The S1P/S1P1 Signaling Axis Plays Regulatory Functions in the Crosstalk Between Brain-Metastasizing Melanoma Cells and Microglia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Melanoma-Conditioned Medium
2.3. Cytokines
2.4. IL-6Rα Inhibition Assay
2.5. RNA Isolation and Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.6. Western Blotting
2.7. Flow Cytometry
2.8. Construction of S1P1lo Microglia Cells
2.9. Viability Assay (XTT)
2.10. Phagocytosis Assay
2.11. Apoptosis Assay
2.12. Cell Death Labeling in Co-Cultures
2.13. Proliferation in Co-Cultures
2.14. Spheroid (3D) Cultures
2.15. Evaluation of Sensitivity to Combined BRAF and S1P1 Inhibition
2.16. Biostatistic Analysis
3. Results
3.1. Brain-Metastasizing Melanoma Cells Upregulate S1PR1 in Microglia via IL-6
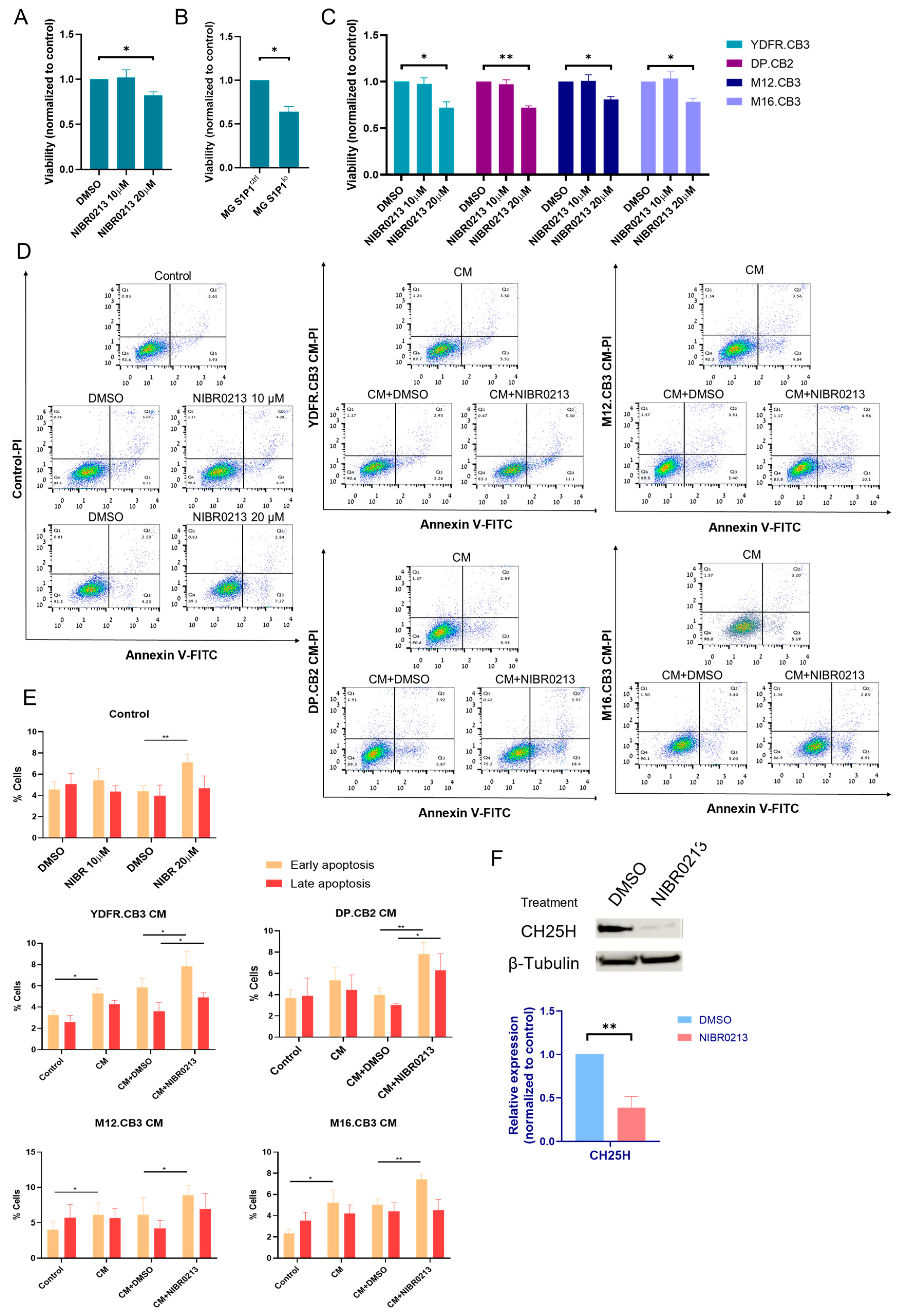
3.2. Pharmacological and Genetic Inhibition of S1P1
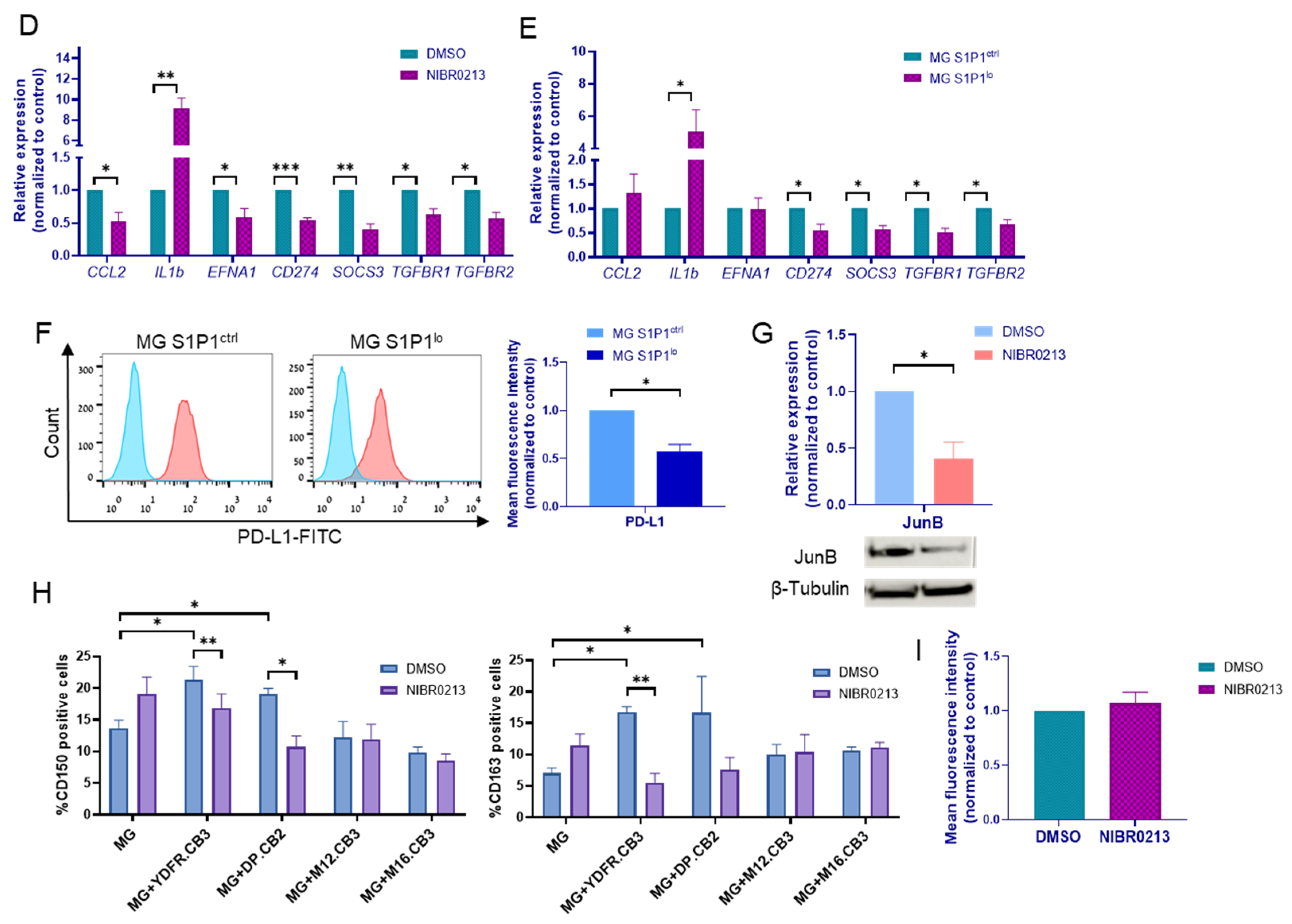
3.3. S1P1 Inhibition Shapes the Molecular Profile of Microglia Cells
3.4. S1P1 Inhibition Abolishes CD150 and CD163 Upregulation in Microglia Co-Cultured with BMMC
3.5. S1P1 Inhibition Does Not Affect the Phagocytic Ability of Microglia Cells
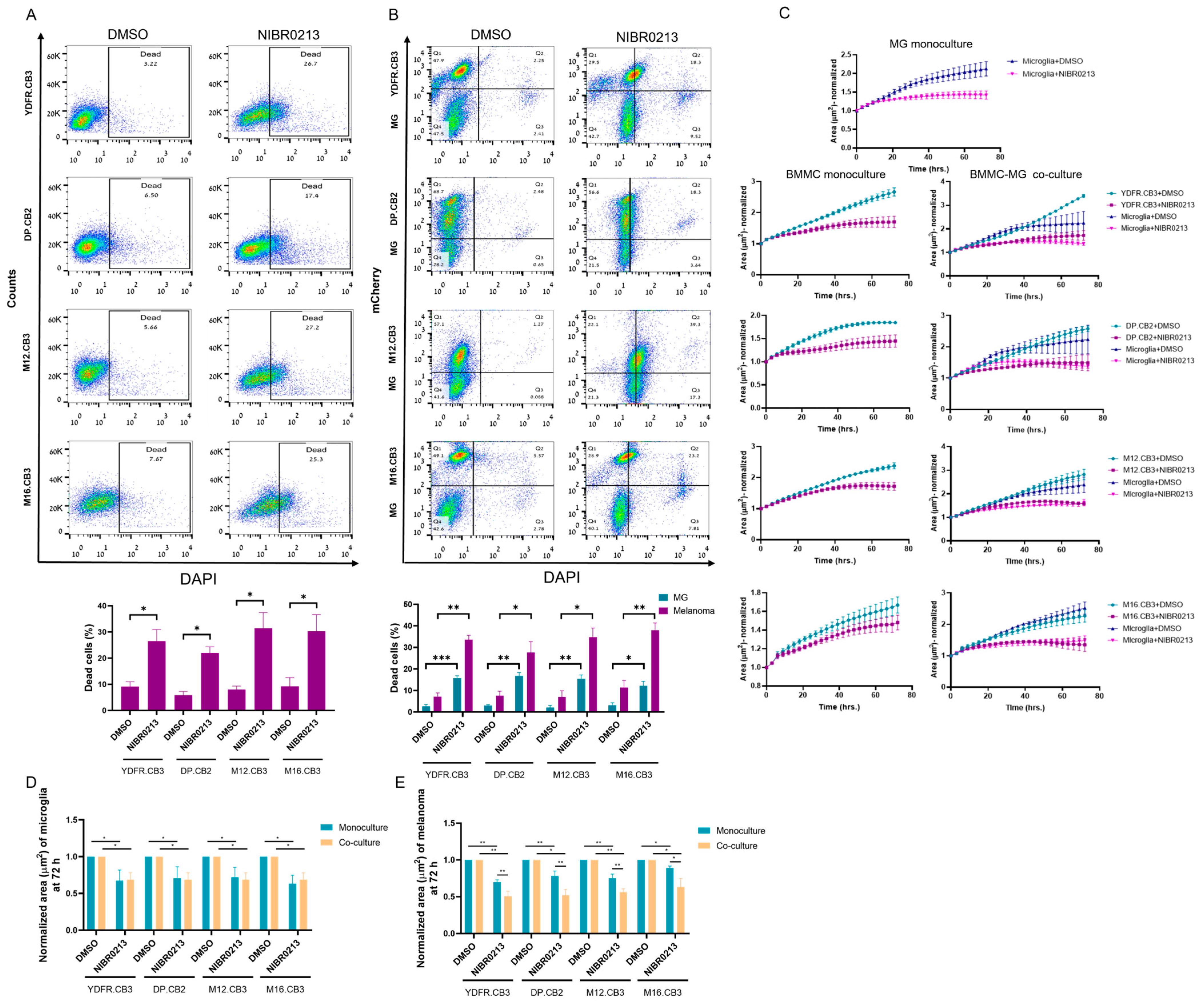
3.6. S1P1 Inhibition Reduces the Proliferation Rate of Microglia and BMMCs
3.7. Melanoma-Secreted Factors Sensitize Microglia to S1P1 Inhibition-Induced Apoptosis
3.8. BMMCs Exhibit Greater Sensitivity to NIBR0213-Induced Growth Arrest Compared to Their Microglial Co-Culture Counterparts
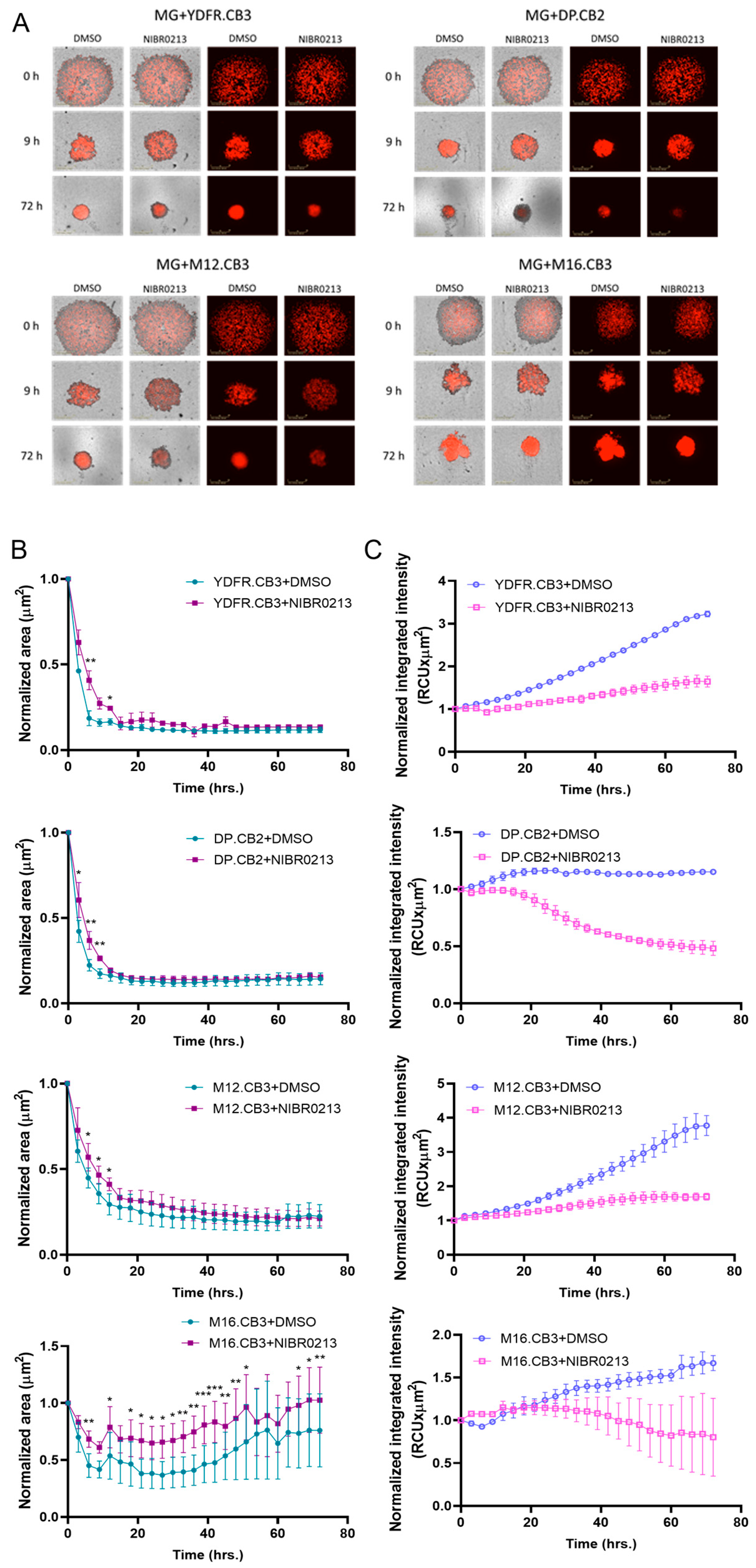
3.9. S1P1 Inhibition Modulates Spheroid Formation in BMMC–Microglia Co-Cultures
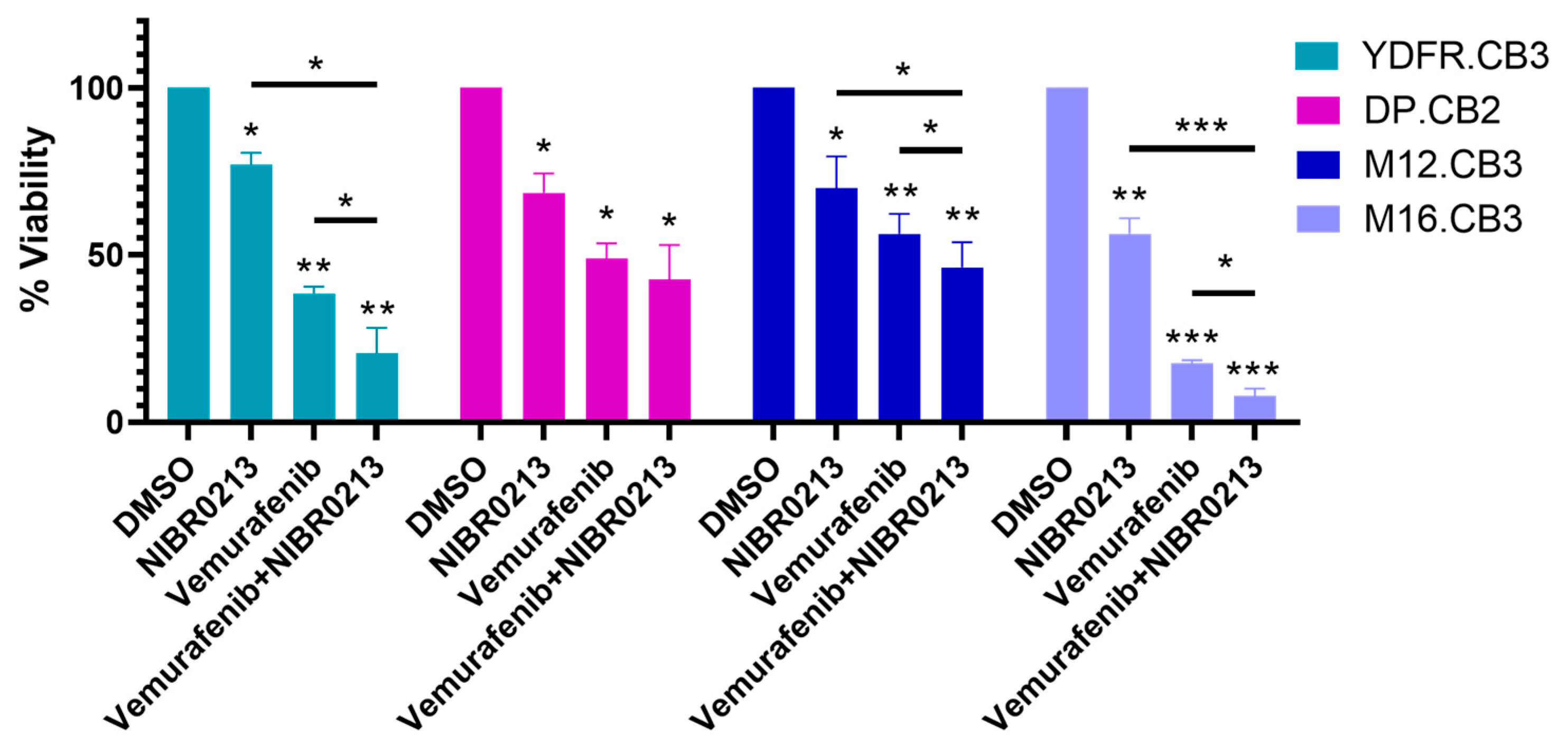
3.10. Targeting S1P1 Potentiates the Efficacy of Vemurafenib Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25-HC | 25-hydroxycholesterol |
| BMMCs | Brain-metastasizing melanoma cells |
| CH25H | Cholesterol 25-hydroxylase |
| LIF | Leukemia inhibitory factor |
| MG | Microglia |
| OSM | Oncostatin M |
| PDGF-AA | Platelet-derived growth factor AA |
| S1P | Sphingosine-1-phosphate |
| S1PR1/S1P1 | Sphingosine-1-phosphate receptor 1 |
| SREBP2 | Sterol regulatory element-binding protein 2 |
| SphK1 | Sphingosine-1-kinase |
| TAMs | Tumor-associated macrophages |
| TGF-β1 | Transforming growth factor-β1 |
| TGFBR1 | TGF-β receptor type I |
| TGFBR2 | TGF-β receptor type II |
Appendix A
Appendix A.1
| CD150 | CD163 | ||||
|---|---|---|---|---|---|
| Treatment | Average | SEM | Average | SEM | |
| MG | DMSO | 13.67 | 1.24 | 6.99 | 0.90 |
| NIBR0213 | 19.13 | 2.66 | 11.35 | 1.83 | |
| MG+YDFR.CB3 | DMSO | 21.30 | 2.14 | 16.73 | 0.87 |
| NIBR0213 | 16.90 | 2.16 | 5.55 | 1.43 | |
| MG+DP.CB2 | DMSO | 19.10 | 0.84 | 16.66 | 5.74 |
| NIBR0213 | 10.74 | 1.72 | 7.57 | 1.96 | |
| MG+M12.CB3 | DMSO | 12.19 | 2.54 | 10.01 | 1.61 |
| NIBR0213 | 11.93 | 2.38 | 10.39 | 2.76 | |
| MG+M16.CB3 | DMSO | 9.82 | 0.89 | 9.95 | 1.31 |
| NIBR0213 | 8.56 | 1.03 | 11.16 | 0.74 | |
Appendix A.2
| MG | BMMC | ||||
|---|---|---|---|---|---|
| Treatment | Average | SEM | Average | SEM | |
| MG+YDFR.CB3 | DMSO | 2.73 | 0.78 | 7.18 | 1.71 |
| NIBR0213 | 15.78 | 1.08 | 33.59 | 2.06 | |
| MG+DP.CB2 | DMSO | 3.05 | 0.41 | 7.55 | 2.14 |
| NIBR0213 | 16.78 | 1.59 | 27.69 | 5.02 | |
| MG+M12.CB3 | DMSO | 2.05 | 1.05 | 6.98 | 2.90 |
| NIBR0213 | 15.43 | 1.80 | 34.83 | 4.17 | |
| MG+M16.CB3 | DMSO | 3.17 | 1.15 | 11.43 | 3.23 |
| NIBR0213 | 12.18 | 2.08 | 37.93 | 3.44 | |
Appendix A.3
| Treatment | Average | SEM | |
|---|---|---|---|
| MG only | DMSO | 1.00 | 0.00 |
| NIBR0213 | 0.69 | 0.09 | |
| MG+YDFR.CB3 | DMSO | 1.00 | 0.00 |
| NIBR0213 | 0.67 | 0.14 | |
| MG+DP.CB2 | DMSO | 1.00 | 0.00 |
| NIBR0213 | 0.71 | 0.16 | |
| MG+M12.CB3 | DMSO | 1.00 | 0.00 |
| NIBR0213 | 0.72 | 0.14 | |
| MG+M16.CB3 | DMSO | 1.00 | 0.00 |
| NIBR0213 | 0.63 | 0.12 |
Appendix A.4
| Alone | Co-Culture | ||||
|---|---|---|---|---|---|
| Treatment | Average | SEM | Average | SEM | |
| MG+YDFR.CB3 | DMSO | 1.00 | 0.00 | 1.00 | 0.00 |
| NIBR0213 | 0.70 | 0.03 | 0.51 | 0.07 | |
| MG+DP.CB2 | DMSO | 1.00 | 0.00 | 1.00 | 0.00 |
| NIBR0213 | 0.78 | 0.07 | 0.52 | 0.08 | |
| MG+M12.CB3 | DMSO | 1.00 | 0.00 | 1.00 | 0.00 |
| NIBR0213 | 0.75 | 0.06 | 0.57 | 0.04 | |
| MG+M16.CB3 | DMSO | 1.00 | 0.00 | 1.00 | 0.00 |
| NIBR0213 | 0.89 | 0.03 | 0.63 | 0.12 | |
Appendix A.5
| YDFR.CB3 | DP.CB2 | M12.CB3 | M16.CB3 | |||||
|---|---|---|---|---|---|---|---|---|
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | |
| DMSO | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 |
| Vemurafenib | 38.50 | 2.02 | 49.00 | 4.58 | 56.33 | 6.01 | 17.67 | 0.88 |
| Vemurafenib+NIBR0213 | 20.50 | 7.79 | 42.67 | 10.33 | 46.33 | 7.51 | 7.83 | 2.24 |
| NIBR0213 | 77.00 | 3.61 | 68.67 | 5.78 | 63.67 | 13.48 | 56.33 | 4.70 |
References
- Langley, R.R.; Fidler, I.J. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr. Rev. 2007, 28, 297–321. [Google Scholar] [CrossRef]
- Vosoughi, E.; Lee, J.M.; Miller, J.R.; Nosrati, M.; Minor, D.R.; Abendroth, R.; Lee, J.W.; Andrews, B.T.; Leng, L.Z.; Wu, M.; et al. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer 2018, 18, 490. [Google Scholar] [CrossRef]
- Goenka, A.; Khan, F.; Verma, B.; Sinha, P.; Dmello, C.C.; Jogalekar, M.P.; Gangadaran, P.; Ahn, B. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun. 2023, 43, 525–561. [Google Scholar] [CrossRef]
- Witz, I.P. Tumor-microenvironment interactions: Dangerous liaisons. Adv. Cancer Res. 2008, 100, 203–229. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Neganova, M.E.; Aleksandrova, Y.; Burcher, J.T.; Chugunova, E.; Fan, R.; Tse, E.; Sethi, G.; Bishayee, A.; Liu, J. Signaling controversy and future therapeutical perspectives of targeting sphingolipid network in cancer immune editing and resistance to tumor necrosis factor-alpha immunotherapy. Cell Commun. Signal. 2024, 22, 251. [Google Scholar] [CrossRef]
- Tsai, H.C.; Nguyen, K.; Hashemi, E.; Engleman, E.; Hla, T.; Han, M.H. Myeloid sphingosine-1-phosphate receptor 1 is important for CNS autoimmunity and neuroinflammation. J. Autoimmun. 2019, 105, 102290. [Google Scholar] [CrossRef]
- Hughes, J.E.; Srinivasan, S.; Lynch, K.R.; Proia, R.L.; Ferdek, P.; Hedrick, C.C. Sphingosine-1-Phosphate Induces an Antiinflammatory Phenotype in Macrophages. Circ. Res. 2008, 102, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, Y.; Lin, W.; Zhong, H.; Xu, K.; Qi, X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Carrié, L.; Virazels, M.; Dufau, C.; Montfort, A.; Levade, T.; Ségui, B.; Andrieu-Abadie, N. New Insights into the Role of Sphingolipid Metabolism in Melanoma. Cells 2020, 9, 1967. [Google Scholar] [CrossRef] [PubMed]
- Weichand, B.; Popp, R.; Dziumbla, S.; Mora, J.; Strack, E.; Elwakeel, E.; Frank, A.-C.; Scholich, K.; Pierre, S.; Syed, S.N.; et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J. Exp. Med. 2017, 214, 2695–2713. [Google Scholar] [CrossRef]
- Nagahashi, M.; Yamada, A.; Katsuta, E.; Aoyagi, T.; Huang, W.-C.; Terracina, K.P.; Hait, N.C.; Allegood, J.C.; Tsuchida, J.; Yuza, K.; et al. Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis. Cancer Res. 2018, 78, 1713–1725. [Google Scholar] [CrossRef]
- Pereira, F.V.; Arruda, D.C.; Figueiredo, C.R.; Massaoka, M.H.; Matsuo, A.L.; Bueno, V.; Rodrigues, E.G. FTY720 induces apoptosis in B16F10-NEX2 murine melanoma cells, limits metastatic development in vivo, and modulates the immune system. Clinics 2013, 68, 1018–1027. [Google Scholar] [CrossRef]
- Arseni, L.; Sharma, R.; Mack, N.; Nagalla, D.; Ohl, S.; Hielscher, T.; Singhal, M.; Pilz, R.; Augustin, H.; Sandhoff, R.; et al. Sphingosine-1-Phosphate Recruits Macrophages and Microglia and Induces a Pro-Tumorigenic Phenotype That Favors Glioma Progression. Cancers 2023, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Izraely, S.; Ben-Menachem, S.; Sagi-Assif, O.; Telerman, A.; Zubrilov, I.; Ashkenazi, O.; Meshel, T.; Maman, S.; Orozco, J.I.; Salomon, M.P.; et al. The metastatic microenvironment: Melanoma–microglia cross-talk promotes the malignant phenotype of melanoma cells. Int. J. Cancer 2018, 144, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Izraely, S.; Sagi-Assif, O.; Klein, A.; Meshel, T.; Tsarfaty, G.; Pasmanik-Chor, M.; Nahmias, C.; Couraud, P.; Ateh, E.; Bryant, J.L.; et al. The metastatic microenvironment: Brain-residing melanoma metastasis and dormant micrometastasis. Int. J. Cancer 2011, 131, 1071–1082. [Google Scholar] [CrossRef]
- Izraely, S.; Ben-Menachem, S.; Sagi-Assif, O.; Meshel, T.; Marzese, D.M.; Ohe, S.; Zubrilov, I.; Pasmanik-Chor, M.; Hoon, D.S.; Witz, I.P. ANGPTL4 promotes the progression of cutaneous melanoma to brain metastasis. Oncotarget 2017, 8, 75778–75796. [Google Scholar] [CrossRef]
- Izraely, S.; Sagi-Assif, O.; Klein, A.; Meshel, T.; Ben-Menachem, S.; Zaritsky, A.; Ehrlich, M.; Prieto, V.G.; Bar-Eli, M.; Pirker, C.; et al. The metastatic microenvironment: Claudin-1 suppresses the malignant phenotype of melanoma brain metastasis. Int. J. Cancer 2014, 136, 1296–1307. [Google Scholar] [CrossRef]
- Izraely, S.; Klein, A.; Sagi-Assif, O.; Meshel, T.; Tsarfaty, G.; Hoon, D.S.; Witz, I.P. Chemokine–chemokine receptor axes in melanoma brain metastasis. Immunol. Lett. 2009, 130, 107–114. [Google Scholar] [CrossRef]
- Adir, O.; Sagi-Assif, O.; Meshel, T.; Ben-Menachem, S.; Pasmanik-Chor, M.; Hoon, D.S.B.; Witz, I.P.; Izraely, S. Heterogeneity in the Metastatic Microenvironment: JunB-Expressing Microglia Cells as Potential Drivers of Melanoma Brain Metastasis Progression. Cancers 2023, 15, 4979. [Google Scholar] [CrossRef]
- Izraely, S.; Ben-Menachem, S.; Malka, S.; Sagi-Assif, O.; Bustos, M.A.; Adir, O.; Meshel, T.; Chelladurai, M.; Ryu, S.; Ramos, R.I.; et al. The Vicious Cycle of Melanoma-Microglia Crosstalk: Inter-Melanoma Variations in the Brain-Metastasis-Promoting IL-6/JAK/STAT3 Signaling Pathway. Cells 2023, 12, 1513. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, J.; Yu, J.; Liu, R.; Ma, H.; Zhao, Y. Nicotinamide mononucleotides alleviated neurological impairment via anti-neuroinflammation in traumatic brain injury. Int. J. Med. Sci. 2023, 20, 307–317. [Google Scholar] [CrossRef]
- Neidert, N.; von Ehr, A.; Zöller, T.; Spittau, B. Microglia-Specific Expression of Olfml3 Is Directly Regulated by Transforming Growth Factor β1-Induced Smad2 Signaling. Front. Immunol. 2018, 9, 1728. [Google Scholar] [CrossRef]
- Quancard, J.; Bollbuck, B.; Janser, P.; Angst, D.; Berst, F.; Buehlmayer, P.; Streiff, M.; Beerli, C.; Brinkmann, V.; Guerini, D.; et al. A Potent and Selective S1P1 Antagonist with Efficacy in Experimental Autoimmune Encephalomyelitis. Chem. Biol. 2012, 19, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 Deficiency Promotes M1 Macrophage Polarization and Inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Yeh, W.-I.; De Sarno, P.; Holdbrooks, A.T.; Liu, Y.; Muldowney, M.T.; Reynolds, S.L.; Yanagisawa, L.L.; Fox, T.H.; Park, K.; et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 5004–5009. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, X.; Zhang, Y.; Wang, Y. The Role of Microglia in Brain Metastases: Mechanisms and Strategies. Aging Dis. 2024, 15, 169–185. [Google Scholar] [CrossRef]
- Carson, W.F.; Salter-Green, S.E.; Scola, M.M.; Joshi, A.; Gallagher, K.A.; Kunkel, S.L. Enhancement of macrophage inflammatory responses by CCL2 is correlated with increased miR-9 expression and downregulation of the ERK1/2 phosphatase Dusp6. Cell. Immunol. 2017, 314, 63–72. [Google Scholar] [CrossRef]
- Fukuda, R.; Beppu, S.; Hinata, D.; Kamada, Y.; Okiyoneda, T. Perturbation of EPHA2 and EFNA1 trans binding amplifies inflammatory response in airway epithelial cells. iScience 2025, 28, 111872. [Google Scholar] [CrossRef]
- Xie, R.; Yuan, M.; Jiang, Y. The Pan-Cancer Crosstalk Between the EFNA Family and Tumor Microenvironment for Prognosis and Immunotherapy of Gastric Cancer. Front. Cell Dev. Biol. 2022, 10, 790947. [Google Scholar] [CrossRef]
- Weigert, A.; Olesch, C.; Brüne, B. Sphingosine-1-Phosphate and Macrophage Biology—How the Sphinx Tames the Big Eater. Front. Immunol. 2019, 10, 1706. [Google Scholar] [CrossRef]
- Zveik, O.; Rechtman, A.; Brill, L.; Vaknin-Dembinsky, A. Anti- and pro-inflammatory milieu differentially regulate differentiation and immune functions of oligodendrocyte progenitor cells. Immunology 2024, 171, 618–633. [Google Scholar] [CrossRef]
- Yonesu, K.; Nakamura, T.; Mizuno, Y.; Suzuki, C.; Nagayama, T.; Satoh, S.; Nara, F. A Novel Sphingosine-1-Phosphate Receptor 1 Antagonist Prevents the Proliferation and Relaxation of Vascular Endothelial Cells by Sphingosine-1-Phosphate. Biol. Pharm. Bull. 2010, 33, 1500–1505. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Zhi, Y.; Song, H.; Zong, M.; Yi, J.; Mao, G.; Chen, L.; Huang, G. S1PR1 promotes proliferation and inhibits apoptosis of esophageal squamous cell carcinoma through activating STAT3 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 369. [Google Scholar] [CrossRef]
- Gaire, B.P.; Lee, C.-H.; Sapkota, A.; Lee, S.Y.; Chun, J.; Cho, H.J.; Nam, T.-G.; Choi, J.W. Identification of Sphingosine 1-Phosphate Receptor Subtype 1 (S1P1) as a Pathogenic Factor in Transient Focal Cerebral Ischemia. Mol. Neurobiol. 2017, 55, 2320–2332. [Google Scholar] [CrossRef]
- Rutherford, C.; Childs, S.; Ohotski, J.; McGlynn, L.; Riddick, M.; MacFarlane, S.; Tasker, D.; Pyne, S.; Pyne, N.J.; Edwards, J.; et al. Regulation of cell survival by sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent suppression of Bim and PI-3-kinase/protein kinase C-mediated upregulation of Mcl-1. Cell Death Dis. 2013, 4, e927. [Google Scholar] [CrossRef]
- Yoshino, T.; Tabunoki, H.; Sugiyama, S.; Ishii, K.; Kim, S.U.; Satoh, J.-I. Non-phosphorylated FTY720 Induces Apoptosis of Human Microglia by Activating SREBP2. Cell. Mol. Neurobiol. 2011, 31, 1009–1020. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Li, M.; Chen, M.; Sun, C. Multifaceted Functions of CH25H and 25HC to Modulate the Lipid Metabolism, Immune Responses, and Broadly Antiviral Activities. Viruses 2020, 12, 727. [Google Scholar] [CrossRef]
- Rostami, N.; Nikkhoo, A.; Ajjoolabady, A.; Azizi, G.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Yousefi, B.; Yousefi, M.; Jadidi-Niaragh, F. S1PR1 as a Novel Promising Therapeutic Target in Cancer Therapy. Mol. Diagn. Ther. 2019, 23, 467–487. [Google Scholar] [CrossRef]
- Shida, D.; Takabe, K.; Kapitonov, D.; Milstien, S.; Spiegel, S. Targeting SphK1 as a New Strategy against Cancer. Curr. Drug Targets 2008, 9, 662–673. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Amereh, M.; Edwards, R.; Akbari, M.; Nadler, B. In-Silico Modeling of Tumor Spheroid Formation and Growth. Micromachines 2021, 12, 749. [Google Scholar] [CrossRef]
- Moshe, A.; Izraely, S.; Sagi-Assif, O.; Malka, S.; Ben-Menachem, S.; Meshel, T.; Pasmanik-Chor, M.; Hoon, D.S.; Witz, I.P. Inter-Tumor Heterogeneity—Melanomas Respond Differently to GM-CSF-Mediated Activation. Cells 2020, 9, 1683. [Google Scholar] [CrossRef]
- Izraely, S.; Ben-Menachem, S.; Sagi-Assif, O.; Meshel, T.; Malka, S.; Telerman, A.; Bustos, M.A.; Ramos, R.I.; Pasmanik-Chor, M.; Hoon, D.S.B.; et al. The melanoma brain metastatic microenvironment: Aldolase C partakes in shaping the malignant phenotype of melanoma cells—A case of inter-tumor heterogeneity. Mol. Oncol. 2020, 15, 1376–1390. [Google Scholar] [CrossRef]
- LaMontagne, K.; Littlewood-Evans, A.; Schnell, C.; O’REilly, T.; Wyder, L.; Sanchez, T.; Probst, B.; Butler, J.; Wood, A.; Liau, G.; et al. Antagonism of Sphingosine-1-Phosphate Receptors by FTY720 Inhibits Angiogenesis and Tumor Vascularization. Cancer Res. 2006, 66, 221–231. [Google Scholar] [CrossRef]
- Fischl, A.S.; Wang, X.; Falcon, B.L.; Almonte-Baldonado, R.; Bodenmiller, D.; Evans, G.; Stewart, J.; Wilson, T.; Hipskind, P.; Manro, J.R.; et al. Inhibition of Sphingosine Phosphate Receptor 1 Signaling Enhances the Efficacy of VEGF Receptor Inhibition. Mol. Cancer Ther. 2019, 18, 856–867. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Kim, G.; McKee, A.E.; Ning, Y.-M.; Hazarika, M.; Theoret, M.; Johnson, J.R.; Xu, Q.C.; Tang, S.; Sridhara, R.; Jiang, X.; et al. FDA Approval Summary: Vemurafenib for Treatment of Unresectable or Metastatic Melanoma with the BRAFV600E Mutation. Clin. Cancer Res. 2014, 20, 4994–5000. [Google Scholar] [CrossRef]
- Khaddour, K.; Kurn, H.; Zito, P.M. Vemurafenib. In StatPearls; StatPearls Publishing: Treasure Island, CA, USA, 2025. [Google Scholar]
- Joyce, J.A.; Pollard, J.W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 2008, 9, 239–252. [Google Scholar] [CrossRef]
- Ghebremedhin, A.; Athavale, D.; Zhang, Y.; Yao, X.; Balch, C.; Song, S. Tumor-Associated Macrophages as Major Immunosuppressive Cells in the Tumor Microenvironment. Cancers 2024, 16, 3410. [Google Scholar] [CrossRef]
- Kuen, J.; Darowski, D.; Kluge, T.; Majety, M.; Ahmad, A. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE 2017, 12, e0182039. [Google Scholar] [CrossRef]
- Park, J.V.; Chandra, R.; Cai, L.; Ganguly, D.; Li, H.; Toombs, J.E.; Girard, L.; Brekken, R.A.; Minna, J.D. Tumor Cells Modulate Macrophage Phenotype in a Novel In Vitro Co-Culture Model of the NSCLC Tumor Microenvironment. J. Thorac. Oncol. 2022, 17, 1178–1191. [Google Scholar] [CrossRef]
- Lovly, C.M.; Salama, A.K.; Salgia, R. Tumor Heterogeneity and Therapeutic Resistance. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2016; Volume 35, pp. e585–e593. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Zhao, S.; Adebiyi, M.G.; Zhang, Y.; Couturier, J.P.; Fan, X.; Zhang, H.; Kellems, R.E.; Lewis, D.E.; Xia, Y. Sphingosine-1-phosphate receptor 1 mediates elevated IL-6 signaling to promote chronic inflammation and multitissue damage in sickle cell disease. FASEB J. 2018, 32, 2855–2865. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhang, H.; Zhang, L.; Cai, T.-T.; Huang, D.-J.; He, J.; Ni, H.-H.; Zhou, F.-J.; Zhang, X.-S.; Li, J. Sphingosine 1 phosphate receptor-1 (S1P1) promotes tumor-associated regulatory T cell expansion: Leading to poor survival in bladder cancer. Cell Death Dis. 2019, 10, 50. [Google Scholar] [CrossRef]
- Mullazehi, M.; Mathsson, L.; Lampa, J.; Rönnelid, J. Surface-bound anti–type II collagen–containing immune complexes induce production of tumor necrosis factor α, interleukin-1β, and interleukin-8 from peripheral blood monocytes via fcγ receptor IIA: A potential pathophysiologic mechanism for humoral anti–type II collagen immunity in arthritis. Arthritis Rheum. 2006, 54, 1759–1771. [Google Scholar] [CrossRef]
- Suzuki, A.; Hanada, T.; Mitsuyama, K.; Yoshida, T.; Kamizono, S.; Hoshino, T.; Kubo, M.; Yamashita, A.; Okabe, M.; Takeda, K.; et al. Cis3/Socs3/Ssi3 Plays a Negative Regulatory Role in Stat3 Activation and Intestinal Inflammation. J. Exp. Med. 2001, 193, 471–482. [Google Scholar] [CrossRef]
- Dees, C.; Pötter, S.; Zhang, Y.; Bergmann, C.; Zhou, X.; Luber, M.; Wohlfahrt, T.; Karouzakis, E.; Ramming, A.; Gelse, K.; et al. TGF-β–induced epigenetic deregulation of SOCS3 facilitates STAT3 signaling to promote fibrosis. J. Clin. Investig. 2020, 130, 2347–2363. [Google Scholar] [CrossRef]
- Yokogami, K.; Yamashita, S.; Takeshima, H. Hypoxia-induced decreases in SOCS3 increase STAT3 activation and upregulate VEGF gene expression. Brain Tumor Pathol. 2012, 30, 135–143. [Google Scholar] [CrossRef]
- Zheng, Z.V.; Chen, J.; Lyu, H.; Lam, S.Y.E.; Lu, G.; Chan, W.Y.; Wong, G.K.C. Novel role of STAT3 in microglia-dependent neuroinflammation after experimental subarachnoid haemorrhage. Stroke Vasc. Neurol. 2021, 7, 62–70. [Google Scholar] [CrossRef]
- Yang, X.; He, G.; Hao, Y.; Chen, C.; Li, M.; Wang, Y.; Zhang, G.; Yu, Z. The role of the JAK2-STAT3 pathway in pro-inflammatory responses of EMF-stimulated N9 microglial cells. J. Neuroinflamm. 2010, 7, 54. [Google Scholar] [CrossRef]
- McFarland, B.C.; Marks, M.P.; Rowse, A.L.; Fehling, S.C.; Gerigk, M.; Qin, H.; Benveniste, E.N. Loss of SOCS3 in myeloid cells prolongs survival in a syngeneic model of glioma. Oncotarget 2016, 7, 20621–20635. [Google Scholar] [CrossRef]
- da Silva, A.B.; Coelho, P.L.C.; Amparo, J.A.O.; Carneiro, M.M.A.d.A.; Borges, J.M.P.; Souza, C.d.S.; Costa, M.d.F.D.; Mecha, M.; Rodriguez, C.G.; da Silva, V.D.A.; et al. The flavonoid rutin modulates microglial/macrophage activation to a CD150/CD206 M2 phenotype. Chem. Interact. 2017, 274, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kowal, K.; Silver, R.; Sławińska, E.; Bielecki, M.; Chyczewski, L.; Kowal-Bielecka, O. CD163 and its role in inflammation. Folia Histochem. Cytobiol. 2011, 49, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice with Intracranial Gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, H.; Chen, B.; Liu, X.; Zhang, S.; Zong, Z.; Gao, M. PD-L1-Mediated Immunosuppression in Glioblastoma Is Associated with the Infiltration and M2-Polarization of Tumor-Associated Macrophages. Front. Immunol. 2020, 11, 588552. [Google Scholar] [CrossRef]
- Maldonado, L.A.G.; Nascimento, C.R.; Fernandes, N.A.R.; Silva, A.L.P.; D’sIlva, N.J.; Rossa, C.R., Jr. Influence of tumor cell-derived TGF-β on macrophage phenotype and macrophage-mediated tumor cell invasion. Int. J. Biochem. Cell Biol. 2022, 153, 106330. [Google Scholar] [CrossRef] [PubMed]
- Gratchev, A. TGF-β signalling in tumour associated macrophages. Immunobiology 2017, 222, 75–81. [Google Scholar] [CrossRef]
- McCartney-Francis, N.L.; Wahl, S.M. Transforming growth factor β: A matter of life and death. J. Leukoc. Biol. 1994, 55, 401–409. [Google Scholar] [CrossRef]
- Senft, D.; Berking, C.; Graf, S.A.; Kammerbauer, C.; Ruzicka, T.; Besch, R.; Sanchis, D. Selective Induction of Cell Death in Melanoma Cell Lines through Targeting of Mcl-1 and A1. PLoS ONE 2012, 7, e30821. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sada-Ovalle, I.; Nishimura, T.; Anderson, A.C.; Kuchroo, V.K.; Remold, H.G.; Behar, S.M. IL-1β Promotes Antimicrobial Immunity in Macrophages by Regulating TNFR Signaling and Caspase-3 Activation. J. Immunol. 2013, 190, 4196–4204. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, F.A.; Levy, D.; Reichert, C.O.; Cunha-Neto, E.; Kalil, J.; Bydlowski, S.P. Effects of Oxysterols on Immune Cells and Related Diseases. Cells 2022, 11, 1251. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Ikeda, Y.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA 2007, 104, 6511–6518. [Google Scholar] [CrossRef]
- Dominiak, A.; Chełstowska, B.; Olejarz, W.; Nowicka, G. Communication in the Cancer Microenvironment as a Target for Therapeutic Interventions. Cancers 2020, 12, 1232. [Google Scholar] [CrossRef]
- Pérez, C.N.; Falcón, C.R.; Mons, J.D.; Orlandi, F.C.; Sangiacomo, M.; Fernandez-Muñoz, J.M.; Guerrero, M.; Benito, P.G.; Colombo, M.I.; Zoppino, F.C.; et al. Melanoma cells with acquired resistance to vemurafenib have decreased autophagic flux and display enhanced ability to transfer resistance. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166801. [Google Scholar] [CrossRef]
- Garandeau, D.; Noujarède, J.; Leclerc, J.; Imbert, C.; Garcia, V.; Bats, M.-L.; Rambow, F.; Gilhodes, J.; Filleron, T.; Meyer, N.; et al. Targeting the Sphingosine 1-Phosphate Axis Exerts Potent Antitumor Activity in BRAFi-Resistant Melanomas. Mol. Cancer Ther. 2019, 18, 289–300. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adir, O.; Sagi-Assif, O.; Ben-Menachem, S.; Witz, I.P.; Izraely, S. The S1P/S1P1 Signaling Axis Plays Regulatory Functions in the Crosstalk Between Brain-Metastasizing Melanoma Cells and Microglia. Cancers 2025, 17, 3175. https://doi.org/10.3390/cancers17193175
Adir O, Sagi-Assif O, Ben-Menachem S, Witz IP, Izraely S. The S1P/S1P1 Signaling Axis Plays Regulatory Functions in the Crosstalk Between Brain-Metastasizing Melanoma Cells and Microglia. Cancers. 2025; 17(19):3175. https://doi.org/10.3390/cancers17193175
Chicago/Turabian StyleAdir, Orit, Orit Sagi-Assif, Shlomit Ben-Menachem, Isaac P. Witz, and Sivan Izraely. 2025. "The S1P/S1P1 Signaling Axis Plays Regulatory Functions in the Crosstalk Between Brain-Metastasizing Melanoma Cells and Microglia" Cancers 17, no. 19: 3175. https://doi.org/10.3390/cancers17193175
APA StyleAdir, O., Sagi-Assif, O., Ben-Menachem, S., Witz, I. P., & Izraely, S. (2025). The S1P/S1P1 Signaling Axis Plays Regulatory Functions in the Crosstalk Between Brain-Metastasizing Melanoma Cells and Microglia. Cancers, 17(19), 3175. https://doi.org/10.3390/cancers17193175








