Novel MAML2 Fusions in Human Malignancy †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Compliance Statement
2.2. DNA Sequencing
2.3. Whole Transcriptome Sequencing
2.4. Loss of Heterozygosity
2.5. Statistical Analysis
3. Results
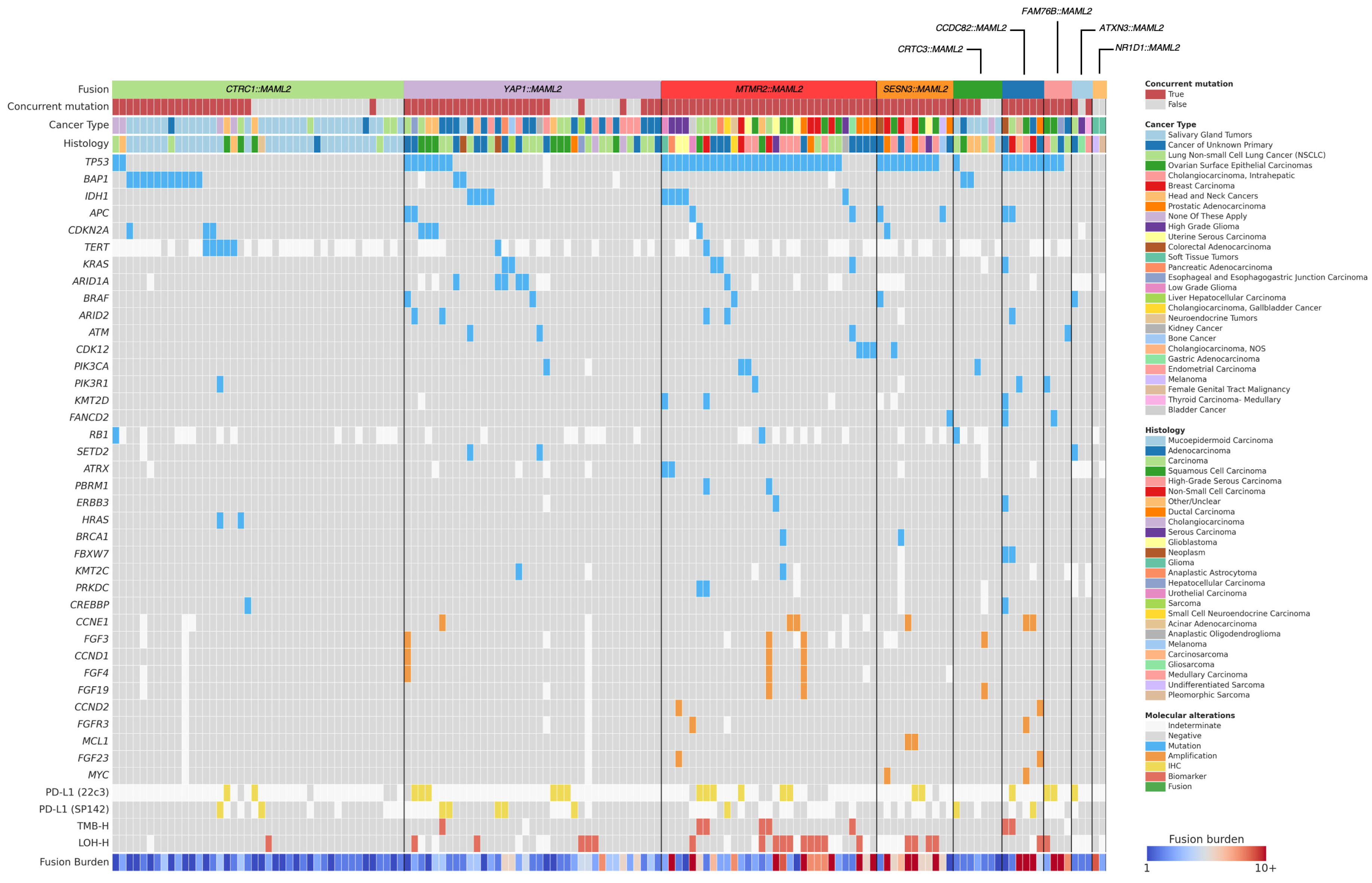
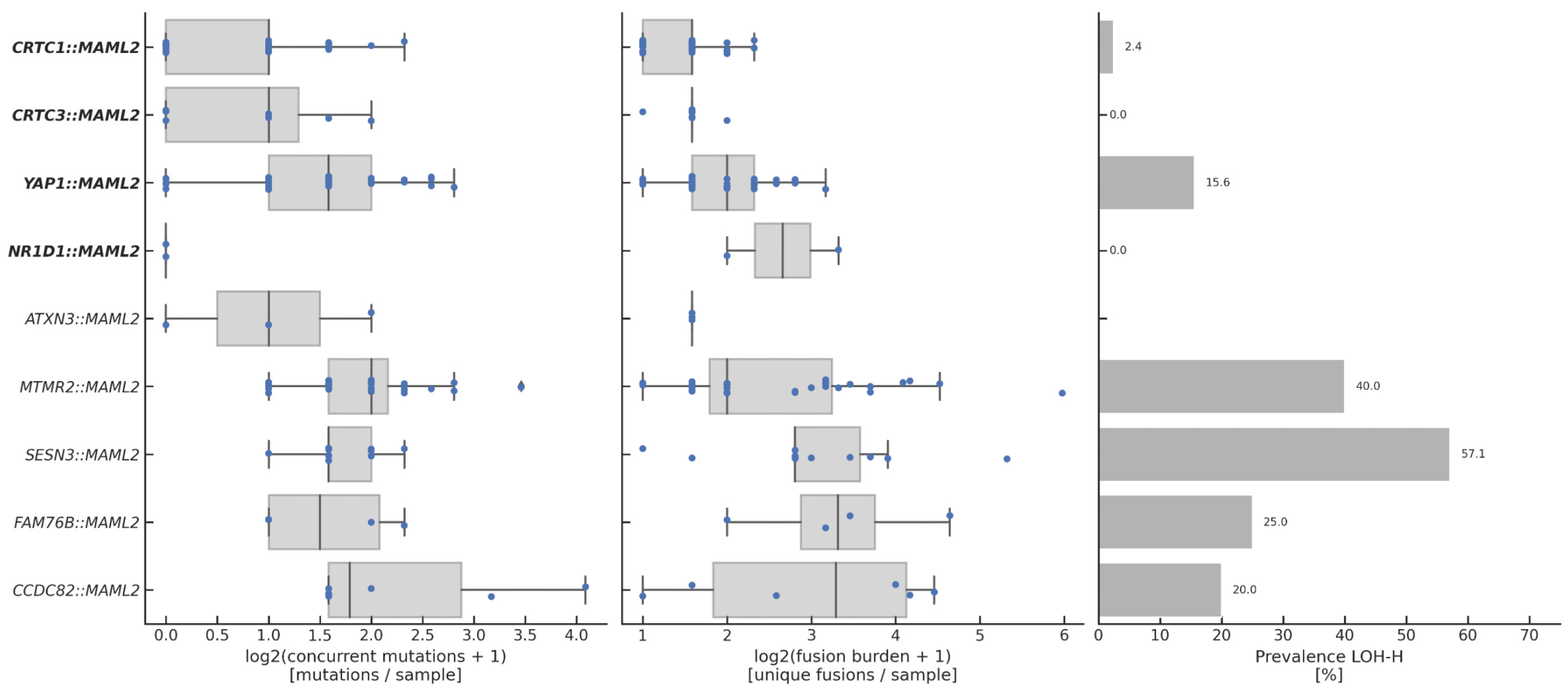
3.1. Overview of Fusions
3.2. Clinical Characteristics of Fusion-Positive Patients
3.3. Expression of MAML2 Fusions
3.4. Genomic Landscape of Fusion-Positive Tumors
4. Discussion
Translational/Clinical Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IRB | Institutional Review Board |
| MECT | Mucoepidermoid carcinoma |
| MAML2 | Mastermind-like 2 |
References
- Wu, L.; Sun, T.; Kobayashi, K.; Gao, P.; Griffin, J.D. Identification of a Family of Mastermind-Like Transcriptional Coactivators for Mammalian Notch Receptors. Mol. Cell. Biol. 2002, 22, 7688–7700. [Google Scholar] [CrossRef]
- Tonon, G.; Modi, S.; Wu, L.; Kubo, A.; Coxon, A.B.; Komiya, T.; O’Neil, K.; Stover, K.; El-Nagger, A.; Griffin, J.D.; et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat. Genet. 2003, 33, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Saade, R.E.; Bell, D.; Garcia, J.; Roberts, D.; Weber, R. Role of CRTC1/MAML2 Translocation in the Prognosis and Clinical Outcomes of Mucoepidermoid Carcinoma. JAMA Otolaryngol.–Head. Neck Surg. 2016, 142, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Komiya, T.; Park, Y.; Modi, S.; Coxon, A.B.; Oh, H.; Kaye, F.J. Sustained expression of Mect1-Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene 2006, 25, 6128–6132. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, S.; Li, J.-L.; Ni, W.; Guo, R.; Lu, J.; Kaye, F.J.; Wu, L. CRTC1-MAML2 fusion-induced lncRNA LINC00473 expression maintains the growth and survival of human mucoepidermoid carcinoma cells. Oncogene 2018, 37, 1885–1895. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, W.; Li, J.-L.; Lin, S.; Zhou, X.; Sun, Y.; Li, J.W.; Leon, M.E.; Hurtado, M.D.; Zolotukhin, S.; et al. The CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight 2021, 6, e139497. [Google Scholar] [CrossRef]
- Coxon, A.; Rozenblum, E.; Park, Y.-S.; Joshi, N.; Tsurutani, J.; Dennis, P.A.; Kirsch, I.R.; Kaye, F.J. Mect1-Maml2 Fusion Oncogene Linked to the Aberrant Activation of Cyclic AMP/CREB Regulated Genes. Cancer Res. 2005, 65, 7137–7144. [Google Scholar] [CrossRef]
- Birkeland, A.C.; Foltin, S.K.; Michmerhuizen, N.L.; Hoesli, R.C.; Rosko, A.J.; Byrd, S.; Yanik, M.; Nor, J.E.; Bradford, C.R.; Prince, M.E.; et al. Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral. Oncol. 2017, 68, 5–8. [Google Scholar] [CrossRef]
- Fehr, A.; Röser, K.; Heidorn, K.; Hallas, C.; Löning, T.; Bullerdiek, J. A new type of MAML2 fusion in mucoepidermoid carcinoma. Genes. Chromosomes Cancer 2007, 47, 203–206. [Google Scholar] [CrossRef]
- Valouev, A.; Weng, Z.; Sweeney, R.T.; Varma, S.; Le, Q.T.; Kong, C.; Sidow, A.; West, R.B. Discovery of recurrent structural variants in nasopharyngeal carcinoma. Genome Res. 2014, 24, 300–309. [Google Scholar] [CrossRef]
- Vivero, M.; Davineni, P.; Nardi, V.; Chan, J.K.C.; Sholl, L.M. Metaplastic thymoma: A distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Lab. Investig. 2019, 33, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Kiyono, T.; Ryo, E.; Ogawa, R.; Wakai, S.; Ichikawa, H.; Suzuki, K.; Arai, S.; Tsuta, K.; Ishida, M.; et al. Recurrent YAP1-MAML2 and YAP1-NUTM1 fusions in poroma and porocarcinoma. J. Clin. Investig. 2019, 129, 3827–3832. [Google Scholar] [CrossRef] [PubMed]
- Dermawan, J.K.; DiNapoli, S.E.; Sukhadia, P.; Mullaney, K.A.; Gladdy, R.; Healey, J.H.; Agaimy, A.; Cleven, A.H.; Suurmeijer, A.J.H.; Dickson, B.C.; et al. Malignant undifferentiated epithelioid neoplasms with MAML2 rearrangements: A clinicopathologic study of seven cases demonstrating a heterogenous entity. Genes. Chromosomes Cancer 2023, 62, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Tauziède-Espariat, A.; Siegfried, A.; Nicaise, Y.; Figarella-Branger, D.; Appay, R.; Senova, S.; Bochaton, D.; Hasty, L.; Martin, A.; Chrétien, F.; et al. A novel YAP1-MAML2 fusion in an adult supra-tentorial ependymoma, YAP1-fused. Brain Tumor Pathol. 2022, 39, 240–242. [Google Scholar] [CrossRef]
- Yu, F.-X.; Zhao, B.; Guan, K.-L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Ni, W.; Yu, M.; Yang, R.; Li, J.W.; Zhou, X.; Calbay, O.; Pi, L.; Lu, J.; Huang, S.; Wu, L.; et al. The YAP1-MAML2 fusion drives tumorigenesis and sustains tumor growth. Mol. Ther. Oncol. 2024, 32, 200900. [Google Scholar] [CrossRef]
- Acunzo, M.; Romano, G.; Wernicke, D.; Balatti, V.; Rassenti, L.Z.; Dell’aQuila, M.; Kipps, T.J.; Pekarsky, Y.; Croce, C.M. Translocation t(2;11) in CLL cells results in CXCR4/MAML2 fusion oncogene. Blood 2014, 124, 259–262. [Google Scholar] [CrossRef]
- Massoth, L.R.; Hung, Y.P.; Dias-Santagata, D.; Onozato, M.; Shah, N.; Severson, E.; Duncan, D.; Gillespie, B.J.; Williams, N.F.; Ross, J.S.; et al. Pan-Cancer Landscape Analysis Reveals Recurrent KMT2A-MAML2 Gene Fusion in Aggressive Histologic Subtypes of Thymoma. JCO Precis. Oncol. 2020, 4, 109–115. [Google Scholar] [CrossRef]
- Oon, M.L.; Hendriansyah, L.; Pratiseyo, P.D.; Wahjoepramono, E.; Goh, J.Y.; Kuick, C.H.; Chang, K.T.; Perry, A.; Tan, C.L. The Multifaceted Appearance of Supratentorial Ependymoma with ZFTA-MAML2 Fusion. Free Neuropathol. 2021, 2, 24. [Google Scholar] [CrossRef]
- Zschernack, V.; Jünger, S.T.; Mynarek, M.; Rutkowski, S.; Garre, M.L.; Ebinger, M.; Neu, M.; Faber, J.; Erdlenbruch, B.; Claviez, A.; et al. Supratentorial ependymoma in childhood: More than just RELA or YAP. Acta Neuropathol. 2021, 141, 455–466. [Google Scholar] [CrossRef]
- Tamai, S.; Nakano, Y.; Kinoshita, M.; Sabit, H.; Nobusawa, S.; Arai, Y.; Hama, N.; Totoki, Y.; Shibata, T.; Ichimura, K.; et al. Ependymoma with C11orf95-MAML2 fusion: Presenting with granular cell and ganglion cell features. Brain Tumor Pathol. 2021, 38, 64–70. [Google Scholar] [CrossRef]
- Warmke, L.M.; Collier, C.D.; Davis, J.L. NR1D1::MAML1 epithelioid and spindle cell sarcoma mimicking pseudomyogenic hemangioendothelioma in core biopsy: A case report and review of the literature. Genes. Chromosomes Cancer 2023, 62, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carralero, E.; Cabrera, E.; Rodríguez-Torres, G.; Hernández-Reyes, Y.; Singh, A.N.; Santa-María, C.; Fernan-dez-Justel, J.M.; Janssens, R.C.; Marteijn, J.A.; Evert, B.O.; et al. ATXN3 controls DNA replication and transcription by reg-ulating chromatin structure. Nucleic Acids Res. 2023, 51, 5396–5413. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.F.; Tang, T.T. Targeting the Hippo pathway in cancer. Nat. Rev. Drug Discov. 2025; ahead of print. [Google Scholar] [CrossRef]
- Lao, Z.; Chen, X.; Pan, B.; Fang, B.; Yang, W.; Qian, Y. Pharmacological regulators of Hippo pathway: Advances and challenges of drug development. FASEB J. 2025, 39, e70438. [Google Scholar] [CrossRef] [PubMed]
- Hagenbeek, T.J.; Zbieg, J.R.; Hafner, M.; Mroue, R.; Lacap, J.A.; Sodir, N.M.; Noland, C.L.; Afghani, S.; Kishore, A.; Bhat, K.P.; et al. An allosteric pan-TEAD inhibitor blocks oncogenic YAP/TAZ signaling and overcomes KRAS G12C inhibitor resistance. Nature Cancer 2023, 4, 812–828. [Google Scholar] [CrossRef]
- Yap, T.A.; Kwiatkowski, D.J.; Desai, J.; Dagogo-Jack, I.; Millward, M.; Kindler, H.L.; Tolcher, A.W.; Frentzas, S.; Thurston, A.W.; Post, L.; et al. First-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of yes-associated protein (YAP)/transcriptional enhancer activator domain (TEAD), in patients (pts) with advanced solid tumors enriched for malig-nant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations. In Proceedings of the Annual Meeting of American Association for Cancer Research, Orlando, FL, USA, 15 April 2023; Volume 83 (Suppl. 8), p. CT006. [Google Scholar]
- Schöls, L.; Vieira-Saecker, A.M.M.; Schöls, S.; Przuntek, H.; Epplen, J.T.; Riess, O. Trinucleotide expansion within the MJD1 gene presents clinically as spinocerebellar ataxia and occurs most frequently in German SCA patients. Hum. Mol. Genet. 1995, 4, 1001–1005. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Han, X.; Cheng, Y.; Zhao, E.; Brat, D.J.; Sun, Z.; Fang, D. ATXN3 deubiquitinates YAP1 to promote tumor growth. Am. J. Cancer Res. 2023, 13, 4222–4234. [Google Scholar]
- Lee, J.W.; Hruban, R.H.; Brosens, L.A.A.; Condello, V.; Nikiforova, M.N.; Singhi, A.D.; Tucker, J.; Zureikat, A.H.; He, J.; Paniccia, A.; et al. RNA Sequencing Identifies Frequent Mitogen-activated Protein Kinase–associated Fusion Genes in Intraductal Tubulopapillary Neoplasms of the Pancreas. Gastroenterology 2023, 164, 1310–1313.e6. [Google Scholar] [CrossRef]
- Komiya, T.; Sweeney, K.; Huang, C.H.; Crymes, A.; Wang, B.; Antonarakis, E.S.; Elliott, A.; Oberley, M.J.; Evans, M.G. Novel MAML2 fusions as oncogenic drivers in human malignancy. J. Clin. Oncol. 2025, 43, e15067. [Google Scholar] [CrossRef]

| Fusion | Count | Type | Partner Location | Mechanism | Partner Function |
|---|---|---|---|---|---|
| CRTC::MAML2 | 42 | Pathogenic | 19p13.11 | Translocation | Transcriptional activator |
| YAP1::MAML2 | 37 | Pathogenic | 11q.22.1 | Inversion | Transcriptional activator/repressor |
| MTMR2::MAML2 | 31 | Unclassified | 11q21 | Duplication | Lipid phosphatase |
| SESN3::MAM22.1L2 | 11 | Unclassified | 11q21 | Duplication | Leucine sensor |
| CRTC3::MAML2 | 7 | Pathogenic | 15q26.1 | Translocation | Transcriptional activator |
| CCDC82::MAML2 | 6 | Unclassified | 11q21 | Deletion | Unknown |
| FAM76B::MAML2 | 4 | Unclassified | 11q21 | Duplication | Inhibits NF-κB signaling by preventing nuclear translocation of HNRNPA2B1 |
| ATXN3::MAML2 | 3 | Unclassified | 14q32.12 | Translocation | Deubiquitinating enzyme involved in transcriptional regulation |
| NR1D1::MAML2 | 2 | Likely Pathogenic | 17q21.1 | Translocation | Transcriptional repressor |
| Fusion | Cancer Type | Histology |
|---|---|---|
| CRTC1::MAML2 | Salivary Gland Tumors: 71.4% (30/42) Lung Non-small Cell Lung Cancer (NSCLC): 9.5% (4/42) None Of These Apply: 9.5% (4/42) Cancer of Unknown Primary: 4.8% (2/42) Head and Neck Cancers: 4.8% (2/42) | Mucoepidermoid Carcinoma: 76.2% (32/42) Carcinoma: 7.1% (3/42) Squamous Cell Carcinoma: 7.1% (3/42) Other/Unclear: 4.8% (2/42) Acinar Adenocarcinoma: 2.4% (1/42) Adenocarcinoma: 2.4% (1/42) |
| YAP1::MAML2 | Cancer of Unknown Primary: 40.5% (15/37) Cholangiocarcinoma, Intrahepatic: 21.6% (8/37) Lung Non-small Cell Lung Cancer (NSCLC): 10.8% (4/37) Head and Neck Cancers: 8.1% (3/37) Kidney Cancer: 2.7% (1/37) Liver Hepatocellular Carcinoma: 2.7% (1/37) Esophageal and Esophagogastric Junction Carcinoma: 2.7% (1/37) Pancreatic Adenocarcinoma: 2.7% (1/37) Salivary Gland Tumors: 2.7% (1/37) Cholangiocarcinoma, NOS: 2.7% (1/37) Bone Cancer: 2.7% (1/37) | Carcinoma: 35.1% (13/37) Adenocarcinoma: 27.0% (10/37) Squamous Cell Carcinoma: 18.9% (7/37) Cholangiocarcinoma: 5.4% (2/37) Neoplasm: 5.4% (2/37) Ductal Carcinoma: 2.7% (1/37) Hepatocellular Carcinoma: 2.7% (1/37) Sarcoma: 2.7% (1/37) |
| MTMR2::MAML2 | Ovarian Surface Epithelial Carcinomas: 16.1% (5/31) Breast Carcinoma: 12.9% (4/31) High-Grade Glioma: 12.9% (4/31) Lung Non-small Cell Lung Cancer (NSCLC): 12.9% (4/31) Prostatic Adenocarcinoma: 12.9% (4/31) Uterine Serous Carcinoma: 9.7% (3/31) Pancreatic Adenocarcinoma: 3.2% (1/31) Neuroendocrine Tumors: 3.2% (1/31) Low-Grade Glioma: 3.2% (1/31) Head and Neck Cancers: 3.2% (1/31) Gastric Adenocarcinoma: 3.2% (1/31) Cholangiocarcinoma, Gallbladder Cancer: 3.2% (1/31) Bladder Cancer: 3.2% (1/31) | Adenocarcinoma: 25.8% (8/31) High-Grade Serous Carcinoma: 19.4% (6/31) Non-Small Cell Carcinoma: 9.7% (3/31) Carcinoma: 9.7% (3/31) Glioblastoma: 6.5% (2/31) Squamous Cell Carcinoma: 6.5% (2/31) Anaplastic Astrocytoma: 3.2% (1/31) Anaplastic Oligodendroglioma: 3.2% (1/31) Ductal Carcinoma: 3.2% (1/31) Glioma: 3.2% (1/31) Serous Carcinoma: 3.2% (1/31) Small Cell Neuroendocrine Carcinoma: 3.2% (1/31) Urothelial Carcinoma: 3.2% (1/31) |
| SESN3::MAM22.1L2 | Breast Carcinoma: 27.3% (3/11) Ovarian Surface Epithelial Carcinomas: 27.3% (3/11) Colorectal Adenocarcinoma: 9.1% (1/11) Endometrial Carcinoma: 9.1% (1/11) Melanoma: 9.1% (1/11) Prostatic Adenocarcinoma: 9.1% (1/11) Uterine Serous Carcinoma: 9.1% (1/11) | High-Grade Serous Carcinoma: 36.4% (4/11) Adenocarcinoma: 27.3% (3/11) Ductal Carcinoma: 18.2% (2/11) Melanoma: 9.1% (1/11) Serous Carcinoma: 9.1% (1/11) |
| CRTC3::MAML2 | Salivary Gland Tumors: 71.4% (5/7) Cancer of Unknown Primary: 14.3% (1/7) None Of These Apply: 14.3% (1/7) | Other/Unclear: 42.9% (3/7) Carcinoma: 28.6% (2/7) Mucoepidermoid Carcinoma: 14.3% (1/7) Squamous Cell Carcinoma: 14.3% (1/7) |
| CCDC82::MAML2 | Cancer of Unknown Primary: 16.7% (1/6) Colorectal Adenocarcinoma: 16.7% (1/6) Female Genital Tract Malignancy: 16.7% (1/6) Lung Non-small Cell Lung Cancer (NSCLC): 16.7% (1/6) Ovarian Surface Epithelial Carcinomas: 16.7% (1/6) Prostatic Adenocarcinoma: 16.7% (1/6) | Adenocarcinoma: 33.3% (2/6) Non-Small Cell Carcinoma: 33.3% (2/6) Carcinosarcoma: 16.7% (1/6) High-Grade Serous Carcinoma: 16.7% (1/6) |
| FAM76B::MAML2 | Ovarian Surface Epithelial Carcinomas: 50.0% (2/4) Cancer of Unknown Primary: 25.0% (1/4) Esophageal and Esophagogastric Junction Carcinoma: 25.0% (1/4) | Carcinoma: 50.0% (2/4) Adenocarcinoma: 25.0% (1/4) High-Grade Serous Carcinoma: 25.0% (1/4) |
| ATXN3::MAML2 | High-Grade Glioma: 33.3% (1/3) Lung Non-small Cell Lung Cancer (NSCLC): 33.3% (1/3) Thyroid Carcinoma- Medullary: 33.3% (1/3) | Adenocarcinoma: 33.3% (1/3) Gliosarcoma: 33.3% (1/3) Medullary Carcinoma: 33.3% (1/3) |
| NR1D1::MAML2 | Soft Tissue Tumors: 100.0% (2/2) | Pleomorphic Sarcoma: 50.0% (1/2) Undifferentiated Sarcoma: 50.0% (1/2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komiya, T.; Sweeney, K.; Huang, C.H.; Crymes, A.; Antonarakis, E.S.; Elliott, A.; Oberley, M.J.; Evans, M.G. Novel MAML2 Fusions in Human Malignancy. Cancers 2025, 17, 3146. https://doi.org/10.3390/cancers17193146
Komiya T, Sweeney K, Huang CH, Crymes A, Antonarakis ES, Elliott A, Oberley MJ, Evans MG. Novel MAML2 Fusions in Human Malignancy. Cancers. 2025; 17(19):3146. https://doi.org/10.3390/cancers17193146
Chicago/Turabian StyleKomiya, Takefumi, Kieran Sweeney, Chao H. Huang, Anthony Crymes, Emmanuel S. Antonarakis, Andrew Elliott, Matthew J. Oberley, and Mark G. Evans. 2025. "Novel MAML2 Fusions in Human Malignancy" Cancers 17, no. 19: 3146. https://doi.org/10.3390/cancers17193146
APA StyleKomiya, T., Sweeney, K., Huang, C. H., Crymes, A., Antonarakis, E. S., Elliott, A., Oberley, M. J., & Evans, M. G. (2025). Novel MAML2 Fusions in Human Malignancy. Cancers, 17(19), 3146. https://doi.org/10.3390/cancers17193146






