Newer Insights on the Occurrence of Sarcopenia in Pediatric Patients with Cancer: A Systematic Review of the Past 5 Years of Literature
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Pediatric Cancer
1.2. Correlation of Sarcopenia with Pediatric Cancer
2. Materials and Methods
3. Results
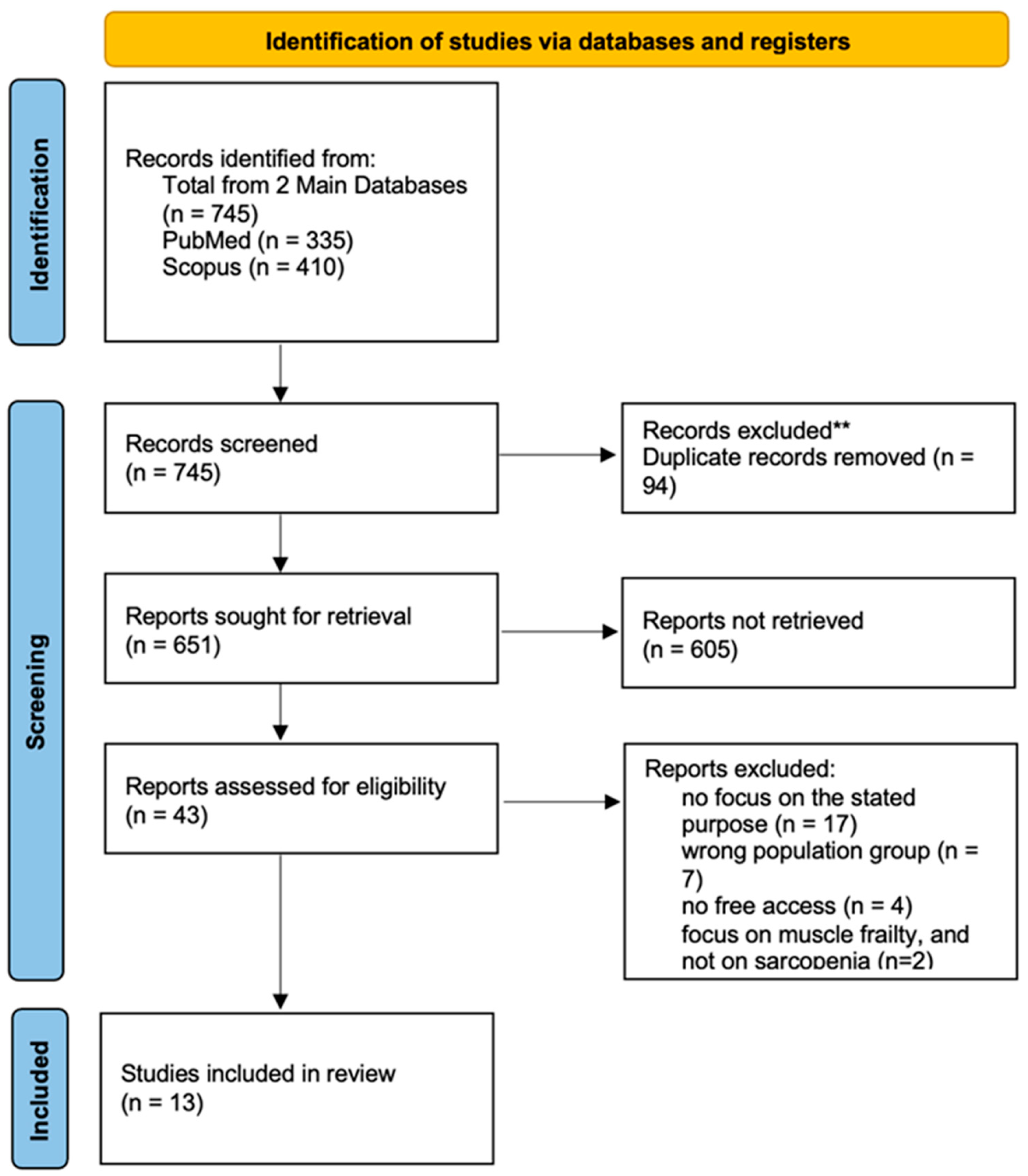
3.1. PRISMA Flow Diagram
3.2. Main Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lupo, P.J.; Spector, L.G. Cancer progress and priorities: Childhood cancer. Cancer Epidemiol. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Pankratz, N.; Marcotte, E.L. Genetic and nongenetic risk factors for childhood cancer. Pediatr. Clin. N. Am. 2015, 62, 11–25. [Google Scholar] [CrossRef]
- van Belzen, I.A.; van Tuil, M.; Badloe, S.; Janse, A.; Verwiel, E.T.; Santoso, M.; de Vos, S.; Baker-Hernandez, J.; Kerstens, H.H.; Solleveld-Westerink, N.; et al. Complex structural variation is prevalent and highly pathogenic in pediatric solid tumors. Cell Genom. 2024, 4, 100675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricci, A.M.; Emeny, R.T.; Bagley, P.J.; Blunt, H.B.; Butow, M.E.; Morgan, A.; Alford-Teaster, J.A.; Titus, L.; Walston, R.R., 3rd; Rees, J.R. Causes of Childhood Cancer: A Review of the Recent Literature: Part I—Childhood Factors. Cancers 2024, 16, 1297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- International Agency for Research on Cancer. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 24 February 2022).
- Lupo, P.J.; Schraw, J.M.; Desrosiers, T.A.; Nembhard, W.N.; Langlois, P.H.; Canfield, M.A.; Copeland, G.; Meyer, R.E.; Brown, A.L.; Chambers, T.M.; et al. Association Between Birth Defects and Cancer Risk Among Children and Adolescents in a Population-Based Assessment of 10 Million Live Births. JAMA Oncol. 2019, 5, 1150–1158. [Google Scholar] [CrossRef]
- Bloom, M.; Maciaszek, J.L.; Clark, M.E.; Pui, C.-H.; Nichols, K.E. Recent advances in genetic predisposition to pediatric acute lymphoblastic leukemia. Expert Rev. Hematol. 2019, 13, 55–70. [Google Scholar] [CrossRef]
- Johnson, K.J.; Lee, J.M.; Ahsan, K.; Padda, H.; Feng, Q.; Partap, S.; Fowler, S.A.; Druley, T.E. Pediatric cancer risk in association with birth defects: A systematic review. PLoS ONE 2017, 12, e0181246. [Google Scholar] [CrossRef]
- Kratz, C.P.; Jongmans, M.C.; Cavé, H.; Wimmer, K.; Behjati, S.; Guerrini-Rousseau, L.; Milde, T.; Pajtler, K.W.; Golmard, L.; Gauthier-Villars, M.; et al. Predisposition to cancer in children and adolescents. Lancet Child Adolesc. Heal. 2021, 5, 142–154. [Google Scholar] [CrossRef]
- Fahmideh, M.A.; Scheurer, M.E. Pediatric Brain Tumors: Descriptive Epidemiology, Risk Factors, and Future Directions. Cancer Epidemiol. Biomark. Prev. 2021, 30, 813–821. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Whiteman, C.A.; Green, A.C. Childhood sun exposure as a risk factor for melanoma: A systematic review of epidemiologic studies. Cancer Causes Control. 2001, 12, 69–82. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Wienke, J.; Dierselhuis, M.P.; Tytgat, G.A.; Künkele, A.; Nierkens, S.; Molenaar, J.J. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur. J. Cancer 2021, 144, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Grabovska, Y.; Mackay, A.; O’Hare, P.; Crosier, S.; Finetti, M.; Schwalbe, E.C.; Pickles, J.C.; Fairchild, A.R.; Avery, A.; Cockle, J.; et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat. Commun. 2020, 11, 4324. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.M.; Reyes-Múgica, M.; Chan, J.K.; Hasle, H.; Lazar, A.J.; Rossi, S.; Ferrari, A.; Jarzembowski, J.A.; Pritchard-Jones, K.; Hill, D.A.; et al. A Summary of the Inaugural WHO Classification of Pediatric Tumors: Transitioning from the Optical into the Molecular Era. Cancer Discov. 2022, 12, 331–355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Qian, D.C.; Kleber, T.; Brammer, B.; Xu, K.M.; Switchenko, J.M.; Janopaul-Naylor, J.R.; Zhong, J.; Yushak, M.L.; Harvey, R.D.; Paulos, C.M.; et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): A propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 2021, 22, 1777–1786. [Google Scholar] [CrossRef]
- Williamson, C.W.; Sherer, M.V.; Zamarin, D.; Sharabi, A.B.; Dyer, B.A.; Mell, L.K.; Mayadev, J.S. Immunotherapy and radiation therapy sequencing: State of the data on timing, efficacy, and safety. Cancer 2021, 127, 1553–1567. [Google Scholar] [CrossRef]
- Helms, L.; Guimera, A.E.; Janeway, K.A.; Bailey, K.M. Innovations in Cancer Treatment of Children. Pediatrics 2023, 152, e2023061539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021, 71 Pt B, 101733. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Goldstein, D.; Tannock, I. Improving access to immunotherapy in low- and middle-income countries. Ann. Oncol. 2022, 33, 360–361. [Google Scholar] [CrossRef]
- Chantada, G.; Lam, C.G.; Howard, S.C. Optimizing outcomes for children with non-Hodgkin lymphoma in low- and middle-income countries by early correct diagnosis, reducing toxic death and preventing abandonment. Br. J. Haematol. 2019, 185, 1125–1135. [Google Scholar] [CrossRef]
- Yalcin-Ozkat, G. Molecular Modeling Strategies of Cancer Multidrug Resistance. Drug Resist. Updat. 2021, 59, 100789. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.L.; Guan, X.M.; Wen, X.H.; Shen, Y.L.; Xiao, J.W.; Guo, Y.X.; Deng, M.Y.; Yu, J. Serious adverse events associated with chemotherapy in children with acute lymphoblastic leukemia. Zhongguo Dang Dai Er Ke Za Zhi 2020, 22, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Wang, Y.; Li, Y.; Wurpel, J.N.D.; Huang, Z.; Chen, Z.-S. The Battlefield of Chemotherapy in Pediatric Cancers. Cancers 2023, 15, 1963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Agarwal, S. Pediatric cancers: Insights and novel therapeutic approaches. Cancers 2023, 15, 3537. [Google Scholar] [CrossRef]
- Shah, N. Dodging the bullet: Therapeutic resistance mechanisms in pediatric cancers. Cancer Drug Resist. 2019, 2, 428–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Ruggiero, A. Malnutrition and sarcopenia in children with cancer. Minerva Pediatr. 2024, 76, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, C.G.; Partin, R.E.; Ness, K.K. Skeletal muscle and childhood cancer: Where are we now and where we go from here. Aging Cancer 2021, 2, 13–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inoue, T.; Wakabayashi, H.; Kawase, F.; Kokura, Y.; Takamasu, T.; Fujiwara, D.; Maeda, K. Diagnostic criteria, prevalence, and clinical outcomes of pediatric sarcopenia: A scoping review. Clin. Nutr. 2024, 43, 1825–1843. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Armenian, S.H.; Bhandari, R.; Lee, K.; Ness, K.; Putt, M.; Lindenfeld, L.; Manoukian, S.; Wade, K.; Dedio, A.; et al. Exercise training and NR supplementation to improve muscle mass and fitness in adolescent and young adult hematopoietic cell transplant survivors: A randomized controlled trial {1}. BMC Cancer 2022, 22, 795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Triarico, S.; Rinninella, E.; Mele, M.C.; Cintoni, M.; Attinà, G.; Ruggiero, A. Prognostic impact of sarcopenia in children with cancer: A focus on the psoas muscle area (PMA) imaging in the clinical practice. Eur. J. Clin. Nutr. 2022, 76, 783–788. [Google Scholar] [CrossRef] [PubMed]
- McBee, M.P.; Woodhouse, C.; Trout, A.T.; Geller, J.I.; Smith, E.A.; Zhang, B.; Towbin, A.J. Skeletal muscle mass as a marker to predict outcomes in children and young adults with cancer. Abdom. Imaging 2022, 47, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Thome, T.; Miguez, K.; Willms, A.J.; Burke, S.K.; Chandran, V.; de Souza, A.R.; Fitzgerald, L.F.; Baglole, C.; Anagnostou, M.; Bourbeau, J.; et al. Skeletal muscle and body composition changes during treatment of childhood acute lymphoblastic leukemia: A prospective study. J. Cachex- Sarcopenia Muscle 2022, 13, 589–604. [Google Scholar] [CrossRef]
- Guolla, L.; Barr, R.; Jaworski, M.; Farncombe, T.; Gordon, C. Sarcopenia in long-term survivors of cancer in childhood and adolescence: A cross-sectional study of calf muscle mass by peripheral quantitative computed tomography with an examination of the muscle–bone unit. Pediatr. Blood Cancer 2023, 71, e30705. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Noguchi, M.; Fukano, R.; Ueda, T.; Taguchi, S.; Yoshimaru, K.; Namie, M.; Shimokawa, M.; Okamura, J. Sarcopenia and obesity in long-term survivors of childhood leukemia/lymphoma: A report from a single institution. Ultrasound Med. Biol. 2021, 51, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, S.; Sandeep, J.; Gauri, K.; Gayatri, V. Dual-Energy X-Ray Absorptiometry and Anthropometry for Assessment of Nutritional Status at Diagnosis in Children with Cancer: A Single-Center Experience from India. South Asian J. Cancer 2022, 11, 164–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bang, M.-J. Definition, assessments, and current research on sarcopenia in children: A narrative review. Ann. Clin. Nutr. Metab. 2024, 16, 49–56. [Google Scholar] [CrossRef]
- Malhotra, P.; Kapoor, G.; Jain, S.; Jain, S.; Sharma, A. Obesity and Sarcopenia in Survivors of Childhood Acute Lymphoblastic Leukemia. Indian Pediatr. 2021, 58, 436–440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zembura, M.; Matusik, P. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022, 13, 914740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Das, G.; Setlur, K.; Jana, M.; Ramakrishnan, L.; Jain, V.; Meena, J.P.; Gupta, A.K.; Dwivedi, S.; Seth, R. Sarcopenic obesity in survivors of childhood acute lymphoblastic leukemia: Prevalence, risk factors, and implications for cancer survivors. Support. Care Cancer 2024, 32, 826. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, C.G.; Garofolo, A.; Leite, H.P. Sarcopenia in children and adolescents submitted to hematopoietic stem cell transplantation. Hematol. Transfus. Cell Ther. 2024, 46 (Suppl 6), S86–S92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Metzger, G.A.; Carsel, A.; Sebastião, Y.V.; Deans, K.J.; Minneci, P.C. Does sarcopenia affect outcomes in pediatric surgical patients? A scoping review. J. Pediatr. Surg. 2021, 56, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, R.K.; Mahmoud, W.S.; Abdrabo, M.S.; Elfakharany, M.S. Effect of adaptive variable-resistance training on chemotherapy-induced sarcopenia, fatigue, and functional restriction in pediatric survivors of acute lymphoblastic leukemia: A prospective randomized controlled trial. Support. Care Cancer 2025, 33, 214. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Samuel, S.R.; Kumar, V.K.; Prasad, H.; Saraswathy, M.V. Evaluation of Chemotherapy Induced Peripheral Neuropathy, Sarcopenia and Fatigue in Children with Acute Lymphoblastic Leukaemia and Lymphoma in Tertiary Care Hospital, Dakshina Kannada. Indian J. Palliat. Care 2023, 29, 426–431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ritz, A.; Kolorz, J.; Hubertus, J.; Ley-Zaporozhan, J.; von Schweinitz, D.; Koletzko, S.; Häberle, B.; Schmid, I.; Kappler, R.; Berger, M.; et al. Sarcopenia is a prognostic outcome marker in children with high-risk hepatoblastoma. Pediatr. Blood Cancer 2021, 68, e28862. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Sollazzo, F.; Corbo, F.; Attinà, G.; Mastrangelo, S.; Cordaro, S.; Modica, G.; Zovatto, I.C.; Monti, R.; Bianco, M.; et al. Bioelectrical Impedance Analysis of Body Composition in Male Childhood Brain Tumor Survivors. Diseases 2024, 12, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kudo, W.; Terui, K.; Furugane, R.; Takenouchi, A.; Komatsu, S.; Kawaguchi, Y.; Nishimura, K.; Katsumi, D.; Hishiki, T. Clinical significance of sarcopenia in children with neuroblastic tumors. Pediatr. Surg. Int. 2024, 40, 237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marmol-Perez, A.; Ubago-Guisado, E.; Gil-Cosano, J.J.; Llorente-Cantarero, F.J.; Pascual-Gázquez, J.F.; Muñoz-Torres, M.; Martinez-Vizcaino, V.; Ness, K.K.; Ruiz, J.R.; Gracia-Marco, L. Co-morbid sarcopenia and low bone mineral density in young paediatric cancer survivors. J. Cachex- Sarcopenia Muscle 2024, 15, 2156–2163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, D.; Kobayashi, R.; Yamamoto, M.; Matsushima, S.; Hori, D.; Yanagi, M.; Kodama, K.; Sano, H.; Akane, Y.; Igarashi, K.; et al. Impact of muscle loss in children with hematologic malignancies undergoing allogeneic hematopoietic cell transplantation. Int. J. Hematol. 2023, 117, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Triarico, S.; Rinninella, E.; Natale, L.; Brizi, M.G.; Cintoni, M.; Raoul, P.; Maurizi, P.; Attinà, G.; Mastrangelo, S.; et al. Clinical Impact of Nutritional Status and Sarcopenia in Pediatric Patients with Bone and Soft Tissue Sarcomas: A Pilot Retrospective Study (SarcoPed). Nutrients 2022, 14, 383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ritz, A.; Froeba-Pohl, A.; Kolorz, J.; Vigodski, V.; Hubertus, J.; Ley-Zaporozhan, J.; von Schweinitz, D.; Häberle, B.; Schmid, I.; Kappler, R.; et al. Total Psoas Muscle Area as a Marker for Sarcopenia Is Related to Outcome in Children with Neuroblastoma. Front. Surg. 2021, 8, 718184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, M.; Zemel, B.S.; Hawkes, C.P.; Long, J.; Kelly, A.; Leonard, M.B.; Jaramillo, D.; Mostoufi-Moab, S. Sarcopenia and preserved bone mineral density in paediatric survivors of high-risk neuroblastoma with growth failure. J. Cachex- Sarcopenia Muscle 2021, 12, 1024–1033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCastlain, K.; Howell, C.R.; Welsh, C.E.; Wang, Z.; Wilson, C.L.; Mulder, H.L.; Easton, J.; Mertens, A.C.; Zhang, J.; Yasui, Y.; et al. The Association of Mitochondrial Copy Number with Sarcopenia in Adult Survivors of Childhood Cancer. JNCI J. Natl. Cancer Inst. 2021, 113, 1570–1580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Atteveld, J.E.; de Winter, D.T.C.; Pluimakers, V.G.; Fiocco, M.; Nievelstein, R.A.J.; Hobbelink, M.G.G.; Kremer, L.C.M.; Grootenhuis, M.A.; Maurice-Stam, H.; Tissing, W.J.E.; et al. Frailty and sarcopenia within the earliest national Dutch childhood cancer survivor cohort (DCCSS-LATER): A cross-sectional study. Am. J. Med. Sci. 2023, 4, e155–e165. [Google Scholar] [CrossRef] [PubMed]
- Buğdaycı, O.; Eker, N. The impact of sarcopenia and sarcopenic obesity on survival in children with Ewing sarcoma and osteosarcoma. Pediatr. Radiol. 2023, 53, 854–861. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omori, A.; Kawakubo, N.; Takemoto, J.; Souzaki, R.; Obata, S.; Nagata, K.; Matsuura, T.; Tajiri, T.; Taguchi, T. Effects of changes in skeletal muscle mass on the prognosis of pediatric malignant solid tumors. Pediatr. Surg. Int. 2022, 38, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Park, J.M.; Jin, W.; Tchahc, H.; Kwon, K.A.; Hahm, K.B. Amelioration of cancer cachexia with preemptive administration of tumor necrosis factor-α blocker. J. Clin. Biochem. Nutr. 2022, 70, 117–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, K.; Fleming, A.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Ladhani, S.; Socha, A.; Shinuda, J.; Jaber, S.; Burrow, S.; et al. Overweight, Obesity and Adiposity in Survivors of Childhood Brain Tumours: A Systematic Review and Meta-Analysis. Clin. Obes. 2017, 8, 55–67. [Google Scholar] [CrossRef]
- Wang, K.-W.; de Souza, R.J.; Fleming, A.; Singh, S.K.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Burrow, S.; Scheinemann, K.; Thabane, L.; et al. Adiposity in Childhood Brain Tumors: A Report from the Canadian Study of Determinants of Endometabolic Health in Children (CanDECIDE Study). Sci. Rep. 2017, 7, srep45078. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Slosman, D.O.; Pichard, C. Fat-Free and Fat Mass Percentiles in 5225 Healthy Subjects Aged 15 to 98 Years. Nutrition 2001, 17, 534–541. [Google Scholar] [CrossRef]
- Talluri, A.; Liedtke, R.; Mohamed, E.I.; Maiolo, C.; Martinoli, R.; De Lorenzo, A. The Application of Body Cell Massindex for Studying Muscle Mass Changes in Health and Disease conditions. Acta Diabetol. 2003, 40, s286–s289. [Google Scholar] [CrossRef]
- Rondanelli, M.; Talluri, J.; Peroni, G.; Donelli, C.; Guerriero, F.; Ferrini, K.; Riggi, E.; Sauta, E.; Perna, S.; Guido, D. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a Useful Prognostic Factor to Describe Nutritional, Inflammation and Muscle Mass Status in Hospitalized Elderly?: Body Cell Mass Index Links in Elderly. Clin. Nutr. 2018, 37, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Yamakawa, K.; Masuda, A.; Toyama, H.; Sofue, K.; Nanno, Y.; Komatsu, S.; Omiya, S.; Sakai, A.; Kobayashi, T.; et al. Association of sarcopenia with a poor prognosis and decreased tumor-infiltrating CD8-positive T cells in pancreatic ductal adenocarcinoma: A retrospective analysis. Ann. Surg. Oncol. 2023, 30, 5776–5787. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Yamashita, Y.; Saito, Y.; Nakagawa, S.; Okabe, H.; Imai, K.; Komohara, Y.; Miyamoto, Y.; Chikamoto, A.; Ishiko, T.; et al. Sarcopenia affects systemic and local immune system and impacts postoperative outcome in patients with extrahepatic cholangiocarcinoma. World J. Surg. 2019, 43, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Liu, C.; Li, X. Stage-dependent changes in albumin, NLR, PLR, and AFR are correlated with shorter survival in patients with gastric cancer. Clin. Lab. 2019, 65, 1623–1633. [Google Scholar] [CrossRef]
- Almasaudi, A.S.; Dolan, R.D.; Edwards, C.A.; McMillan, D.C. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers 2020, 12, 1986. [Google Scholar] [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: A prospective study. Am. J. Med. 2020, 133, 713–722.e7. [Google Scholar] [CrossRef]
- Joyce, E.D.; Nolan, V.G.; Ness, K.K.; Ferry, R.J.; Robison, L.L.; Pui, C.-H.; Hudson, M.M.; Kaste, S.C. Association of muscle strength and bone mineral density in adult survivors of childhood acute lymphoblastic leukemia. Arch. Phys. Med. Rehabil. 2011, 92, 873–879. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Joseph, C.; Kenny, A.M.; Taxel, P.; Lorenzo, J.A.; Duque, G.; Kuchel, G.A. Role of endocrine-immune dysregulation in os-teoporosis, sarcopenia, frailty and fracture risk. Mol. Aspects Med. 2005, 26, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, A.; Rotolo, S.; Cintoni, M.; Rinninella, E.; Pulcini, G.; Schena, C.A.; Ferracci, F.; Grassi, F.; Raoul, P.; Moroni, R.; et al. The prognostic value of skeletal muscle index on clinical and survival outcomes after cytoreduction and HIPEC for peritoneal metastases from colorectal cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2022, 48, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Rayar, M.; Webber, C.E.; Nayiager, T.; Sala, A.; Barr, R.D. Sarcopenia in children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2013, 35, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, N.; Kinoshita, Y.; Souzaki, R.; Koga, Y.; Oba, U.; Ohga, S.; Taguchi, T. The influence of sarcopenia on high-risk neuroblastoma. J. Surg. Res. 2019, 236, 101–105. [Google Scholar] [CrossRef]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: A narrative review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef]
- Valente, V.B.; Verza, F.A.; Lopes, F.Y.K.; Ferreira, J.Z.; dos Santos, P.S.P.; Sundefeld, M.L.M.M.; Biasoli, É.R.; Miyahara, G.I.; Soubhia, A.M.P.; de Andrade, M.; et al. Stress hormones concentrations in the normal microenvironment predict risk for chemically induced cancer in rats. Psychoneuroendocrinology 2018, 89, 229–238. [Google Scholar] [CrossRef]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann. Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Gorham, E.D.; Kim, J.; Hofflich, H.; Cuomo, R.E.; Garland, C.F. Could vitamin D sufficiency improve the survival of colorectal cancer patients? J. Steroid Biochem. Mol. Biol. 2015, 148, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Jürgens, H.; Frühwald, M.C. Important aspects of nutrition in children with cancer. Adv. Nutr. Int. Rev. J. 2011, 2, 67–77. [Google Scholar] [CrossRef]
- Egler, R.A.; Burlingame, S.M.; Nuchtern, J.G.; Russell, H.V. Interleukin-6 and Soluble Interleukin-6 Receptor Levels as Markers of Disease Extent and Prognosis in Neuroblastoma. Clin. Cancer Res. 2008, 14, 7028–7034. [Google Scholar] [CrossRef]
- Armenian, S.H.; Iukuridze, A.; Teh, J.B.; Mascarenhas, K.; Herrera, A.; McCune, J.S.; Zain, J.M.; Mostoufi-Moab, S.; McCormack, S.; Slavin, T.P.; et al. Abnormal body composition is a predictor of adverse outcomes after autologous haematopoietic cell transplantation. J. Cachex-Sarcopenia Muscle 2020, 11, 962–972. [Google Scholar] [CrossRef]
- Wadhwa, A.; Adams, K.M.; Dai, C.; Richman, J.S.; McDonald, A.M.; Williams, G.R.; Bhatia, S. Association between body composition and chemotherapy-related toxicity in children with lymphoma and rhabdomyosarcoma. Cancer 2022, 128, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Serrano, A.J.; Estefanía-Fernández, K.; Oterino, C.; Ramírez-Amoros, C.; Navarro, G.; Sastre, A.; Pérez-Martínez, A.; Barrena, S.; Oliveros, F.H.; Martínez, L. Sarcopenia as a Prognostic Factor in Patients with Hepatoblastoma: Does It Influence Surgical Outcomes and Survival? Preliminary Retrospective Study. J. Pediatr. Surg. 2023, 58, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Schab, M.; Skoczen, S. Nutritional status, body composition and diet quality in children with cancer. Front. Oncol. 2024, 14, 1389657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joffe, L.; Schadler, K.L.; Shen, W.; Ladas, E.J. Body Composition in Pediatric Solid Tumors: State of the Science and Future Directions. JNCI Monogr. 2019, 2019, 144–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, A.; Ferrucci, L.M.; Caan, B.J.; Irwin, M.L. Effect of Exercise on Sarcopenia among Cancer Survivors: A Systematic Review. Cancers 2022, 14, 786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murphy, A.J.; White, M.; Davies, P.S. Body composition of children with cancer. Am. J. Clin. Nutr. 2010, 92, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cortés, L.; Martínez-Vieyra, X.; Mejía-Aranguré, J.M.; López-Alarcón, M.; Martin-Trejo, J.; Delgadillo-Portillo, S.; Guzmán-Castro, B.; Delgadillo-Portillo, J.; Atilano-Miguel, S.; Rodríguez-Cruz, M.; et al. Pilot study on the effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on body composition in children with acute lymphoblastic leukemia: Randomized clinical trial. Clin. Nutr. 2023, 42, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Delorme, J.; Dima, A.; Bélanger, V.; Napartuk, M.; Bouchard, I.; Meloche, C.; Curnier, D.; Sultan, S.; Laverdière, C.; Sinnett, D.; et al. Impact of Early Nutritional Intervention During Cancer Treatment on Dietary Intakes and Cardiometabolic Health in Children and Adolescents. Cancers 2025, 17, 157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pedretti, L.; Massa, S.; Leardini, D.; Muratore, E.; Rahman, S.; Pession, A.; Esposito, S.; Masetti, R. Role of Nutrition in Pediatric Patients with Cancer. Nutrients 2023, 15, 710. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiuza-Luces, C.; Padilla, J.R.; Soares-Miranda, L.; Santana-Sosa, E.; Quiroga, J.V.; Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Lorenzo-González, R.; Verde, Z.; et al. Exercise Intervention in Pediatric Patients with Solid Tumors. Med. Sci. Sports Exerc. 2017, 49, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zheng, J.; Liu, K. Supervised Exercise Interventions in Childhood Cancer Survivors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Children 2022, 9, 824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumann, F.T.; Bloch, W.; Beulertz, J. Clinical exercise interventions in pediatric oncology: A systematic review. Pediatr. Res. 2013, 74, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.T.; Li, W.H.C.; Ho, L.L.K.; Ho, K.Y.; Chan, G.C.F.; Chung, J.O.K. Physical activity for pediatric cancer survivors: A systematic review of randomized controlled trials. J. Cancer Surviv. 2021, 15, 876–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orsso, C.E.; Tibaes, J.R.; Oliveira, C.L.; Rubin, D.A.; Field, C.J.; Heymsfield, S.B.; Prado, C.M.; Haqq, A.M. Low muscle mass and strength in pediatrics patients: Why should we care? Clin. Nutr. 2019, 38, 2002–2015. [Google Scholar] [CrossRef] [PubMed]
- Triarico, S.; Rinninella, E.; Attinà, G.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Nutritional status in the pediatric oncology patients. Front. Biosci. 2022, 14, 4. [Google Scholar] [CrossRef]
- Schmidt-Andersen, P.; Pouplier, A.; Faigenbaum, A.D.; Beth, C.K.; Olsen, C.C.; Lykkedegn, S.; Hasle, H.; Müller, K.; Larsen, H.B.; Fridh, M.; et al. Evaluating Feasibility of an Exercise Intervention Including Physical Assessment During the First 6 Months of Cancer Treatment in Children and Adolescents in a Randomized Controlled Trial. Pediatr. Blood Cancer 2025, 72, e31498. [Google Scholar] [CrossRef] [PubMed]
- Götte, M.; Kesting, S.V.; Gerss, J.; Rosenbaum, D.; Boos, J. Feasibility and effects of a home-based intervention using activity trackers on achievement of individual goals, quality of life and motor performance in patients with paediatric cancer. BMJ Open Sport Exerc. Med. 2018, 4, e000322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- 105. Kallenbach, J.G.; Bachman, J.F.; Paris, N.D.; Blanc, R.S.; O’COnnor, T.; Furati, E.; Hernady, E.; Johnston, C.J.; Williams, J.P.; Chakkalakal, J.V. Muscle-specific functional deficits and lifelong fibrosis in response to paediatric radiotherapy and tumour elimination. J. Cachex-Sarcopenia Muscle 2022, 13, 296–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alalwan, T.A. Phenotypes of Sarcopenic Obesity: Exploring the Effects on Peri-Muscular Fat, the Obesity Paradox, Hormone-Related Responses and the Clinical Implications. Geriatrics 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pranikoff, S.; Miller, V.L.A.; Heiling, H.; Deal, A.M.; Valle, C.G.; Williams, G.R.; Muss, H.B.; Nichols, H.B.; Smitherman, A.B. Frail young adult cancer survivors experience poor health-related quality of life. Cancer 2022, 128, 2375–2383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hager, A.; Guo, Y.; Wang, Y.; Mazurak, V.; Gilmour, S.M.; Mager, D.R. Exercise rehabilitation to treat sarcopenia in pediatric transplant populations. Pediatr. Transplant. 2023, 27, e14602. [Google Scholar] [CrossRef] [PubMed]
| PICO | |
|---|---|
| Population (P) | Children and adolescents diagnosed with cancer (all types), during or after treatment, that appeared sarcopenia |
| Intervention (I) | Factors associated with the development of sarcopenia: chemotherapy, radiotherapy, corticosteroid use, malnutrition, reduced physical activity |
| Comparator (C) | Pediatric cancer patients without sarcopenia, or healthy age-matched controls. |
| Outcome (O) | Positive association between pediatric cancer/oncology and sarcopenia. |
| # | Author | Date of Publication | Type of Study | Type of Cancer | Population | Results |
|---|---|---|---|---|---|---|
| 1 | Ritz A et al. [49] | May 2021 | Research Article | Hepatoblastoma | 67 children from the Dr. von Hauner Children’s hospital with an average age of 2.15 years | Association between hepatoblastoma and sarcopenia, which leads to relapse. The measurement of tPMA was proposed for the assessment of sarcopenia. Sarcopenic children were more numerous than non-sarcopenic ones and were associated with a higher likelihood of relapse. |
| 2 | Romano A et al. [50] | Nov 2024 | Research Article | Brain Tumor | 14 male childhood brain tumor survivors, above 12 years | Association of pediatric brain cancer with sarcopenia and metabolic syndrome (MetS). The use of BIA for assessing body composition and fat content. |
| 3 | Das G et al. [44] | Nov 2024 | Research Article | Acute Lymphoblastic Leukemia (ALL) | 65 survivors of ALL between 7 and 18 years old. | Sarcopenic obesity that develops in survivors is an indicator of metabolic disease, and early exposure to anticancer treatment—which affects muscle health—also contributes to its development. |
| 4 | Kudo W et al. [51] | Aug 2024 | Research Article | Neuroblastic Tumor | 35 patients with an average age of 2.5 years | SMI served as a prognostic indicator and decreased during treatment, similar to HT and BW, which reflect growth. BMI did not follow the same pattern as the other parameters. |
| 5 | Marmol-Perez A et al. [52] | Aug 2024 | Research Article | General Pediatric Cancer | 116 young pediatric cancer survivors with an average age of 12.1 years | Approximately one third of the survivors developed sarcopenia, and these individuals had a higher likelihood of being assessed with low bone mineral density (BMD) values. |
| 6 | Suzuki D et al. [53] | Jan 2023 | Research Article | Hematologic malignancies | 65 patients, with a mean age of 11.3 years for males (40 individuals) and 11.7 years for females (25 individuals). | Patients who experienced loss of muscle mass (sarcopenia) prior to hematopoietic cell transplantation (HCT) were associated with poor overall survival. These patients had previously undergone chemotherapy. |
| 7 | Romano A et al. [54] | Jan 2022 | Pilot retrospective study | Bone and soft tissue sarcomas (Ewing sarcoma, rhabdomyosarcoma, desmoplastic tumor) | 22 pediatric patients age between 1 and 16 years | In many cases, sarcopenia appears at diagnosis. Evidence of a decrease in tPMA (sarcopenia measurement) 12 months after treatment. No association was found with prognosis. |
| 8 | Ritz A et al. [55] | Aug 2021 | Retrospective analysis | Neuroblastoma | 101 children between 1 and 15 years of age, who underwent a workup for NB | The majority of patients showed reduced tPMA before surgery. Sarcopenia is associated with reduced prognosis. |
| 9 | Guo M et al. [56] | Aug 2021 | Prospective Study | High Risk-Neuroblastoma | 20 survivors of HR-NBL 6–16 years and 20 healthy controls | Survivors of high-risk neuroblastoma treated with cis-retinoic acid exhibit sarcopenia and diminished skeletal growth years after treatment. Sarcopenia and sarcopenic obesity are indicators of poor prognosis. |
| 10 | McCastlain K et al. [57] | Apr 2021 | Research Article | General Pediatric Cancer | 1762 Survivors aged 18 years and older at follow-up, and 10 or more years from primary diagnosis | The decrease in mtDNAcn after cancer treatment is indicative of sarcopenia. Risk factors for sarcopenia include female gender, tumor type, age at diagnosis, exposure to cranial radiation and alkylating agents, physical inactivity, Asian ancestry, and the presence of the T allele at the genetic locus rs9991501 (in the HSD17B11 gene). |
| 11 | Van Atteveld JE et al. [58] | Apr 2023 | Research Article | General Pediatric Cancer | 3996 adult survivors aged between 18 and 45 years old | The occurrence of frailty, pre-frailty, and sarcopenia has been observed in survivors, even in the third decade of life following treatment for childhood cancer. |
| 12 | Buğdaycı O et al. [59] | 2023 | Research Article | Ewing Sarcoma and Osteosarcoma | 60 patients aged between 16 months and 18 years. | There is no clear association between overall survival, with or without events, and sarcopenia, although a higher skeletal muscle index (SMI) was associated with increased survival in survivors. |
| 13 | Omori A et al. [60] | Dec 2022 | Retrospective cohort study | Malignant Solid Tumors | progression-free survival group (PFS group) (n = 21), relapse/death group (R/D group) (n = 7). Control 185 | Before the start of treatment, patients with tumors did not show sarcopenia. Increased muscle mass after the end of treatment is an indicator of good prognosis after treatment. |
| References | Selection | Comparability | Outcome | Total Quality Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representatives of Exposed Cohort | Sample size | Assessment of Outcome | Non-Respondents | Adjust for the Most Important Risk Factors | Adjust for Other Risk Factors | Assessment of Outcome | Statistical Test | ||
| Ritz A et al. [49] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Romano A et al. [50] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Das G et al. [44] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Kudo W et al. [51] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Marmol-Perez A et al. [52] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Suzuki D et al. [53] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Romano A et al. [54] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Ritz A et al. [55] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Guo M et al. [56] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| McCastlain K et al. [57] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Van Atteveld JE et al. [58] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Buğdaycı O et al. [59] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Omori A et al. [60] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiosis, G.; Ioannou, D.; Skourtsidis, K.; Fouskas, V.; Stergiou, K.; Kavvadas, D.; Papamitsou, T.; Karachrysafi, S.; Kourti, M. Newer Insights on the Occurrence of Sarcopenia in Pediatric Patients with Cancer: A Systematic Review of the Past 5 Years of Literature. Cancers 2025, 17, 3188. https://doi.org/10.3390/cancers17193188
Kiosis G, Ioannou D, Skourtsidis K, Fouskas V, Stergiou K, Kavvadas D, Papamitsou T, Karachrysafi S, Kourti M. Newer Insights on the Occurrence of Sarcopenia in Pediatric Patients with Cancer: A Systematic Review of the Past 5 Years of Literature. Cancers. 2025; 17(19):3188. https://doi.org/10.3390/cancers17193188
Chicago/Turabian StyleKiosis, Georgios, Despoina Ioannou, Kanellos Skourtsidis, Vasilis Fouskas, Konstantinos Stergiou, Dimitrios Kavvadas, Theodora Papamitsou, Sofia Karachrysafi, and Maria Kourti. 2025. "Newer Insights on the Occurrence of Sarcopenia in Pediatric Patients with Cancer: A Systematic Review of the Past 5 Years of Literature" Cancers 17, no. 19: 3188. https://doi.org/10.3390/cancers17193188
APA StyleKiosis, G., Ioannou, D., Skourtsidis, K., Fouskas, V., Stergiou, K., Kavvadas, D., Papamitsou, T., Karachrysafi, S., & Kourti, M. (2025). Newer Insights on the Occurrence of Sarcopenia in Pediatric Patients with Cancer: A Systematic Review of the Past 5 Years of Literature. Cancers, 17(19), 3188. https://doi.org/10.3390/cancers17193188









