Integrating Molecular Phenotyping into Treatment Algorithms for Advanced Oestrogen Receptor-Positive Breast Cancer
Simple Summary
Abstract
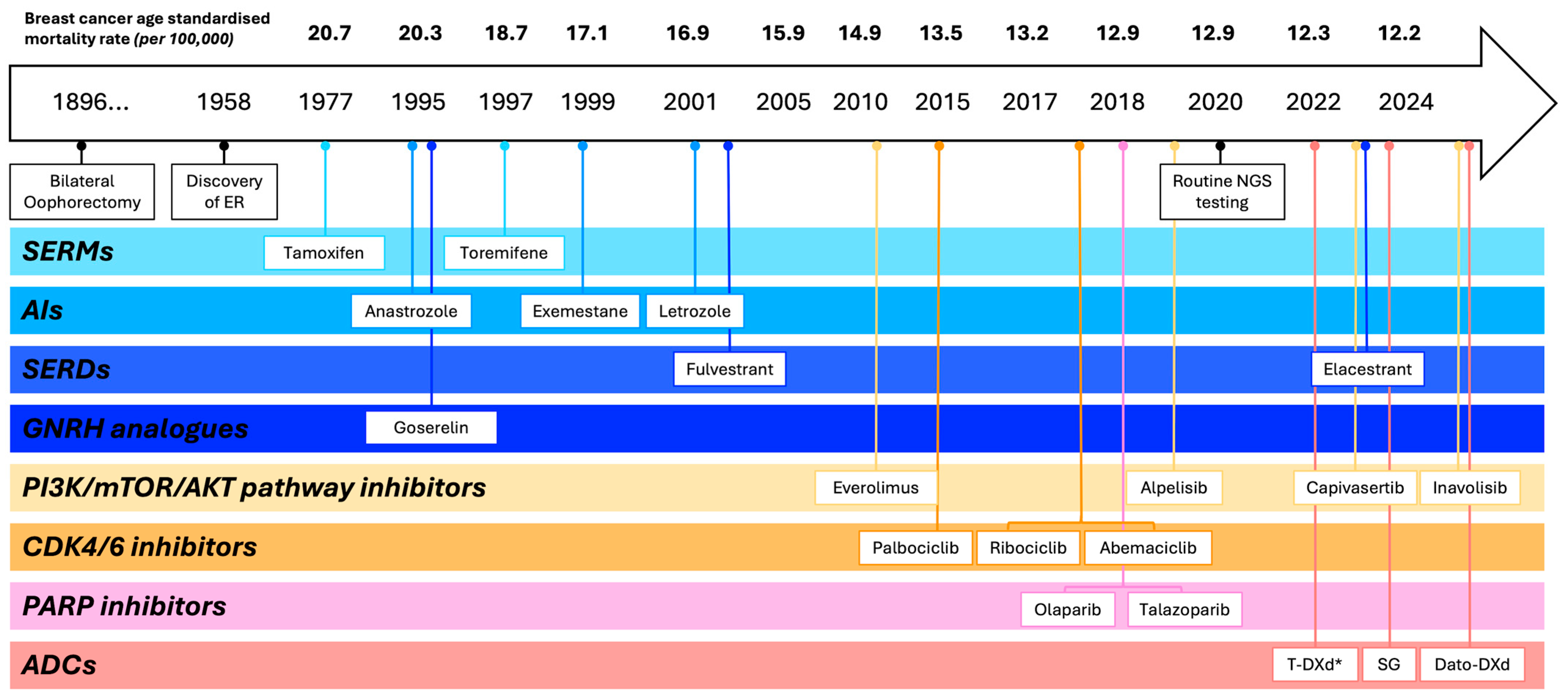
1. Introduction
2. The Molecular Landscape of Advanced ER-Positive Breast Cancer
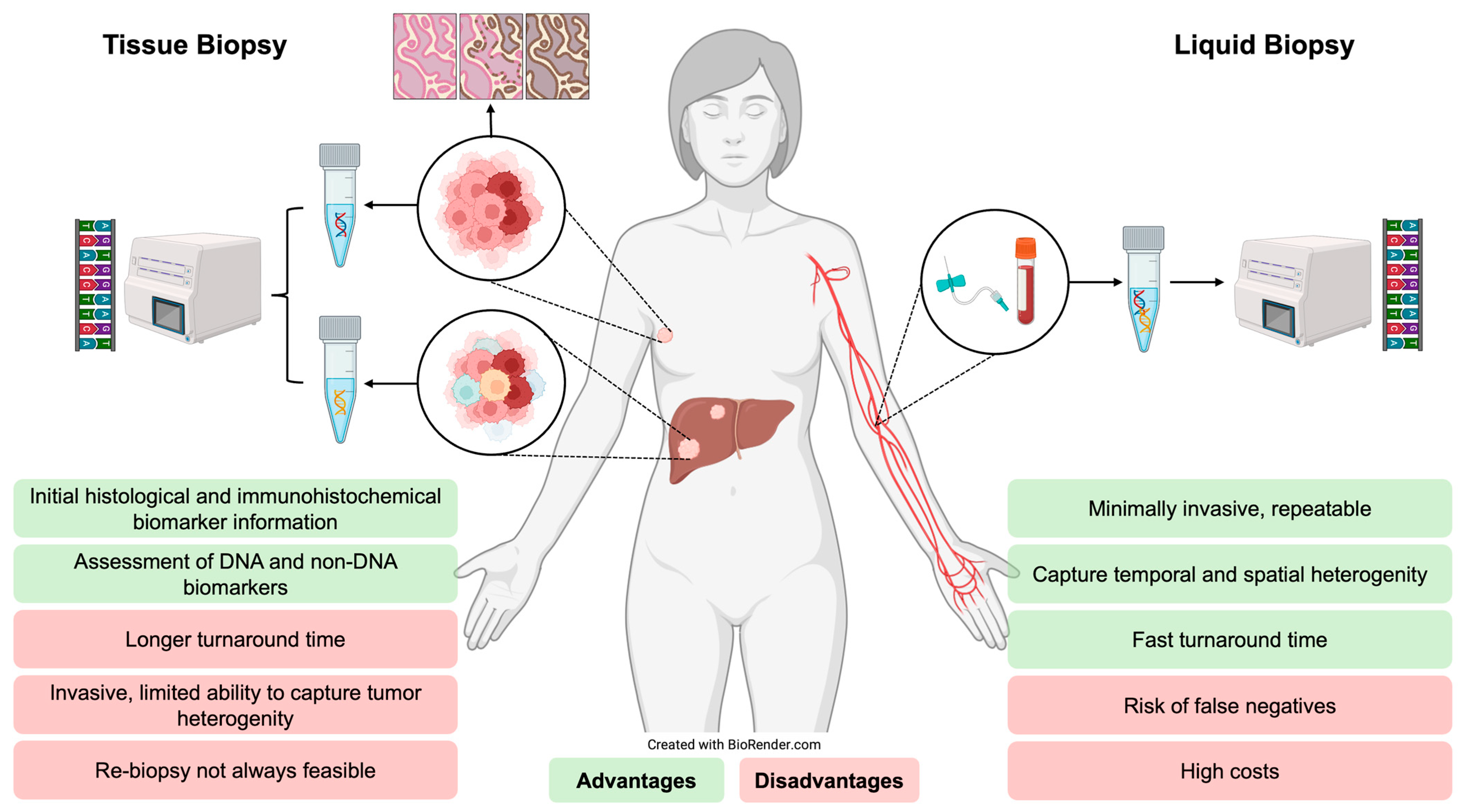
3. Molecular Profiling in Advanced ER-Positive Breast Cancer
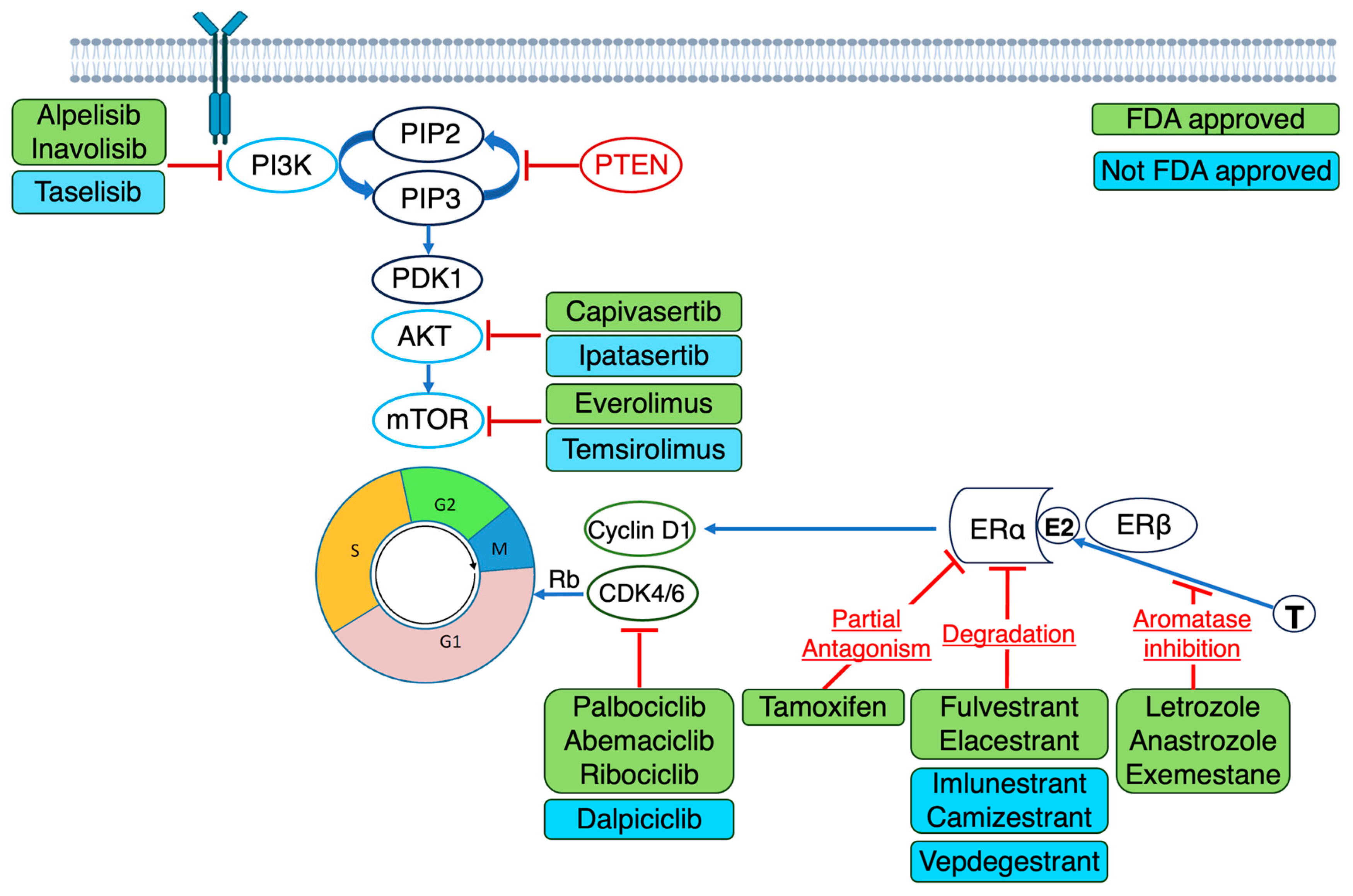
4. Therapies Targeting Genomic Aberrations in Advanced ER-Positive Breast Cancer
4.1. ESR1 Mutations
4.2. Alterations in PIK3CA, AKT and PTEN
4.3. BRCA1/2 and PALB2 Mutations
4.4. HER2 Mutations
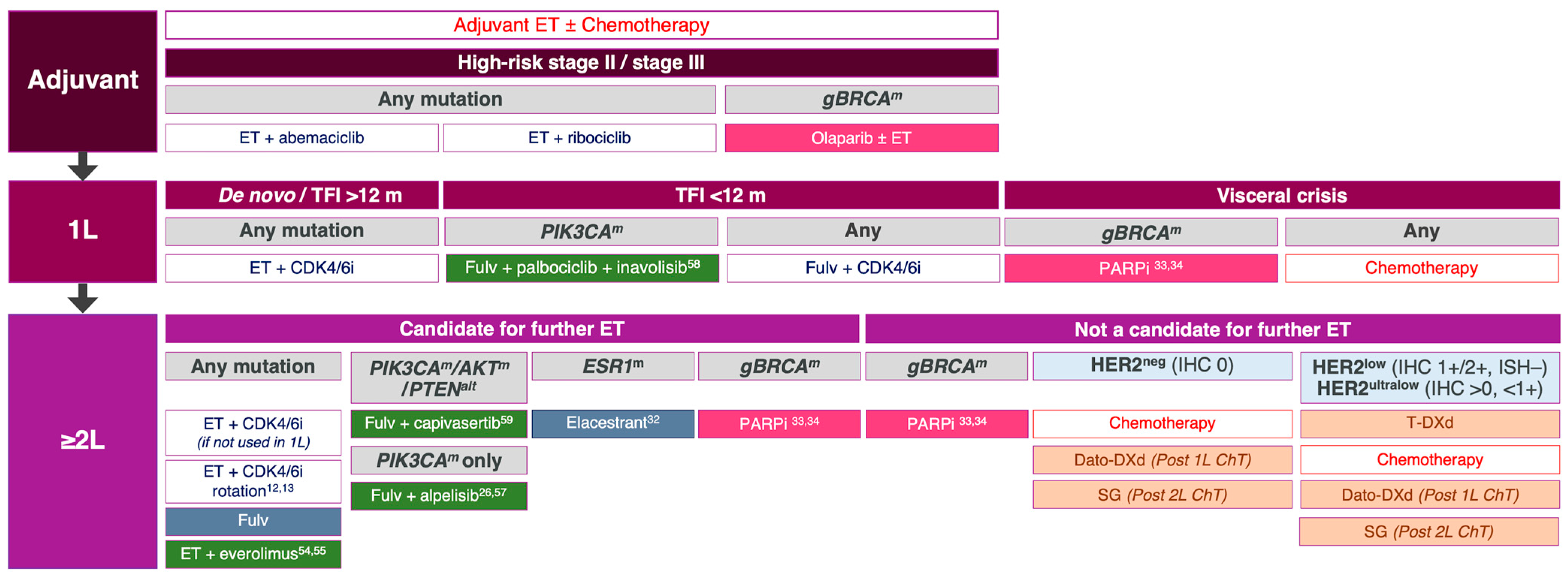
5. Current Limitations and Future Directions of a Molecular Phenotypic Approach to Treating ER-Positive Breast Cancer
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| ADC | Antibody–drug conjugate |
| AIs | Aromatase inhibitors |
| AKT1 | Protein kinase B |
| ASCO | American Society of Clinical Oncology |
| BRCA1/2 | Breast cancer gene 1 and 2 |
| CDK4/6 | Cyclin-dependent kinase 4/6 |
| cfDNA | Cell-free DNA |
| ChT | Chemotherapy |
| ctDNA | Circulating tumour DNA |
| Dato-DXd | Datopotamab deruxtecan |
| ddPCR | digital droplet polymerase chain reaction |
| E2 | Oestradiol |
| ER | Oestrogen receptor |
| ERα | Oestrogen receptor alpha |
| ERβ | Oestrogen receptor beta |
| ESCAT | ESMO Scale for Clinical Actionability of Molecular Targets |
| ESMO | European Society for Medical Oncology |
| ESR1 | Oestrogen receptor 1 |
| ET | Endocrine therapy |
| FDA | Food and Drug Administration |
| Fulv | Fulvestrant |
| GNRH | Gonadotropin releasing hormone |
| HER2 | Human epidermal growth factor 2 |
| HR | Hazard ratio |
| IHC | Immunohistochemistry |
| MBC | Metastatic breast cancer |
| Mo | Months |
| MTOR | Mammalian target of rapamycin |
| NA | Not applicable |
| NGS | Next-generation sequencing |
| NR | Not reported |
| NTRK | Neurotrophic tyrosine receptor kinase |
| OS | Overall survival |
| PALB2 | Partner and localiser of BRCA2 |
| PARP | Poly (ADP-ribose) polymerase |
| PDK1 | 3-phosphoinositide-dependant kinase 1 |
| PIK3CA | phosphatidylinositol-4,5-bisphosphonate 3-kinase catalytic subunit alpha |
| PIP2 | Phosphatidylinositol (4,5)-bisphosphonate |
| PIP3 | Phosphatidylinositol (3,4,5)-Triphosphonate |
| PFS | Progression-free survival |
| PR | Progesterone receptor |
| PTEN | Phosphatase and tensin homolog |
| Rb | Retinoblastoma |
| SERD | Selective oestrogen receptor degrader |
| SERM | Selective oestrogen receptor modulator |
| SG | Sacituzumab Govitecan |
| T | Testosterone |
| T-DXd | Trastuzumab Deruxtecan |
| TFI | Treatment-free interval |
| TKI | Tyrosine kinase inhibitor |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Reductions in recurrence in women with early breast cancer entering clinical trials between 1990 and 2009: A pooled analysis of 155,746 women in 151 trials. Lancet 2024, 404, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Rassy, E.; Mosele, M.; Di Meglio, A.; Pistilli, B.; Andre, F. Precision oncology in patients with breast cancer: Towards a ‘screen and characterize’ approach. ESMO Open 2024, 9, 103716. [Google Scholar] [CrossRef] [PubMed]
- Beatson, G.T. On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases. Trans. Med.-Chir. Soc. Edinb. 1896, 15, 153–179. [Google Scholar]
- Beatson, G.T. The treatment of cancer of the breast by oophorectomy and thyroid extract. Br. Med. J. 1901, 2, 1145–1148. [Google Scholar]
- Shatsky, R.; Parker, B.A.; Bui, N.Q.; Helsten, T.; Schwab, R.B.; Boles, S.G.; Kurzrock, R. Next-Generation Sequencing of Tissue and Circulating Tumor DNA: The UC San Diego Moores Center for Personalized Cancer Therapy Experience with Breast Malignancies. Mol. Cancer Ther. 2019, 18, 1001–1011. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Vahdat, L.T. Evolving Management of Breast Cancer in the Era of Predictive Biomarkers and Precision Medicine. J. Pers. Med. 2024, 14, 719. [Google Scholar] [CrossRef]
- Klocker, E.V.; Hasenleithner, S.; Bartsch, R.; Gampenrieder, S.P.; Egle, D.; Singer, C.F.; Rinnerthaler, G.; Hubalek, M.; Schmitz, K.; Bago-Horvath, Z.; et al. Clinical applications of next-generation sequencing-based ctDNA analyses in breast cancer: Defining treatment targets and dynamic changes during disease progression. Mol. Oncol. 2024, 19, 1897–1917. [Google Scholar] [CrossRef]
- Mazzitelli, C.; Santini, D.; Corradini, A.G.; Zamagni, C.; Trerè, D.; Montanaro, L.; Taffurelli, M. Liquid Biopsy in the Management of Breast Cancer Patients: Where Are We Now and Where Are We Going. Diagnostics 2023, 13, 1241. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Cancer Mortality by Age Visualisation. In Cancer Data in Australia [Internet]; AIHW: Canberra, Australia, 9 December 2024. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/cancer-mortality-by-age-visualisation (accessed on 19 July 2025).
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Sakach, E.; Sathe, C.; Ahn, H.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; et al. Randomized Phase II Trial of Endocrine Therapy With or Without Ribociclib After Progression on Cyclin-Dependent Kinase 4/6 Inhibition in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: MAINTAIN Trial. J. Clin. Oncol. 2023, 41, 4004–4013. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Bianchini, G.; Hamilton, E.; Graff, S.L.; Park, K.H.; Jeselsohn, R.; Demirci, U.; Martin, M.; Layman, R.M.; Hurvitz, S.A.; et al. Abemaciclib Plus Fulvestrant in Advanced Breast Cancer After Progression on CDK4/6 Inhibition: Results From the Phase III postMONARCH Trial. J. Clin. Oncol. 2025, 43, 1101–1112. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas (TCGA) Research Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.e6. [Google Scholar] [CrossRef]
- Bertucci, F.; Ng, C.K.Y.; Patsouris, A.; Droin, N.; Piscuoglio, S.; Carbuccia, N.; Soria, J.C.; Dien, A.T.; Adnani, Y.; Kamal, M.; et al. Genomic characterization of metastatic breast cancers. Nature 2019, 569, 560–564, Erratum in Nature 2019, 572, E7. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2024, 25, e629–e638. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Im, S.-A.; Saura, C.; Loibl, S.; Kalinsky, K.; Schmid, P.; Loi, S.; Thanopoulou, E.; Shankar, N.; Jin, Y.; et al. Overall Survival with Inavolisib in PIK3CA-Mutated Advanced Breast Cancer. N. Engl. J. Med. 2025, 393, 151–161. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Moreno, H.L.G.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.I.I.I.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Burstein, H.J.; DeMichele, A.; Somerfield, M.R.; Henry, N.L.; for the Biomarker Testing and Endocrine and Targeted Therapy in Metastatic Breast Cancer Expert Panels. Testing for ESR1 Mutations to Guide Therapy for Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2023, 41, 3423–3425. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer. Version 4.2025. NCCN: Plymouth Meeting, PA, USA, 2025. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419 (accessed on 29 July 2025).
- Condorelli, R.; Mosele, F.; Verret, B.; Bachelot, T.; Bedard, P.; Cortes, J.; Hyman, D.; Juric, D.; Krop, I.; Bieche, I.; et al. Genomic alterations in breast cancer: Level of evidence for actionability according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2019, 30, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; Pistilli, B.; Collins, J.; D’cRuz, C.; Gresty, C.; Sommavilla, R.; Sudhan, D.; Miller, C.; Ha, J.; Neven, P. Abstract PO2-19-10: CAPItello-292 Phase 3: An open-label, randomized study of capivasertib, fulvestrant, and investigator’s choice of CDK4/6 inhibitor (palbociclib or ribociclib) in HR+/HER2– advanced breast cancer. Cancer Res. 2024, 84, PO2-19-10. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.-A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Mayer, E.L.; Park, Y.H.; Janni, W.; Ma, C.; Cristofanilli, M.; Bianchini, G.; Kalinsky, K.; Iwata, H.; Chia, S.; et al. First-line camizestrant for ESR1-mutated advanced breast cancer: A phase 3, ctDNA-guided, randomized trial. N. Engl. J. Med. 2025, 392, 2147–2158. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J. Clin. Oncol. 2020, 38, 4274–4282. [Google Scholar] [CrossRef]
- Jhaveri, K.; Eli, L.D.; Wildiers, H.; Hurvitz, S.A.; Guerrero-Zotano, A.; Unni, N.; Brufsky, A.; Park, H.; Waisman, J.; Yang, E.S.; et al. Neratinib + fulvestrant + trastuzumab for HR-positive, HER2-negative, HER2-mutant metastatic breast cancer: Outcomes and biomarker analysis from the SUMMIT trial. Ann. Oncol. 2023, 34, 885–898. [Google Scholar] [CrossRef]
- Li, B.T.; Meric-Bernstam, F.; Bardia, A.; Naito, Y.; Siena, S.; Aftimos, P.G.; Anderson, I.; Curigliano, G.; De Migue Luken, M.J.; Kalra, M.; et al. 654O Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with solid tumors harboring specific HER2-activating mutations (HER2m): Primary results from the international phase II DESTINY-PanTumor01 (DPT-01) study. Ann Oncol. 2023, 34, S459–S460. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; Dubois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Brett, J.O.; Spring, L.M.; Bardia, A.; Wander, S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021, 23, 85. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, K.; Sharma, A.; Agnihotram, R.-K.V.; Altuntur, S.; Park, M.; Meterissian, S.; Burnier, J.V. Circulating Tumor DNA and Survival in Metastatic Breast Cancer. JAMA Netw. Open 2024, 7, e2431722. [Google Scholar] [CrossRef] [PubMed]
- Chandarlapaty, S.; Chen, D.; He, W.; Sung, P.; Samoila, A.; You, D.; Bhatt, T.; Patel, P.; Voi, M.; Gnant, M.; et al. Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer: A secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016, 2, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Boscolo Bielo, L.; Guerini Rocco, E.; Trapani, D.; Zagami, P.; Taurelli Salimbeni, B.; Esposito, A.; Belli, C.; Crimini, E.; Venetis, K.; Munzone, E.; et al. Genomic and clinical landscape of metastatic hormone receptor-positive breast cancers carrying ESR1 alterations. ESMO Open. 2022, 7, 100661. [Google Scholar] [CrossRef]
- Buono, G.; Gerratana, L.; Bulfoni, M.; Provinciali, N.; Basile, D.; Giuliano, M.; Corvaja, C.; Arpino, G.; Del Mastro, L.; De Placido, S.; et al. Circulating tumor DNA analysis in breast cancer: Is it ready for prime-time? Cancer Treat. Rev. 2019, 73, 73–83. [Google Scholar] [CrossRef]
- Rosin, J.; Svegrup, E.; Valachis, A.; Zerdes, I. Discordance of PIK3CA mutational status between primary and metastatic breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2023, 201, 161–169. [Google Scholar] [CrossRef]
- Fillbrunn, M.; Signorovitch, J.; André, F.; Wang, I.; Lorenzo, I.; Ridolfi, A.; Park, J.; Dua, A.; Rugo, H.S. PIK3CA mutation status, progression and survival in advanced HR + /HER2- breast cancer: A meta-analysis of published clinical trials. BMC Cancer 2022, 22, 1002. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Chic, N.; Pascual, T.; Adamo, B.; Vidal, M.; González-Farré, B.; Sanfeliu, E.; Schettini, F.; Conte, B.; Brasó-Maristany, F.; et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020, 22, 45. [Google Scholar] [CrossRef]
- Dempsey, N.; Bhatt, P.; Lewis, C.; Tolman, D.; Chamorro, Y.; Rubens, M.; Carcas, L.; Sandoval-Leon, A.C.; Ahluwalia, M.S.; Mahtani, R.L.; et al. Co-occurrence of ESR1 and PIK3CA mutations in HR+/HER2− metastatic breast cancer: Incidence and outcomes with targeted therapy. J. Clin. Oncol. 2024, 42, e13097. [Google Scholar] [CrossRef]
- Bhave, M.A.; Quintanilha, J.C.F.; Tukachinsky, H.; Li, G.; Scott, T.; Ross, J.S.; Pasquina, L.; Huang, R.S.P.; McArthur, H.; Levy, M.A.; et al. Comprehensive genomic profiling of ESR1, PIK3CA, AKT1, and PTEN in HR(+)HER2(−) metastatic breast cancer: Prevalence along treatment course and predictive value for endocrine therapy resistance in real-world practice. Breast Cancer Res. Treat. 2024, 207, 599–609. [Google Scholar] [CrossRef]
- Rosa-Rosa, J.M.; Caniego-Casas, T.; Leskela, S.; Cristobal, E.; González-Martínez, S.; Moreno-Moreno, E.; López-Miranda, E.; Holgado, E.; Pérez-Mies, B.; Garrido, P.; et al. High Frequency of ERBB2 Activating Mutations in Invasive Lobular Breast Carcinoma with Pleomorphic Features. Cancers 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.; Sheng, S.; Yuan, H.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017, 108, 671–677. [Google Scholar] [CrossRef]
- Iams, W.T.; Mackay, M.; Ben-Shachar, R.; Drews, J.; Manghnani, K.; Hockenberry, A.J.; Cristofanilli, M.; Nimeiri, H.; Guinney, J.; Benson, A.B. Concurrent Tissue and Circulating Tumor DNA Molecular Profiling to Detect Guideline-Based Targeted Mutations in a Multicancer Cohort. JAMA Netw. Open 2024, 7, e2351700. [Google Scholar] [CrossRef]
- Xu, J.; Gao, H.; Guan, X.; Meng, J.; Ding, S.; Long, Q.; Yi, W. Circulating tumor DNA: From discovery to clinical application in breast cancer. Front. Immunol. 2024, 15, 1355887. [Google Scholar] [CrossRef]
- Schrijver, W.A.M.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor conversion in distant breast cancer metastases: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Priedigkeit, N.; Hartmaier, R.J.; Chen, Y.; Vareslija, D.; Basudan, A.; Watters, R.J.; Thomas, R.; Leone, J.P.; Lucas, P.C.; Bhargava, R.; et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. 2017, 3, 666–671. [Google Scholar] [CrossRef]
- Lindström, L.S.; Karlsson, E.; Wilking, U.M.; Johansson, U.; Hartman, J.; Lidbrink, E.K.; Hatschek, T.; Skoog, L.; Bergh, J. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J. Clin. Oncol. 2012, 30, 2601–2608. [Google Scholar] [CrossRef] [PubMed]
- Bayle, A.; Peyraud, F.; Belcaid, L.; Brunet, M.; Aldea, M.; Clodion, R.; Dubos, P.; Vasseur, D.; Nicotra, C.; Geraud, A.; et al. Liquid versus tissue biopsy for detecting actionable alterations according to the ESMO Scale for Clinical Actionability of molecular Targets in patients with advanced cancer: A study from the French National Center for Precision Medicine (PRISM). Ann. Oncol. 2022, 33, 1328–1331. [Google Scholar] [CrossRef]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020, 21, 1296–1308. [Google Scholar] [CrossRef]
- Raei, M.; Heydari, K.; Tabarestani, M.; Razavi, A.; Mirshafiei, F.; Esmaeily, F.; Taheri, M.; Hoseini, A.; Nazari, H.; Shamshirian, D.; et al. Diagnostic accuracy of ESR1 mutation detection by cell-free DNA in breast cancer: A systematic review and meta-analysis of diagnostic test accuracy. BMC Cancer 2024, 24, 908. [Google Scholar] [CrossRef]
- Galvano, A.; Castellana, L.; Gristina, V.; La Mantia, M.; Insalaco, L.; Barraco, N.; Perez, A.; Cutaia, S.; Calò, V.; Russo, T.D.B.; et al. The diagnostic accuracy of PIK3CA mutations by circulating tumor DNA in breast cancer: An individual patient data meta-analysis. Ther. Adv. Med. Oncol. 2022, 14, 17588359221110162. [Google Scholar] [CrossRef]
- Chia, S.K.L.; Solovieff, N.; Joshi, M.; Im, S.-A.; Bianchi, G.V.; de la Cruz-Merino, L.; Jerusalem, G.H.M.; Sonke, G.S.; Nusch, A.; Beck, J.T.; et al. On-treatment dynamic circulating tumor DNA changes associated with progres-sion-free survival and overall survival in patients with HR+/HER2− advanced breast cancer in MONALEESA-3. J. Clin. Oncol. 2024, 42 (Suppl. 16), 1012. [Google Scholar] [CrossRef]
- Turner, N.C.; Swift, C.; Kilburn, L.S.; Fribbens, C.; Beaney, M.; Garcia-Murillas, I.; Budzar, A.U.; Robertson, J.F.; Gradishar, W.; Piccart, M.; et al. ESR1 Mutations and Overall Survival on Fulvestrant versus Exemestane in Advanced Hormone Receptor–Positive Breast Cancer: A Combined Analysis of the Phase III SoFEA and EFECT Trials. Clin. Cancer Res. 2020, 26, 5172–5177. [Google Scholar] [CrossRef]
- Bardia, A.; Cortés, J.; Bidard, F.-C.; Neven, P.; Garcia-Sáenz, J.; Aftimos, P.; O’sHaughnessy, J.; Lu, J.; Tonini, G.; Scartoni, S.; et al. Elacestrant in ER+, HER2− Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups. Clin. Cancer Res. 2024, 30, 4299–4309. [Google Scholar] [CrossRef]
- Oliveira, M.; Pominchuk, D.; Nowecki, Z.; Hamilton, E.; Kulyaba, Y.; Andabekov, T.; Hotko, Y.; Melkadze, T.; Nemsadze, G.; Neven, P.; et al. Camizestrant, a next-generation oral SERD, versus fulvestrant in post-menopausal women with oestrogen receptor-positive, HER2-negative advanced breast cancer (SERENA-2): A multi-dose, open-label, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Neven, P.; Casalnuovo, M.L.; Kim, S.-B.; Tokunaga, E.; Aftimos, P.; Saura, C.; O’sHaughnessy, J.; Harbeck, N.; Carey, L.A.; et al. Imlunestrant with or without Abemaciclib in Advanced Breast Cancer. N. Engl. J. Med. 2025, 392, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Lim, E.; Chavez-MacGregor, M.; Bardia, A.; Wu, J.; Zhang, Q.; Nowecki, Z.; Cruz, F.M.; Safin, R.; Kim, S.-B.; et al. Giredestrant for Estrogen Receptor–Positive, HER2-Negative, Previously Treated Advanced Breast Cancer: Results From the Randomized, Phase II acelERA Breast Cancer Study. J. Clin. Oncol. 2024, 42, 2149–2160. [Google Scholar] [CrossRef]
- Campone, M.; De Laurentiis, M.; Jhaveri, K.; Hu, X.; Ladoire, S.; Patsouris, A.; Zamagni, C.; Cui, J.; Cazzaniga, M.; Cil, T.; et al. Vepdegestrant, a PROTAC Estrogen Receptor Degrader, in Advanced Breast Cancer. N. Engl. J. Med. 2025, 393, 556–568. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Chan, A.; Petrakova, K.; Delaloge, S.; Campone, M.; Iwata, H.; Peddi, P.F.; Kaufman, P.A.; De Kermadec, E.; Liu, Q.; et al. AMEERA-3: Randomized Phase II Study of Amcenestrant (Oral Selective Estrogen Receptor Degrader) Versus Standard Endocrine Monotherapy in Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer. J. Clin. Oncol. 2023, 41, 4014–4024. [Google Scholar] [CrossRef]
- Cortés, J.; Hurvitz, S.A.; O’SHaughnessy, J.; Delaloge, S.; Iwata, H.; Rugo, H.S.; Neven, P.; Kanagavel, D.; Cohen, P.; Paux, G.; et al. Randomized Phase III Study of Amcenestrant Plus Palbociclib Versus Letrozole Plus Palbociclib in Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Primary Results From AMEERA-5. J. Clin. Oncol. 2024, 42, 2680–2690. [Google Scholar] [CrossRef]
- Goetz, M.; Bagegni, N.; Batist, G.; Brufsky, A.; Cristofanilli, M.; Damodaran, S.; Daniel, B.; Fleming, G.; Gradishar, W.; Graff, S.; et al. Lasofoxifene versus fulvestrant for ER+/HER2− metastatic breast cancer with an ESR1 mutation: Results from the randomized, phase II ELAINE 1 trial. Ann. Oncol. 2023, 34, 1141–1151. [Google Scholar] [CrossRef]
- Damodaran, S.; Plourde, P.V.; Moore, H.C.F.; Anderson, I.C.; Portman, D.J. Open-label, phase 2, multicenter study of lasofoxifene (LAS) combined with abemaciclib (Abema) for treating pre- and postmenopausal women with locally advanced or metastatic ER+/HER2− breast cancer and an ESR1 mutation after progression on prior therapies. J. Clin. Oncol. 2022, 40, 1022. [Google Scholar] [CrossRef]
- Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M.; Ladoire, S.; Levy, C.; et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Im, S.-A.; Hamilton, E.P.; Cussac, A.L.; Baird, R.D.; Ettl, J.; Goetz, M.P.; Iwata, H.; Joy, A.A.; Neven, P.; Haddad, V.; et al. SERENA-4: A phase 3 comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive, HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. J. Clin. Oncol. 2021, 39, TPS1101. [Google Scholar] [CrossRef]
- Turner, N.C.; Jhaveri, K.L.; Bardia, A.; Niikura, N.; Dieras, V.; Barrios, C.H.; Im, S.-A.; Mueller, V.; Bellet, M.; Chang, C.-W.; et al. persevERA Breast Cancer (BC): Phase III study evaluating the efficacy and safety of giredestrant (GDC-9545) + palbociclib versus letrozole + palbociclib in patients (pts) with estrogen-receptor-positive, HER2-negative locally advanced or metastatic BC (ER+/HER2– LA/mBC). J. Clin. Oncol. 2021, 39, TPS1103. [Google Scholar] [CrossRef]
- Goetz, M.P.; Wander, S.A.; Bachelot, T.; Batist, G.; Cortes, J.; Cristofanilli, M.; Curigliano, G.; de Nonneville, A.; Gal-Yam, E.N.; Jhaveri, K.L.; et al. Open-label, randomized, multicenter, phase 3, ELAINE 3 study of the efficacy and safety of lasofoxifene plus abemaciclib for treating ER+/HER2−, locally advanced or metastatic breast cancer with an ESR1 mutation. J. Clin. Oncol. 2024, 42, TPS1127. [Google Scholar] [CrossRef]
- Hackbart, H.; Cui, X.; Lee, J.S. Androgen receptor in breast cancer and its clinical implication. Transl. Breast Cancer Res. 2023, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, N.; Zhao, F.; Manola, J.; Klein, P.; Ramaswamy, B.; Brufsky, A.; Stella, P.J.; Burnette, B.; Telli, M.; Makower, D.F.; et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. 2018, 36, 1556–1563. [Google Scholar] [CrossRef]
- Vasseur, A.; Cabel, L.; Hego, C.; Takka, W.; Grati, O.T.; Renouf, B.; Lerebours, F.; Loirat, D.; Brain, E.; Cottu, P.; et al. Fulvestrant and everolimus efficacy after CDK4/6 inhibitor: A prospective study with circulating tumor DNA analysis. Oncogene 2024, 43, 1214–1222. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Pérez-García, J.M.; Ruiz Borrego, M.; Tolosa, P.; Blanch, S.; Fernández-Ortega, A.; Urruticoecheah, A.; Blancasi, I.; Sauraj, C.; Rojas, B.; et al. Preventing alpelisib-related hyperglycaemia in HR+/HER2−/PIK3CA-mutated advanced breast cancer using metformin (METALLICA): A multicentre, open-label, single-arm, phase 2 trial. Eur. J. Cancer. 2024, 204, 112635. [Google Scholar] [CrossRef]
- Chia, S.K.L.; Redfern, A.D.; Ayoub, J.P.M.; Chalchal, H.I.; Rayson, D.; Rushton, M.; Desbiens, C.; Sabanathan, D.; Raphael, J.; Chan, A.; et al. A double-blind place-bo-controlled randomised phase III trial of fulvestrant and ipatasertib as treatment for advanced HER2-negative and es-trogen receptor positive (ER+) breast cancer following progression on first line CDK 4/6 inhibitor and aromatase in-hibitor: The CCTG/BCT MA.40/FINER study (NCT04650581). J Clin Oncol. 2025, 43 (Suppl. 17), LBA100. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Mayer, I.A.; Ganju, V.; Dickler, M.; Johnston, S.; Morales, S.; Yardley, D.A.; Melichar, B.; Forero-Torres, A.; Lee, S.C.; et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016, 17, 811–821. [Google Scholar] [CrossRef]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Rodríguez, L.P.-A.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Park, H.; Dumbrava, E.E.; Uboha, N.V.; Kalyan, A.; Dayyani, F.; Arora, M.; Villalona-Calero, M.A.; Mahdi, H.S.; Patel, R.A.; et al. Abstract CT071: Phase I trial of trastuzumab deruxtecan in combination with neratinib in solid tumors with HER2 alterations (NCI 10495). Cancer Res. 2025, 85, CT071. [Google Scholar] [CrossRef]
- Yap, T.; Elhaddad, A.; Grisham, R.; Hamm, J.; Marks, D.; Shapiro, G.; Corre, C.; Li, J.; Lin, T.; Liu, F.; et al. First-in-human phase 1/2a study of a potent and novel CDK2-selective inhibitor PF-07104091 in patients (pts) with advanced solid tumors, enriched for CDK4/6 inhibitor resistant HR+/HER2− breast cancer. J. Clin. Oncol. 2023, 41 (Suppl. 16), 3010. [Google Scholar] [CrossRef]
- Juric, D.; Patel, M.; Duska, L.; Jhaveri, K.; Henick, B.; Matulonis, U.; Munster, P.; Birrer, M.; Moore, K.; Curigliano, G.; et al. BLU-222, an investigational, oral, potent, and highly selective CDK2 inhibitor (CDK2i), as monotherapy in patients with advanced solid tumors and in combination with ribociclib and fulvestrant in HR+/HER2– breast cancer. J. Clin. Oncol. 2024, 42 (Suppl. 16), 1056. [Google Scholar] [CrossRef]
- Maleki, H.; Aiyelabegan, H.T.; Javadi, P.; Abdi, F.; Mirzavi, F.; Zarei Behjani, Z.; Rizvanov, A.A.; Takallu, S.; Kumar, R.; Hadi Barhaghtalab, R.; et al. Nanotechnology-mediated precision drug delivery strategies for breast cancer treatment. Biomed. Pharmacother. 2025, 188, 118224. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Fujigaki, H.; Kato, K.; Yamazaki, K.; Fujigaki, S.; Kunisawa, K.; Yamamoto, Y.; Mouri, A.; Oda, A.; Nabeshima, T.; et al. Selective and competitive inhibition of kynurenine aminotransferase 2 by glycyrrhizic acid and its analogues. Sci. Rep. 2019, 9, 10243. [Google Scholar] [CrossRef] [PubMed]
| Molecular Aberration | Estimated Prevalence in ER-Positive Breast Cancer | ESCAT Scale Classification | Drug Class Matched | Key Clinical Trial |
|---|---|---|---|---|
| PIK3CAmut | 30–40% [17] | IA |
| |
| PTENdel | 7% [17] | I/II | ||
| AKT1mut | 7% [17] | I/II | ||
| ESR1mut | Primary breast cancer: < 1% [15] Post adjuvant AI: 5% [16,17] Progression on AI for metastatic disease: 30–40% [16,17] | IA |
| |
| Germline BRCA1/2mut | 4% [15] | IA |
| |
| Somatic BRCA1/2mut | 3% [15] | IIB |
| |
| HER2mut | 3–4% [15] | IIB |
| |
| NTRKfusions | <1% [26] | IC |
|
|
| Germline PALB2mut | 1% [26] | IIB |
|
|
| ESR1 | Prevalence | Median OS (Months) [38] |
|---|---|---|
| ESR1wt | NA | 32.1 (95% CI 28.1–36.4) |
| ESR1mut (all) | Primary breast cancer: <1% [15] Post adjuvant AI: 5% [16,17] Progression on AI for metastatic disease: 30–40% [16,17] | 20.7 (HR 1.6, 95% CI 17.7–28.1, p < 0.001) |
| ESR1mut D538G | 21.1% [38]–41.2% [39] | 26.0 (HR 1.4, 95% CI 19.2–32.4, p = 0.03) |
| ESR1mut Y537S | 13.3% [38]–22.1% [39] | 20.0 (HR 1.8, 95% CI 13.0–29.3, p = 0.003) |
| Dual mutations | 3.8% [39]–5.5% [38] | 15.2 (HR 2.23, 95% CI 10.9–27.4, p < 0.001) |
| Trial | Novel Drug and Mechanism | Phase | Line | Population | Treatment Arms | Prior CDK4/6i (%) | ESR1mut (%) | Efficacy ESR1 mut | Efficacy ESR1wt | Efficacy Overall Population |
|---|---|---|---|---|---|---|---|---|---|---|
| EMERALD [28] | Elacestrant (PO SERD) | 3 | 2–3 | ER+/HER2− MBC, post-ET+ CDK4/6i | Elacestrant vs. PCET | 100 | 48 | PFS 3.8 vs. 1.9 mo; HR 0.55, 95% CI 0.39–0.77, p = 0.0005 | NR | PFS 2.8 vs. 1.9 mo; HR 0.70, 95% CI 0.55–0.88, p = 0.002 |
| SERENA-2 [60] | Camizestrant (PO SERD + ER antagonist) | 2 | 2 | ER+/HER2− MBC, post-ET | Camizestrant (75 mg, 100 mg, and 300 mg) vs. Fulvestrant | 51 | 38 | PFS 6.3 (75 mg) vs. 2.2 mo; HR 0.33, 90% CI 0.18–0.58 | PFS 7.2 (75 mg) vs. 7.2 mo; HR 0.80, 90% CI 0.51–1.27 | PFS 7.2 (75 mg) vs. 3.7 mo; HR 0.59, 90% CI 0.42–0.82, p = 0.017 |
| EMBER-3 [61] | Imlunestrant (PO SERD) | 3 | 1–2 | ER+/HER2− MBC, post-ET ± CDK4/6i | Imlunestrant vs. Imlunestrant + Abemaciclib vs. PCET | 58 | 38 | Imlunestrant vs. PCET: PFS 5.5 vs. 3.8 mo; HR 0.76, p <0.001 Imlunestrant + Abemaciclib vs. Imlunestrant: PFS 9.4 vs. 5.5 mo; HR 0.57, 95% CI 0.44–0.73, p < 0.001 | NR | Imlunestrant vs. PCET: PFS 5.6 vs. 5.5 mo; HR 0.87, 95% CI 0.72–1.04, p = 0.12 |
| * AMEERA-3 [64] | Amcenestrant (PO SERD) | 2 | 2–3 | ER+/HER2− MBC, post-ET | Amcenestrant vs. PCET | 79 | 44 | PFS 3.7 vs. 2.0, HR 0.9, 95% CI 0.57–1.5 | PFS 3.5 vs. 3.9 mo; HR 1.3, 95% CI 0.88–1.9 | PFS 3.6 vs. 3.7 mo; HR 1.05 (95% CI: 0.79–1.40), p = 0.64 |
| * AMEERA-5 [65] | Amcenestrant (PO SERD) | 3 | 1 | ER+/HER2− MBC without prior therapy | Amcenestrant + Palbociclib vs. Letrozole + Palbociclib | 0 | NR | NR | NR | Stopped for futility; mPFS estimates not robust. HR 1.2, 95% CI 0.93–1.55, p = 0.93 |
| acelERA [62] | Giredestrant (PO SERD) | 2 | 2–3 | ER+/HER2− MBC, post-ET ± CDK4/6i | Giredestrant vs. PCET | 42 | 38 | PFS 5.3 vs. 3.5 mo; HR 0.60, 95% CI 0.35–1.03 | PFS 7.2 vs. 6.6 months, HR 1.01, 95% CI 0.64–1.60 | PFS 5.6 vs. 5.4 mo; HR 0.81, 95% 0.60–1.1, p = 0.17 |
| VERITAC-2 [63] | Vepdegestrant (PROTAC ER degrader) | 3 | 2–3 | ER+/HER2− MBC, post-ET ± CDK4/6i | Vepdegestrant vs. Fulvestrant | 100 | 43 | PFS 5.0 vs. 2.1 mo; HR 0.58, 955 CI 0.43–0.78, p < 0.001 | NR | PFS 3.8 vs. 3.6 mo; HR 0.83, 95% CI 0.69–1.01), p = 0.07 |
| ELAINE-1 [66] | Lasofoxifene (SERM) | 2 | 2 | ER+/HER2− MBC, post-ET+ CDK4/6i, ESR1mut | Lasofoxifene vs. Fulvestrant | 100 | 100 | PFS 5.5 vs. 3.7 mo; HR 0.69, 95% CI 0.43–1.1, p = 0.138 | NA | PFS 5.5 vs. 3.7 mo; HR 0.69, 95% CI 0.43–1.1, p = 0.138 |
| ELAINE-2 [67] | Lasofoxifene (SERM) | 2 | 2–3 | ER+/HER2− MBC, post-ET ± CDK4/6i, ESR1mut | Lasofoxifene + Abemaciclib (single arm) | 96 | 100 | ORR 55.6% (95% CI 33.7–75.4) | NA | ORR 55.6% (95% CI 33.7–75.4) |
| PADA-1 [68] | Fulvestrant (IM SERD) | 3 | 1.5 (rising ESR1mut on 1L) | ER+/HER2− MBC on ET + Palbociclib | Continue ET + Palbociclib vs. Fulvestrant + Palbociclib | 100 | 100 | PFS 11.9 vs. 5.7 mo; HR 0.61, 95% CI 0.43–0.86, p = 0.004 | NA | PFS 11.9 vs. 5.7 mo; HR 0.61, 95% CI 0.43–0.86, p = 0.0040 |
| SERENA-6 [29] | Camizestrant (PO SERD + ER antagonist) | 3 | 1.5 (rising ESR1mut on 1L) | ER+/HER2− MBC on ET + CDK4/6i | Continue ET + CDK4/6i vs. Camizestrant + CDK4/6i | 100 | 100 | PFS 16 vs. 9.2 mo; HR 0.44, 95% CI 0.31–0.60, p < 0.00001 | NA | PFS 16 vs. 9.2 mo; HR 0.44, 95% CI 0.31–0.60, p < 0.00001 |
| SERENA-4 [69] | Camizestrant (PO SERD + ER antagonist) | 3 | 1 | ER+/HER2− MBC without prior therapy | Camizestrant + Palbociclib vs. Anastrazole + Palbociclib | 0 | NR | Results awaited | ||
| persevERA [70] | Giredestrant (PO SERD) | 3 | 1 | ER+/HER2− MBC without prior therapy | Giredestrant + Palbociclib vs. Letrozole + Palbociclib | 0 | NR | Results awaited | ||
| ELAINE-3 [71] | Lasofoxifene (SERM) | 3 | 2–3 | ER+/HER2− MBC, post-ET ± CDK4/6i (Ribociclib or Palbociclib), ESR1mut | Lasofoxifene + Abemaciclib vs. Fulvestrant + Abemaciclib | 100 | 100 | Results awaited | ||
| Trial | Novel Drug and Mechanism | Phase | Line | Population | Treatment Arms | Prior CDK4/6i (%) | Mutant Population (%) | Detection Method for Genomic Profiling | Efficacy in Mutant Cohort | Efficacy in Non-Mutant Cohort | Efficacy Overall Population |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SOLAR-1 [18] | Alpelisib (PIK3CA inhibitor) | 3 | 2 | ER+/HER2− MBC, post-ET | Alpelisib + Fulvestrant vs. placebo + Fulvestrant | 6 | 29 (PIK3CA) | Tissue | PFS 11.0 vs. 5.7 mo; HR 0.65, 95% CI 0.50–0.85, p < 0.001 | PFS 7.4 vs. 5.6 mo; HR 0.85, 95% CI 0.58–1.25 | NR |
| BYLieve [19] | Alpelisib (PIK3CA inhibitor) | 2–single arm | 2 | ER+/HER2− MBC, post-ET + CDK4/6i | Alpelisib + Fulvestrant | 100 | 100 (PIK3CA) | Tissue or ctDNA | PFS 8.0 mo (95% CI 5.6–8.6) | NA | PFS 8.0 mo (95% CI 5.6–8.6) |
| SANDPIPER [77] | Taselisib (PIK3CA inhibitor) | 3 | 2 | ER+ MBC, post-ET | Taselisib + Fulvestrant vs. placebo + Fulvestrant | 3 | 100 (PIK3CA) | Tissue | PFS 7.4 vs. 5.4 mo; HR 0.70, 95% CI 0.56–0.89, p = 0.0037 | NA | PFS 7.4 vs. 5.4 mo; HR 0.70, 95% CI 0.56–0.89, p = 0.0037 |
| * FERGI [78] | Pictilisib (PIK3CA inhibitor) | 2 | 2 | ER+/HER2− MBC, post-ET | Pictilisib + Fulvestrant vs. Fulvestrant + placebo | U | 41 (PIK3CA) | Tissue | PFS 6.5 vs. 5.1 mo; HR 0.73, 95% CI 0.42–1.28, p = 0.268 | PFS 5.8 vs. 3.6 mo; HR 0.72, 95% CI 0.42–1.23, p = 0.23 | PFS 6.6 vs. 5.1 mo; HR 0.74, 95% CI 0.52–1.06, p = 0.096 |
| CAPItello-291 [21] | Capivasertib (Pan-AKT inhibitor) | 3 | 2 | ER+ /HER2− MBC, post-ET± CDK4/6i | Capivasertib + Fulvestrant vs. Fulvestrant + placebo | 70 | 41 (PIK3CA, AKT1 or PTEN) | Tissue | PFS 7.3 vs. 3.1 mo; HR 0.50, 95% CI 0.38–0.65, p < 0.001 | PFS 7.2 vs. 3.7 mo; HR 0.70, 95% CI 0.56–0.88 (post-hoc) | PFS 7.2 vs. 3.6 mo; HR 0.60, 95% CI 0.51–0.71, p <0.001 |
| FINER [76] | Ipatasertib (Pan-AKT inhibitor) | 3 | 2 | ER+/HER2− MBC, post-ET+ CDK4/6i | Ipatasertib + Fulvestrant vs. Fulvestrant + placebo | 100 | 44 (PIK3CA, AKT1 or PTEN) | ctDNA | PFS 5.45 vs. 1.91 mo; HR 0.47, 95% CI 0.31–0.72, p = 0.0005 | NR | PFS 5.32 vs. 1.94 mo; HR 0.61, 95% CI 0.46–0.81, p = 0.0007 |
| INAVO120 [20] | Inavolisib (PIK3CA inhibitor) | 3 | 1 | ER+/HER2− MBC, PIK3CA-mutant, relapsed during or within 12 months of adjuvant ET | Inavolisib + Fulvestrant + Palbociclib vs. placebo + Fulvestrant + Palbociclib | 1 | 100 (PIK3CA) | Tissue or ctDNA | PFS 15.0 vs. 7.3 mo; HR 0.43, 95% CI 0.32–0.59, p < 0.001 | NA | PFS 15.0 vs. 7.3 mo; HR 0.43, 95% CI 0.32–0.59, p < 0.001 |
| CAPItello-292 [27] | Capivasertib (Pan-AKT inhibitor) | 3 | 1 | ER+/HER2− MBC, relapsed during or within 12 months of adjuvant ET | Capivasertib + Fulvestrant + CDK4/6i (Ribociclib or palbociclib) vs. Fulvestrant + CDK4/6i (Ribociclib or palbociclib) | Results awaited | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Childs, S.; Semba, R.; Haggstrom, L.; Lim, E. Integrating Molecular Phenotyping into Treatment Algorithms for Advanced Oestrogen Receptor-Positive Breast Cancer. Cancers 2025, 17, 3174. https://doi.org/10.3390/cancers17193174
Childs S, Semba R, Haggstrom L, Lim E. Integrating Molecular Phenotyping into Treatment Algorithms for Advanced Oestrogen Receptor-Positive Breast Cancer. Cancers. 2025; 17(19):3174. https://doi.org/10.3390/cancers17193174
Chicago/Turabian StyleChilds, Sarah, Ryoko Semba, Lucy Haggstrom, and Elgene Lim. 2025. "Integrating Molecular Phenotyping into Treatment Algorithms for Advanced Oestrogen Receptor-Positive Breast Cancer" Cancers 17, no. 19: 3174. https://doi.org/10.3390/cancers17193174
APA StyleChilds, S., Semba, R., Haggstrom, L., & Lim, E. (2025). Integrating Molecular Phenotyping into Treatment Algorithms for Advanced Oestrogen Receptor-Positive Breast Cancer. Cancers, 17(19), 3174. https://doi.org/10.3390/cancers17193174






