Oncologic Outcomes and Safety of Neoadjuvant Treatment with Anthracyclines Versus Anthracycline-Free Regimens in HER2-Positive Early Breast Cancer in a Colombian Cancer Center: An Observational, Analytical, Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Eligibility
2.2. Outcomes
2.3. Statistical Analysis
3. Results
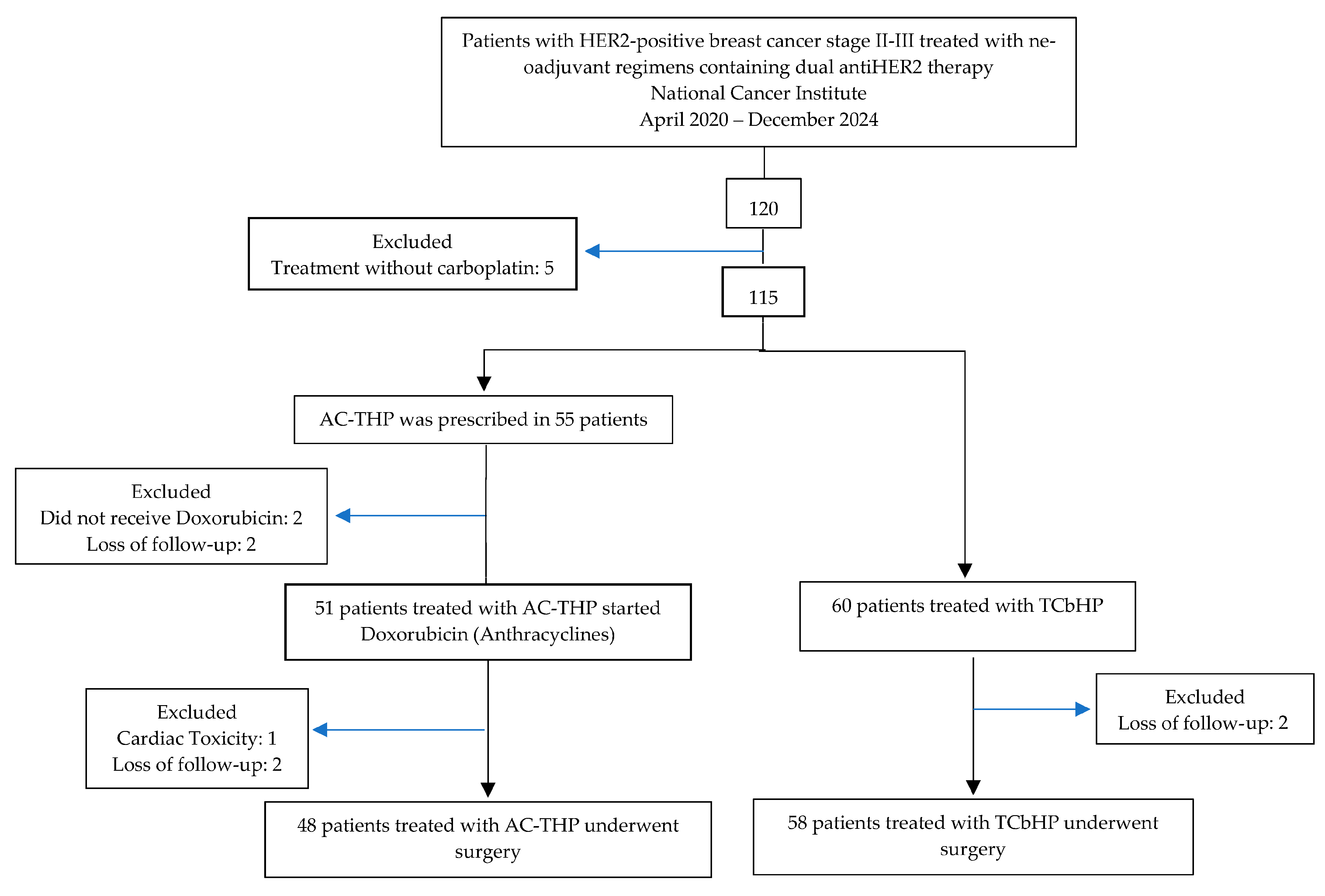
3.1. Patients
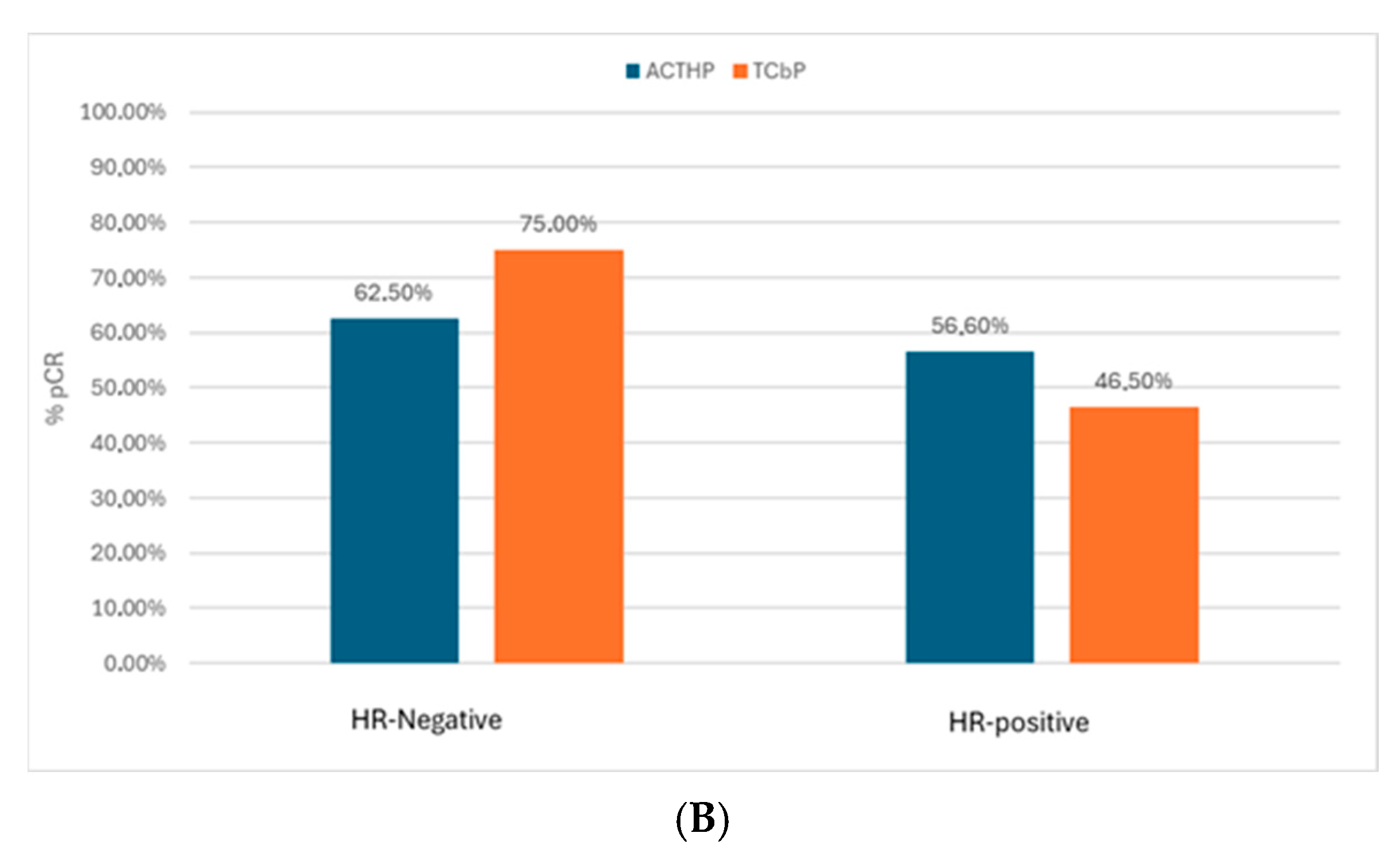
3.2. pCR
3.3. Adjuvant Therapy
3.4. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| AC-THP | Doxorubicin and Cyclophosphamide, followed by Taxane, Trastuzumab and Pertuzumab |
| TCbHP | Carboplatin, weekly Paclitaxel, Trastuzumab and Pertuzumab |
| HR | Hormone receptors |
| ER | Estrogen receptors |
| PR | Progesterone receptors |
| pCR | Pathological complete response |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bogotá, D.C.; Instituto Nacional de Cancerología. Anuario Estadístico 2022. Available online: https://www.cancer.gov.co/conozca-sobre-cancer-1/publicaciones/anuario-estadistico-2022 (accessed on 8 May 2025).
- Díaz-Casas, S.E.; Castilla-Tarra, J.A.; Pena-Torres, E.; Orozco-Ospino, M.; Mendoza-Diaz, S.; Nuñez-Lemus, M.; García-Angulo, O.; García-Mora, M.; Guzmán-AbiSaab, L.; Lehmann-Mosquera, C.; et al. Pathological response to neoadjuvant chemotherapy and the molecular classification of locally advanced breast cancer in a Latin American cohort. Oncologist 2019, 24, e1360–e1370. [Google Scholar] [CrossRef]
- Esteva, F.J.; Katz, E. Tailoring Neoadjuvant Therapy in Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer: Recent Advances and Strategies. JCO Oncol. Pract. 2024, 20, 1046–1054. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Romond, E.H.; Jeong, J.H.; Rastogi, P.; Swain, S.M.; Geyer, C.E.; Ewer, M.S. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2012, 31, 3792–3799. [Google Scholar] [CrossRef]
- Advani, P.P.; Ballman, K.V.; Dockter, T.J.; Colon-Otero, G.; Perez, E.A. Long-term cardiac safety analysis of NCCTG N9831 (Alliance) adjuvant trastuzumab trial. J. Clin. Oncol. 2016, 34, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Gray, R.; Bennett, J.M.; Variakojis, D.; Robert, N.; Wood, W.C. Leukemogenic Potential of Adjuvant Chemotherapy for Early-Stage Breast Cancer: The Eastern Cooperative Oncology Group Experience. J. Clin. Oncol. 1995, 13, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; McAndrew, N.P.; Bardia, A.; Press, M.F.; Pegram, M.; Crown, J.P. A careful reassessment of anthracycline use in curable breast cancer. NPJ Breast Cancer. 2021, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Villacampa, G.; Matikas, A.; Oliveira, M.; Prat, A.; Pascual, T.; Papakonstantinou, A. Landscape of neoadjuvant therapy in HER2-positive breast cancer: A systematic review and network meta-analysis. Eur. J. Cancer 2023, 190, 112885. [Google Scholar] [CrossRef] [PubMed]
- Cuello, J.; Fidalgo, A.; Lopez, L.; Llinas, N. Quimioterapia neoadyuvante libre de antraciclinas en cáncer de mama Her2 positivo: Estudio cuasiexperimental de eficacia y seguridad cardiovascular por puntajes de propensión. Rev. Col. Hematol. Oncol. 2023, 9, 153–157. [Google Scholar] [CrossRef]
- Zhu, J.; Min, N.; Chen, Y.; Li, X. Neoadjuvant therapy with vs. without anthracyclines for HER2-positive breast cancer: A systematic review and meta-analysis. Ann. Transl. Med. 2023, 11, 200. [Google Scholar] [CrossRef]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.M.; Verrill, M. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Waldron-Lynch, M.; Eng-Wong, J.; Kirk, S.; Cortés, J. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur. J. Cancer 2018, 89, 27–35. [Google Scholar] [CrossRef]
- Lu, H.; Yan, H.; Liao, S.; Deng, J.; Zhang, J.; Yao, F. Efficacy, cardiotoxicity and factors affecting pathologic complete response of neoadjuvant chemotherapy with anthracycline-containing verses anthracycline-free regimens plus dual HER2 blockade for HER2-positive early-stage breast cancer: A retrospective study. Transl. Cancer Res. 2023, 12, 1490–1502. [Google Scholar] [CrossRef]
- Slamon, D.J.; Eiermann, W.; Robert, N.J.; Giermek, J.; Martin, M.; Jasiowka, M.; Mackey, J.R.; Chan, A.; Liu, M.-C.; Pinter, T.; et al. Abstract S5-04: Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res. 2016, 76, S5-04. [Google Scholar] [CrossRef]
- van Der Voort, A.; van Ramshorst, M.S.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Vulink, A.J. Three-Year Follow-up of Neoadjuvant Chemotherapy with or without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients with ERBB2-Positive Breast Cancer: A Secondary Analysis of the TRAIN-2 Randomized, Phase 3 Trial. JAMA Oncol. 2021, 7, 978–984. [Google Scholar] [CrossRef]
- de Pinho, I.S.; Luz, P.; Alves, L.; Lopes-Brás, R.; Patel, V.; Esperança-Martins, M. Anthracyclines versus No Anthracyclines in the Neoadjuvant Strategy for HER2+ Breast Cancer: Real-World Evidence. Clin. Drug Investig. 2023, 43, 691–698. [Google Scholar] [CrossRef]
- Reinert, T.; de Souza, A.B.A.; Sartori, G.P.; Obst, F.M.; Barrios, C.H. Highlights of the 17th St Gallen International Breast Cancer Conference 2021: Customising local and systemic therapies. Ecancermedicalscience 2021, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Kolberg-Liedtke, C.; Lüftner, D.; Brucker, S.Y.; Budach, W.; Denkert, C.; Fasching, P.A. Practice-Changing Perspectives regarding Systemic Therapy in Early Breast Cancer: Opinions of German Experts regarding the 17th St. Gallen International Consensus Conference. Breast Care 2022, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Elghazaly, H.; Azim, H.A.; Rugo, H.S.; Camero, D.; Swain, S.M.; Curigliano, G. Tailoring neoadjuvant systemic therapy in breast cancer: “The advent of a personalized approach”—The Breast-Gynecological and Immuno-Oncology International Cancer Conference (BGICC) consensus and recommendations. Cancer 2024, 130, 3251–3271. [Google Scholar] [CrossRef] [PubMed]
- Quintero Ortiz, M.A.; Ballén, D.F.; Briceño Morales, X.; Bruges Maya, R.; Contreras Mejía, F.; Sánchez Castillo, J.O. Indicaciones para el uso de pertuzumab en cáncer de mama HER2 positivo no metastásico en los escenarios neoadyuvante y adyuvante. Revisión de la evidencia y abordaje terapéutico en el Instituto Nacional de Cancerología—Colombia. Rev. Col. Hematol. Oncol. 2023, 27, 16–25. [Google Scholar] [CrossRef]
- Ditsch, N.; Gnant, M.; Thomssen, C.; Harbeck, N. St. Gallen/Vienna 2025 Summary of Key Messages on Therapy in Early Breast Cancer from the 2025 St. Gallen International Breast Cancer Conference. Breast Care 2025, 20, 1–10. [Google Scholar] [CrossRef]
- NCCN. Breast Cancer Version 4. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 8 May 2025).
- Gao, H.F.; Li, W.; Wu, Z.; Dong, J.; Cao, Y.; Zhao, Y. De-escalated neoadjuvant taxane plus trastuzumab and pertuzumab with or without carboplatin in HER2-positive early breast cancer (neoCARHP): A multicentre, open-label, randomised, phase 3 trial. Ther. Adv. Med. Oncol. 2021, 13, 17588359211009003. [Google Scholar] [CrossRef]
- Tung, N.M.; Zhao, F.; DeMichele, A.; Prat, A.; Winer, E.P.; Wright, J.; Recht, A.; Weiss, A.; Tjoe, J.A.; Feldman, S.M.; et al. Predicting pathologic complete response (pCR) from clinicopathologic variables and HER2DX genomic test in stage II/III HER2+ breast cancer treated with taxane, trastuzumab, and pertuzumab (THP): Secondary results from the EA1181/CompassHER2 pCR trial. J. Clin. Oncol. 2025, 43, 501. [Google Scholar] [CrossRef]


| Characteristics | AC-THP (n = 51), n (%) | TCbHP (n = 60), n (%) |
|---|---|---|
| Median age (years) [range] Age | 49.74 [30–79] | 54.08 [30–87] |
| <50 | 29 (56.86) | 19 (31.67) |
| ≥50 | 22 (43.14) | 41 (68.33) |
| ER status | ||
| Negative | 18 (35.29) | 28 (46.67) |
| Positive | 33 (64.71) | 32 (53.33) |
| PR Status | ||
| Negative | 27 (52.94) | 33 (55) |
| Positive | 24 (47.06) | 27 (45) |
| Primary Tumor | ||
| T1 | 1 (1.9) | 2 (3.3) |
| T2 | 17 (33.3) | 18 (30.0) |
| T3 | 3 (5.8) | 9 (15.0) |
| T4 | 30 (58.8) | 31 (51.6) |
| Lymph Nodes | ||
| N0 | 9 (17.65) | 15 (25) |
| N1 | 17 (33.33) | 23 (38.33) |
| N2 | 16 (31.37) | 16 (26.67) |
| N3 | 9 (17.65) | 6 (10) |
| Stage | ||
| I | 0 (0) | 1 (1.67) |
| II | 14 (27.45) | 20 (33.33) |
| III | 37 (72.55) | 39 (65) |
| AH | ||
| Yes | 9 (17.65) | 14 (23.33) |
| Type2 DM | ||
| Yes | 2 (3.92) | 2 (3.33) |
| AC-THP n = 51(%) | TCbHP n = 60(%) | |
|---|---|---|
| Patients with at least one NYHA class III/IV heart failure | 1 (1.9%) | 0 (0.0%) |
| Patients with at least one LVEF decline | 5 (9.8%) | 2 (3.3%) |
| Patients with at least one confirmed LVEF decline | 0 (0.0%) | 0 (0.0%) |
| Arrhythmia * | 0 (0.0%) | 1 (1.6%) |
| ACS ** | 1 (1.9%) | 0 (0.0%) |
| AC-THP n = 51 (%) | TCbHP n = 60(%) | |||
|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 2 | Grade 3 | |
| Peripheral sensory neuropathy | 5 (9.8) | 0 (0.0) | 14 (23.3) | 1 (1.6) |
| Neutropenia | 2 (3.9) | 2 (3.9) | 1 (1.6) | 0 (0.0) |
| Febrile neutropenia | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) |
| Thrombocytopenia | 0 (0.0) | 0 (0.0) | 3 (5.0) | 0 (0.0) |
| Anemia | 0 (0.0) | 0 (0.0) | 2 (3.3) | 1 (1.6) |
| Diarrhea | 1 (1.9) | 3 (5.8) | 5 (8.3) | 2 (3.3) |
| Emesis | 0 (0.0) | 1 (1.9) | 0 (0.0) | 0 (0.0) |
| Fatigue | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (1.6) |
| AC-THP n = 51(%) | TCbHP n = 60(%) | |
|---|---|---|
| Dose interruption due to an AE | 3 (5.8) | 4 (6.6) |
| Asymptomatic LVEF decline | 2 | 2 |
| Grade 3 neutropenia | 1 | 1 |
| Grade 2 neutropenia | 0 | 2 |
| Grade 2 thrombocytopenia | 0 | 1 |
| Withdrawn due to an AE | 3 (5.8) | 2 (1.6) |
| Grade 3 fatigue | 1 | 1 |
| NYHA class III/IV heart failure | 1 | 0 |
| Febrile neutropenia | 1 | 0 |
| Peripheral sensory neuropathy/asymptomatic LVEF decline | 0 | 1 |
| Dose reduction | 7 (13.6) | 11 (18.3) |
| Deaths | 2 (3.9) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-Ramos, A.; Zuluaga-Liberato, A.; Díaz-Casas, S.E. Oncologic Outcomes and Safety of Neoadjuvant Treatment with Anthracyclines Versus Anthracycline-Free Regimens in HER2-Positive Early Breast Cancer in a Colombian Cancer Center: An Observational, Analytical, Retrospective Study. Cancers 2025, 17, 3190. https://doi.org/10.3390/cancers17193190
Acevedo-Ramos A, Zuluaga-Liberato A, Díaz-Casas SE. Oncologic Outcomes and Safety of Neoadjuvant Treatment with Anthracyclines Versus Anthracycline-Free Regimens in HER2-Positive Early Breast Cancer in a Colombian Cancer Center: An Observational, Analytical, Retrospective Study. Cancers. 2025; 17(19):3190. https://doi.org/10.3390/cancers17193190
Chicago/Turabian StyleAcevedo-Ramos, Alfredo, Andrea Zuluaga-Liberato, and Sandra E. Díaz-Casas. 2025. "Oncologic Outcomes and Safety of Neoadjuvant Treatment with Anthracyclines Versus Anthracycline-Free Regimens in HER2-Positive Early Breast Cancer in a Colombian Cancer Center: An Observational, Analytical, Retrospective Study" Cancers 17, no. 19: 3190. https://doi.org/10.3390/cancers17193190
APA StyleAcevedo-Ramos, A., Zuluaga-Liberato, A., & Díaz-Casas, S. E. (2025). Oncologic Outcomes and Safety of Neoadjuvant Treatment with Anthracyclines Versus Anthracycline-Free Regimens in HER2-Positive Early Breast Cancer in a Colombian Cancer Center: An Observational, Analytical, Retrospective Study. Cancers, 17(19), 3190. https://doi.org/10.3390/cancers17193190







