutaminase inhibitor | NCT04265534 (Calithera Biosciences; terminated) | |
| DRP-104 (Sirpiglenastat)[150] | Inhibition of glutamine-using enzymes | NCT04471415 (Dracen Pharm; terminated) | |
| CB-228 (Sapanisertib, TAK-228, MLN-0128) [151] | mTORC1/2 inhibitor | NCT05275673 (Calithera Biosciences, terminated) | |
| PIK-75 [152] | PI3K/DNA-PK inhibitor | - | |
| Romidepsin [153] | HDAC inhibitor | - | |
| Others | CET-CH-6 [154] | Unclear | - |
| Periplocin [155] | Unclear | - | |
| NRF2-IN-1 [156,157] | Unclear | - |
| Cell Line | Source |
| AKR [210,220] | ESCC cells derived from EDL2-cyclinD1; p53−/− C57BL/6 mice |
| mEC25 [218] | 4-nitroquinoline-1 oxide-induced ESCC cells derived from C57BL/6 mice |
| B4B8, B7E3, B7E11, B6C3, B6D8 [221] | 4-nitroquinoline-1 oxide-transformed oral SCC cells derived from BALB/C mice |
| MOC1, MOC2, MOC12 [219] | 7, 12-dimethylbenz(a) anthracene-induced oral SCC cells derived from C57BL/6 mice (commercially available from Kerafast, Inc., Newark, CA, USA) |
| NRF2E79Q-MOC1, NRF2E79K-MOC1 [217] | Nrf2 CRISPR knockout plus lentiviral transfection of mutant NRF2 in MOC1 cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ladd, Z.; Xiong, Z.; Bui-Linh, C.; Paiboonrungruang, C.; Subramaniyan, B.; Li, H.; Wang, H.; Balch, C.; Shersher, D.D.; et al. Lymphatic Metastasis of Esophageal Squamous Cell Carcinoma: The Role of NRF2 and Therapeutic Strategies. Cancers 2025, 17, 1853. https://doi.org/10.3390/cancers17111853
Li Y, Ladd Z, Xiong Z, Bui-Linh C, Paiboonrungruang C, Subramaniyan B, Li H, Wang H, Balch C, Shersher DD, et al. Lymphatic Metastasis of Esophageal Squamous Cell Carcinoma: The Role of NRF2 and Therapeutic Strategies. Cancers. 2025; 17(11):1853. https://doi.org/10.3390/cancers17111853
Chicago/Turabian StyleLi, Yahui, Zachary Ladd, Zhaohui Xiong, Candice Bui-Linh, Chorlada Paiboonrungruang, Boopathi Subramaniyan, Huan Li, Haining Wang, Curt Balch, David D. Shersher, and et al. 2025. "Lymphatic Metastasis of Esophageal Squamous Cell Carcinoma: The Role of NRF2 and Therapeutic Strategies" Cancers 17, no. 11: 1853. https://doi.org/10.3390/cancers17111853
APA StyleLi, Y., Ladd, Z., Xiong, Z., Bui-Linh, C., Paiboonrungruang, C., Subramaniyan, B., Li, H., Wang, H., Balch, C., Shersher, D. D., Spitz, F., & Chen, X. (2025). Lymphatic Metastasis of Esophageal Squamous Cell Carcinoma: The Role of NRF2 and Therapeutic Strategies. Cancers, 17(11), 1853. https://doi.org/10.3390/cancers17111853







