Population-Based Survival of Childhood and Adolescent Cancers (0–19 Years) in Madrid: Analysis by Sex, Age, Tumour Type, and Stage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Follow-Up
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AYA | Adolescent and young adult |
| CM | Community of Madrid |
| CNS | Central Nervous System |
| ICCC-3 | International Classification of Childhood Cancer 3rd edition |
| INDEF | National Death Index (by its acronym in Spanish) |
| PCRM | Population-Based Cancer Registry for Childhood and Adolescence of the Community of Madrid |
References
- López-González, R.; Parra-Blázquez, D.; Moñino, D.; Pino-Rosón, C.; Pollán, M.; Aragonés, N. Cancer incidence and stage at diagnosis in children and adolescents in the Community of Madrid, 2015–2018. Paediatr. Perinat. Epidemiol. 2024, 39, 445–455. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística. Defunciones por Causas (Lista Reducida) por Sexo y Grupos de Edad. Available online: https://www.ine.es/jaxiT3/Tabla.htm?t=7947 (accessed on 25 September 2024).
- Botta, L.; Gatta, G.; Capocaccia, R.; Stiller, C.; Cañete, A.; Dal Maso, L.; Innos, K.; Mihor, A.; Erdmann, F.; Spix, C.; et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): Results from a population-based study. Lancet Oncol. 2022, 23, 1525–1536. [Google Scholar] [CrossRef]
- World Health Organization. Childhood Cancer Inequalities in the WHO European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; p. 115. [Google Scholar]
- Vera López, I.; Gandarillas Grande, A.; Díez-Gañán, L.; Zorrilla Torras, B. Mortalidad por cáncer en niños y adolescentes de la Comunidad de Madrid, 1977–2001. An. Pediatría 2005, 62, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Pilas, M.; Toldos, Ó.; Muñoz, A.M.; Salamanca, J. Childhood cancer in a tertiary care hospital in Madrid. Evolution of survival. Years 1999–2016. An. Pediatr. 2020, 93, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Registro Español de Tumores Infantiles (RETI-SEHOP). Estimación de la Supervivencia del Cáncer Infantil (0-14 Años) en España, de los Casos Incidentes en 2019–2021, Con el Método Periodo; Julio 2024; Ministerio de Sanidad y Universidad de Valencia: Valencia, Spain, 2025.
- European Network of Cancer Registries. Registro Poblacional de Cáncer en la Infancia y Adolescencia de la Comunidad de Madrid. Available online: https://www.encr.eu/node/500 (accessed on 27 April 2025).
- Servicio de Vigilancia y Registro de Cáncer. Registro Poblacional de Cáncer en la Infancia y Adolescencia de la Comunidad de Madrid (RECAM-i). Manual de Procedimientos. Available online: https://www.comunidad.madrid/sites/default/files/doc/sanidad/epid/manual_de_procedimientos_recam-i.pdf (accessed on 26 March 2025).
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International Classification of Childhood Cancer, third edition. Cancer 2005, 103, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad, Consumo y Bienestar Social. INDEF Índice Nacional de Defunciones: Manual de Usuario. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/docs/indNacDefunciones/Manual_INDEF_2019.pdf (accessed on 21 October 2024).
- Gupta, S.; Aitken, J.F.; Bartels, U.; Brierley, J.; Dolendo, M.; Friedrich, P.; Fuentes-Alabi, S.; Garrido, C.P.; Gatta, G.; Gospodarowicz, M.; et al. Paediatric cancer stage in population-based cancer registries: The Toronto consensus principles and guidelines. Lancet Oncol. 2016, 17, e163–e172. [Google Scholar] [CrossRef]
- Gupta, S.; Aitken, J.; Bartels, U.; Bhakta, N.; Bucurenci, M.; Brierley, J.D.; De Camargo, B.; Chokunonga, E.; Clymer, J.; Coza, D.; et al. Development of paediatric non-stage prognosticator guidelines for population-based cancer registries and updates to the 2014 Toronto Paediatric Cancer Stage Guidelines. Lancet Oncol. 2020, 21, e444–e451. [Google Scholar] [CrossRef]
- Australian Government—Cancer Australia. Five-Year Relative Survival by Stage at Diagnosis for Childhood Cancers. Available online: https://ncci.canceraustralia.gov.au/outcomes/survival-stage-diagnosis-childhood-cancers/five-year-relative-survival-stage-diagnosis (accessed on 14 September 2022).
- Ohlsen, T.J.D.; Martos, M.R.; Hawkins, D.S. Recent advances in the treatment of childhood cancers. Curr. Opin. Pediatr. 2024, 36, 57–63. [Google Scholar] [CrossRef]
- Pritchard-Jones, K.; Pieters, R.; Reaman, G.H.; Hjorth, L.; Downie, P.; Calaminus, G.; Naafs-Wilstra, M.C.; Steliarova-Foucher, E. Sustaining innovation and improvement in the treatment of childhood cancer: Lessons from high-income countries. Lancet Oncol. 2013, 14, e95–e103. [Google Scholar] [CrossRef]
- Pearce, M.S.; Parker, L. Childhood cancer registrations in the developing world: Still more boys than girls. Int. J. Cancer 2001, 91, 402–406. [Google Scholar] [CrossRef]
- Close, A.G.; Dreyzin, A.; Miller, K.D.; Seynnaeve, B.K.N.; Rapkin, L.B. Adolescent and young adult oncology-past, present, and future. CA Cancer J. Clin. 2019, 69, 485–496. [Google Scholar] [CrossRef]
- Siegel, S.E.; Stock, W.; Johnson, R.H.; Advani, A.; Muffly, L.; Douer, D.; Reed, D.; Lewis, M.; Freyer, D.R.; Shah, B.; et al. Pediatric-Inspired Treatment Regimens for Adolescents and Young Adults With Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia: A Review. JAMA Oncol. 2018, 4, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Cash, T.; Qayed, M.; Ward, K.C.; Mertens, A.C.; Rapkin, L. Comparison of survival at adult versus pediatric treatment centers for rare pediatric tumors in an adolescent and young adult (AYA) population in the State of Georgia. Pediatr. Blood Cancer 2015, 62, 456–462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Desandes, E.; Brugieres, L.; Laurence, V.; Berger, C.; Kanold, J.; Tron, I.; Clavel, J.; Lacour, B. Survival of adolescents with cancer treated at pediatric versus adult oncology treatment centers in France. Pediatr. Blood Cancer 2017, 64, e26326. [Google Scholar] [CrossRef] [PubMed]
- Garrido Colino, C.; Andión Catalán, M.; Molinés Honrubia, A.; Ortega Acosta, M.J.; García Abos, M.; Juan Ribelles, A.; Vagace Valero, J.M. Adolescent cancer care: What has changed in Spain in the past decade? An. Pediatría Engl. Ed. 2023, 98, 129–135. [Google Scholar] [CrossRef]
- Gatta, G.; Capocaccia, R.; De Angelis, R.; Stiller, C.; Coebergh, J.W. Cancer survival in European adolescents and young adults. Eur. J. Cancer 2003, 39, 2600–2610. [Google Scholar] [CrossRef]
- Park, M.; Lim, J.; Lee, J.A.; Park, B.K.; Jung, K.W.; Won, Y.J.; Park, H.J. Cancer Incidence and Survival among Adolescents and Young Adults in Korea: An Update for 2016. Cancer Res. Treat. 2021, 53, 32–44. [Google Scholar] [CrossRef]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef]
- Gatta, G.; Botta, L.; Capocaccia, R.; Cañete, A.; Pritchard-Jones, K. Staging childhood cancers in Europe: Application of the Toronto stage principles for neuroblastoma and Wilms tumour. The JARC pilot study. Pediatr. Blood Cancer 2021, 68, e29020. [Google Scholar] [CrossRef]
- Hoogendijk, R.; van der Lugt, J.; van Vuurden, D.; Kremer, L.; Wesseling, P.; Hoving, E.; Karim-Kos, H.E. Survival rates of children and young adolescents with CNS tumors improved in the Netherlands since 1990: A population-based study. Neurooncol. Adv. 2022, 4, vdab183. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Cheng, S.; Cochrane, D.D. Improving diagnosis of pediatric central nervous system tumours: Aiming for early detection. CMAJ 2017, 189, E459–E463. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Salud Pública. Boletín Epidemiológico. Número 3. Volumen 28. Supervivencia de la Población Infantil y Adolescente con Cáncer de la Comunidad de Madrid, 2015–2018; Consejería de Sanidad: Madrid, Spain, 2023. Available online: https://www.comunidad.madrid/publicacion/ref/50912 (accessed on 2 March 2023).
- Businge, L.; Hagenimana, M.; Motlhale, M.; Bardot, A.; Liu, B.; Anastos, K.; Castle, P.E.; Murenzi, G.; Claire, K.; Sabushimike, D.; et al. Stage at diagnosis and survival by stage for the leading childhood cancers in Rwanda. Pediatr. Blood Cancer 2024, 71, e31020. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Youlden, D.R.; Chitsike, I.; Chokunonga, E.; Couitchéré, L.; Gnahatin, F.; Nambooze, S.; Wabinga, H.; Aitken, J.F. Stage at diagnosis and survival by stage for the leading childhood cancers in three populations of sub-Saharan Africa. Int. J. Cancer 2021, 148, 2685–2691. [Google Scholar] [CrossRef]
- Joko-Fru, W.Y.; Bardot, A.; Bukirwa, P.; Amidou, S.; N’da, G.; Woldetsadik, E.; Chesumbai, G.; Korir, A.; Kamaté, B.; Koon, M.; et al. Cancer survival in sub-Saharan Africa (SURVCAN-3): A population-based study. Lancet Glob. Health 2024, 12, e947–e959. [Google Scholar] [CrossRef]
- Kitur, G.; Mbandar, F.; Muyodi, D.; Asirwa, C. Addressing the Barrier of Physical Access to Oncology Care in Sub-Saharan Africa (SSA) Through Establishment of Short-Stay Homes for Cancer Patients: A Case of International Cancer Institute, Child and Family Wellness Center in Eldoret, Kenya. JCO Glob. Oncol. 2022, 8, 10. [Google Scholar] [CrossRef]
- Omotoso, O.; Teibo, J.O.; Atiba, F.A.; Oladimeji, T.; Paimo, O.K.; Ataya, F.S.; Batiha, G.E.-S.; Alexiou, A. Addressing cancer care inequities in sub-Saharan Africa: Current challenges and proposed solutions. Int. J. Equity Health 2023, 22, 189. [Google Scholar] [CrossRef]
- Bhakta, N.; Rodriguez-Galindo, C. The Toronto Guidelines: A practical means for childhood cancer staging. Lancet Child. Adolesc. Health 2018, 2, 158–159. [Google Scholar] [CrossRef]
- Costantini, A.; Michels, F.S.; Ruhl, J.; Hill, S.; Kohler, B.; Negoita, S. The Trajectory of Pediatric Cancer Data and Collection in the United States. J. Registry Manag. 2023, 50, 82–84. [Google Scholar]
- Botta, L.; Didonè, F.; Lopez-Cortes, A.; Nieto, A.C.; Desandes, E.; Hjalgrim, L.L.; Jakab, Z.; Stiller, C.A.; Zeller, B.; Gatta, G.; et al. International benchmarking of stage at diagnosis for six childhood solid tumours (the BENCHISTA project): A population-based, retrospective cohort study. Lancet Child. Adolesc. Health 2025, 9, 89–99. [Google Scholar] [CrossRef]
- Botta, L.; Gatta, G.; Didonè, F.; Lopez Cortes, A.; Pritchard-Jones, K. International benchmarking of childhood cancer survival by stage at diagnosis: The BENCHISTA project protocol. PLoS ONE 2022, 17, e0276997. [Google Scholar] [CrossRef]
- Lopez-Cortes, A.; Didonè, F.; Botta, L.; Hjalgrim, L.L.; Jakab, Z.; Canete Nieto, A.; Stiller, C.; Zeller, B.; Gatta, G.; Pritchard-Jones, K. Cancer data quality and harmonization in Europe: The experience of the BENCHISTA Project—International benchmarking of childhood cancer survival by stage. Front. Oncol. 2023, 13, 1232451. [Google Scholar] [CrossRef]
- Aitken, J.F.; Youlden, D.R.; Moore, A.S.; Baade, P.D.; Ward, L.J.; Thursfield, V.J.; Valery, P.C.; Green, A.C.; Gupta, S.; Frazier, A.L. Assessing the feasibility and validity of the Toronto Childhood Cancer Stage Guidelines: A population-based registry study. Lancet Child. Adolesc. Health 2018, 2, 173–179. [Google Scholar] [CrossRef]
- Liu, B.; Abraham, N.; Chitsike, I.; Sylvie, C.G.L.; Kambugu, J.; Stévy, N.M.A.; Pondy, A.H.O.; Renner, L.; Parkin, D.M. Enhancing information on stage at diagnosis for childhood cancer in Africa. Pediatr. Blood Cancer 2023, 70, e30555. [Google Scholar] [CrossRef]
- Mallon, B.; Kaboré, R.; Couitchere, L.; Akonde, F.B.; Narison, M.L.R.; Budiongo, A.; Dackono, T.A.; Pondy, A.; Diedhiou, F.; Patte, C.; et al. The feasibility of implementing Toronto childhood cancer stage guidelines and estimating the impact on outcome for childhood cancers in seven pediatric oncology units in sub-Saharan Africa. A study from the Franco-African Pediatric Oncology Group. Pediatr. Blood Cancer 2023, 70, e30664. [Google Scholar] [CrossRef]
- Sacerdote, C.; Mosso, M.L.; Alessi, D.; Merletti, F.; Tagliabue, G.; D’Agostino, A.; Fabiano, S.; Savoia, F.; Piga, P.; Sessa, M.; et al. An application of the Toronto Childhood Cancer Stage Guidelines in three population-based cancer registries: The case of central nervous tumors. Pediatr. Blood Cancer 2020, 67, e28303. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Baade, P.D.; Frazier, A.L.; Gupta, S.; Gottardo, N.G.; Moore, A.S.; Aitken, J.F. Temporal changes in childhood cancer incidence and survival by stage at diagnosis in Australia, 2000–2017. Acta Oncol. 2023, 62, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Frazier, A.L.; Gupta, S.; Pritchard-Jones, K.; Kirby, M.L.; Baade, P.D.; Green, A.C.; Valery, P.C.; Aitken, J.F. Stage at diagnosis for childhood solid cancers in Australia: A population-based study. Cancer Epidemiol. 2019, 59, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Gupta, S.; Frazier, A.L.; Moore, A.S.; Baade, P.D.; Valery, P.C.; Green, A.C.; Aitken, J.F. Stage at diagnosis for children with blood cancers in Australia: Application of the Toronto Paediatric Cancer Stage Guidelines in a population-based national childhood cancer registry. Pediatr. Blood Cancer 2019, 66, e27683. [Google Scholar] [CrossRef]
- De Souza, P.C.F.; Espinosa, M.M.; Teixeira, M.T.B.; Galvão, N.D.; De Lima, F. Staging at Diagnosis and Survival of Hematologic Neoplasms in Children and Adolescents in Mato Grosso, Brazil: A Population-based Study. Asian Pac. J. Cancer Prev. 2025, 26, 171–179. [Google Scholar] [CrossRef]
- Grabois, M.; Oliveira, E.; Carvalho, M. Childhood cancer and pediatric oncologic care in Brazil: Access and equity. Cad. Saúde Pública 2011, 27, 1711–1720. [Google Scholar] [CrossRef][Green Version]
- Fabian, I.D.; Abdallah, E.; Abdullahi, S.U.; Abdulqader, R.A.; Adamou Boubacar, S.; Ademola-Popoola, D.S.; Adio, A.; Afshar, A.R.; Aggarwal, P.; Aghaji, A.E.; et al. Global Retinoblastoma Presentation and Analysis by National Income Level. JAMA Oncol. 2020, 6, 685–695. [Google Scholar] [CrossRef]



| n | % | |
|---|---|---|
| Sex | ||
| Boys | 478 | 55.5 |
| Girls | 384 | 44.6 |
| Age at diagnosis | ||
| <1 years | 63 | 7.3 |
| 01–04 years | 193 | 22.4 |
| 05–09 years | 187 | 21.7 |
| 10–14 years | 190 | 22.0 |
| 15–19 years | 229 | 26.6 |
| Type of cancer according to ICCC-3 | ||
| I. Leukaemias, Myeloproliferative and Myelodysplastic Diseases | 208 | 24.1 |
| II. Lymphomas and reticuloendothelial neoplasms | 191 | 22.2 |
| III. CNS and Miscellaneous Intracranial and Intraspinal Neoplasms | 109 | 12.6 |
| IV. Neuroblastoma and Other Peripheral Nervous Cell Tumours | 43 | 5.0 |
| V. Retinoblastoma | 20 | 2.3 |
| VI. Renal Tumours | 33 | 3.8 |
| VII. Hepatic Tumours | 9 | 1.0 |
| VIII. Malignant Bone Tumours | 74 | 8.6 |
| IX. Soft Tissue and Other Extraosseous Sarcomas | 52 | 6.0 |
| X. Germ Cell Tumours, Trophoblastic Tumours and Neoplasms of Gonads | 54 | 6.3 |
| XI. Other Malignant Epithelial Neoplasms and Malignant Melanomas | 65 | 7.5 |
| XII. Other and Unspecified Malignant Neoplasms | 4 | 0.5 |
| All | 862 | 100.0 |
| Type of Tumour | Staging Category | ||||
|---|---|---|---|---|---|
| Acute lymphoblastic leukemia (n = 116; 18 missing) | CNS1 | CNS2 | CNS3 | ||
| n | 104 | 8 | 4 | ||
| % | 89.7 | 6.9 | 3.4 | ||
| Acute myeloid leukemia (n = 32; 7 missing) | CNS- | CNS+ | |||
| n | 27 | 5 | |||
| % | 84.4 | 15.6 | |||
| Hodgkin lymphoma (n = 79; 3 missing) | IA/IB | IIA/IIB | IIIA/IIIB | IVA/IVB | |
| n | 12 | 43 | 11 | 13 | |
| % | 15.2 | 54.4 | 13.9 | 16.5 | |
| Non-Hodgkin lymphoma (n = 63; 4 missing) | I | II | III | IV | |
| n | 7 | 19 | 24 | 13 | |
| % | 11.1 | 30.2 | 38.1 | 20.6 | |
| Neuroblastoma (n = 38; 5 missing) | L1 | L2 | MS | M | |
| n | 13 | 8 | 4 | 13 | |
| % | 34.2 | 21.1 | 10.5 | 34.2 | |
| Wilms tumour (n = 27; 5 missing) | I/y-I | II/y-II | III/y-III | IV | |
| n | 14 | 6 | 3 | 4 | |
| % | 51.9 | 22.2 | 11.1 | 14.8 | |
| Rhabdomyosarcoma (n = 23; 4 missing) | I | II | III | IV | |
| n | 16 | 2 | 3 | 2 | |
| % | 69.6 | 8.7 | 13.0 | 8.7 | |
| Non-rhabdomyosarcoma (n = 20; 4 missing) | I | II | III | IV | |
| n | 10 | 3 | 3 | 4 | |
| % | 50.0 | 15.0 | 15.0 | 20.0 | |
| Osteosarcoma (n = 32; 4 missing) | Localized | Metastatic | |||
| n | 26 | 6 | |||
| % | 81.3 | 18.8 | |||
| Ewing sarcoma (n = 26; 8 missing) | Localized | Metastatic | |||
| n | 19 | 7 | |||
| % | 73.1 | 26.9 | |||
| Retinoblastoma (n = 20; 0 missing) | 0 | I | II | III | IV |
| n | 11 | 7 | - | 2 | - |
| % | 55.0 | 35.0 | - | 10.0 | - |
| Hepatoblastoma (n = 6; 1 missing) | Localized | Metastatic | |||
| n | 6 | - | |||
| % | 100.0 | - | |||
| Testicular cancer (n = 20; 2 missing) | I | II | III | ||
| n | 12 | 5 | 3 | ||
| % | 60.0 | 25.0 | 15.0 | ||
| Ovarian cancer (n = 6: 4 missing) | I | II | III | IV | |
| n | 5 | 1 | - | - | |
| % | 83.3 | 16.7 | - | - | |
| Medulloblastoma (n = 28; 3 missing) | M0 | M1 | M2 | M3 | M4 |
| n | 22 | 2 | 1 | 3 | - |
| % | 78.6 | 7.1 | 3.6 | 10.7 | - |
| Ependymoma (n = 13; 0 missing) | M0 | M1 | M2 | M3 | M4 |
| n | 13 | - | - | - | - |
| % | 100.0 | - | - | - | - |
| Characteristic | OS at 1 Year | OS at 3 Years | OS at 5 Years | Log, Rank Test p | |||
|---|---|---|---|---|---|---|---|
| n at Risk | % (CI) | n at Risk | % (CI) | n at Risk | % (CI) | ||
| By sex | 0.908 | ||||||
| Boys | 445 | 92.9 (90.2, 94.9) | 420 | 87.7 (84.4, 90.3) | 410 | 85.8 (82.3, 88.6) | |
| Girls | 364 | 94.5 (91.7, 96.4) | 341 | 88.5 (84.9, 91.3) | 330 | 85.9 (82.0, 89.0) | |
| By age | 0.002 | ||||||
| <1 years | 57 | 88.9 (78.1, 94.5) | 55 | 85.7 (74.3, 92.3) | 53 | 84.1 (72.5, 91.1) | |
| 01–04 years | 186 | 95.9 (91.9, 97.9) | 175 | 90.2 (85.0, 93.6) | 171 | 88.6 (83.2, 92.3) | |
| 05–09 years | 180 | 95.7 (91.6, 97.8) | 173 | 92.0 (87.1, 95.1) | 168 | 89.8 (84.5, 93.4) | |
| 10–14 years | 172 | 90.0 (84.8, 93.5) | 152 | 79.5 (73.0, 84.6) | 147 | 77.4 (70.7, 82.7) | |
| 15–19 years | 217 | 94.3 (90.4, 96.7) | 209 | 90.8 (86.3, 93.9) | 201 | 87.8 (82.8, 91.4) | |
| By two categories of age | 0.314 | ||||||
| Childhood (0–14 years) | 592 | 93.4 (91.1, 95.1) | 552 | 87.1 (84.2, 89.4) | 539 | 85.2 (82.1, 87.7) | |
| Adolescence (15–19 years) | 217 | 94.3 (90.4, 96.7) | 209 | 90.8 (86.3, 93.9) | 201 | 87.8 (82.8, 91.4) | |
| By type of tumour | <0.001 | ||||||
| I. Leukaemias, Myeloproliferative and Myelodysplastic Diseases | 189 | 90.9 (86.1, 94.1) | 178 | 85.6 (80.0, 89.7) | 174 | 83.7 (77.9, 88.0) | |
| II. Lymphomas and reticuloendothelial neoplasms | 190 | 99.5 (96.3, 99.9) | 189 | 99.0 (95.9, 99.7) | 186 | 97.4 (93.8, 98.9) | |
| III. CNS and Miscellaneous Intracranial and Intraspinal Neoplasms | 94 | 86.2 (78.2, 91.5) | 77 | 70.6 (61.1, 78.2) | 72 | 66.1 (56.4, 74.1) | |
| IV. Neuroblastoma and Other Peripheral Nervous Cell Tumours | 40 | 93.0 (79.9, 97.7) | 36 | 83.7 (68.9, 91.9) | 34 | 79.1 (63.6, 88.5) | |
| V. Retinoblastoma | 20 | 100 (-) | 20 | 100 (-) | 20 | 100 (-) | |
| VI. Renal Tumours | 33 | 100 (-) | 33 | 100 (-) | 33 | 100 (-) | |
| VII. Hepatic Tumours | 8 | 88.9 (43.3, 98.4) | 8 | 88.9 (43.3, 98.4) | 7 | 77.8 (36.5, 93.9) | |
| VIII. Malignant Bone Tumours | 70 | 94.6 (86.2, 97.9) | 59 | 79.7 (68.7, 87.3) | 57 | 77.0 (65.7, 85.0) | |
| IX. Soft Tissue and Other Extraosseous Sarcomas | 45 | 86.5 (73.8, 93.3) | 44 | 84.6 (71.6, 92.0) | 43 | 82.7 (69.4, 90.6) | |
| X. Germ Cell Tumours, Trophoblastic Tumours and Neoplasms of Gonads | 52 | 96.3 (86.0, 99.1) | 51 | 94.4 (83.8, 98.2) | 51 | 94.4 (83.8, 98.2) | |
| XI. Other Malignant Epithelial Neoplasms and Malignant Melanomas | 62 | 95.4 (86.4, 98.5) | 60 | 92.3 (82.5, 96.7) | 59 | 90.8 (80.6, 95.7) | |
| XII. Other and Unspecified Malignant Neoplasms | 4 | 100 (-) | 4 | 100 (-) | 4 | 100 (-) | |
| By two categories of stage at diagnosis 1 | <0.001 | ||||||
| Early stage | 433 | 96.9 (94.8, 98.1) | 409 | 91.5 (88.5, 93.7) | 401 | 89.7 (86.5, 93.0) | |
| Advanced stage | 89 | 86.4 (78.1, 91.7) | 79 | 76.7 (67.3, 83.7) | 72 | 69.9 (60.0, 77.8) | |
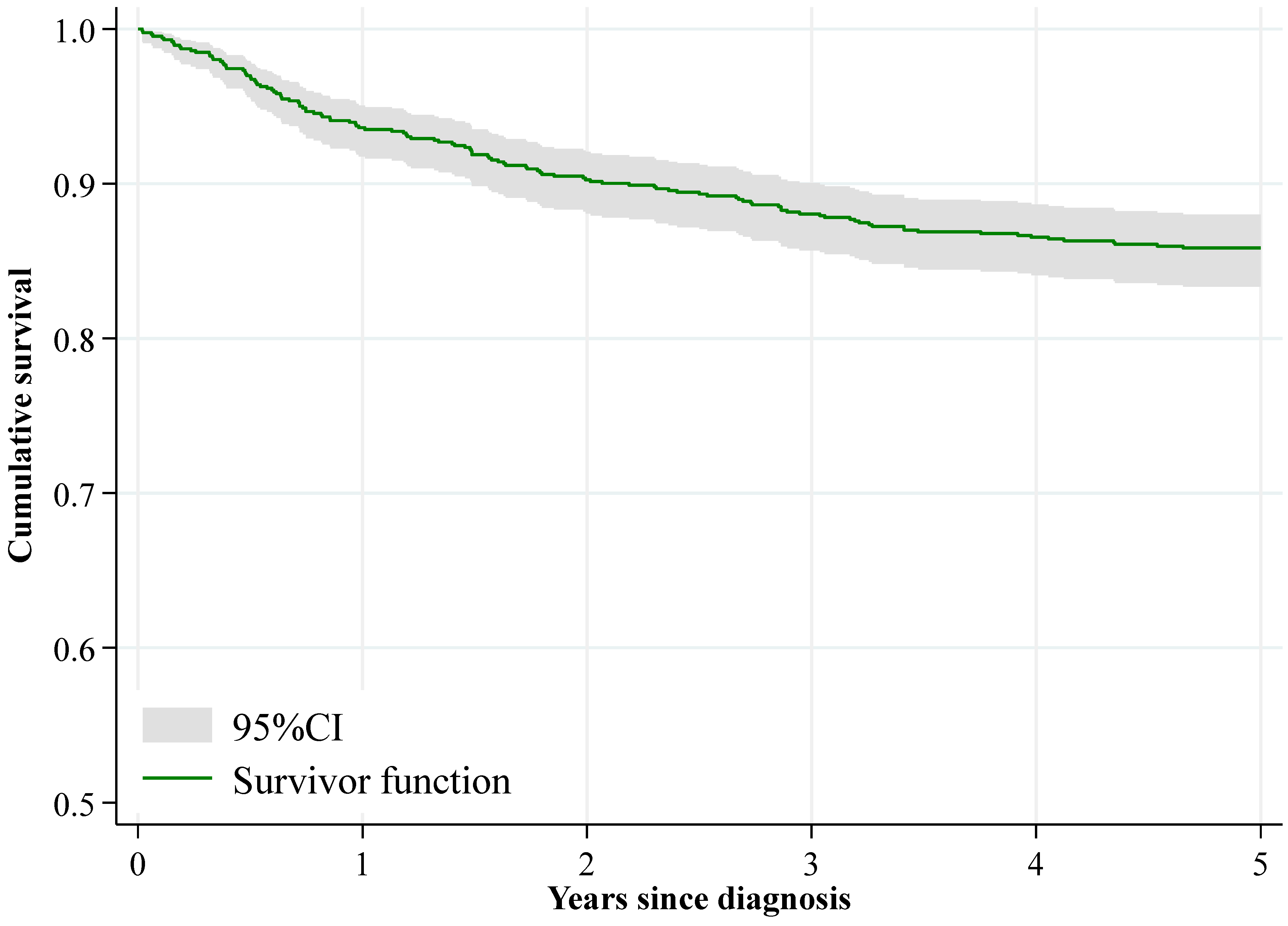
| All | 807 | 93.6 (91.8, 95.1) | 759 | 88.1 (85.7, 90.0) | 740 | 85.9 (83.3, 88.0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-González, R.; Parra-Blázquez, D.; Moñino, D.; Pino-Rosón, C.; Sevilla-Hernández, C.; Pollán, M.; Aragonés, N. Population-Based Survival of Childhood and Adolescent Cancers (0–19 Years) in Madrid: Analysis by Sex, Age, Tumour Type, and Stage. Cancers 2025, 17, 3113. https://doi.org/10.3390/cancers17193113
López-González R, Parra-Blázquez D, Moñino D, Pino-Rosón C, Sevilla-Hernández C, Pollán M, Aragonés N. Population-Based Survival of Childhood and Adolescent Cancers (0–19 Years) in Madrid: Analysis by Sex, Age, Tumour Type, and Stage. Cancers. 2025; 17(19):3113. https://doi.org/10.3390/cancers17193113
Chicago/Turabian StyleLópez-González, Raquel, David Parra-Blázquez, Daniel Moñino, Candela Pino-Rosón, Clotilde Sevilla-Hernández, Marina Pollán, and Nuria Aragonés. 2025. "Population-Based Survival of Childhood and Adolescent Cancers (0–19 Years) in Madrid: Analysis by Sex, Age, Tumour Type, and Stage" Cancers 17, no. 19: 3113. https://doi.org/10.3390/cancers17193113
APA StyleLópez-González, R., Parra-Blázquez, D., Moñino, D., Pino-Rosón, C., Sevilla-Hernández, C., Pollán, M., & Aragonés, N. (2025). Population-Based Survival of Childhood and Adolescent Cancers (0–19 Years) in Madrid: Analysis by Sex, Age, Tumour Type, and Stage. Cancers, 17(19), 3113. https://doi.org/10.3390/cancers17193113






