Physiopathological Features in a Three-Dimensional In Vitro Model of Hepatocellular Carcinoma: Hypoxia-Driven Oxidative Stress and ECM Remodeling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and 3D Model Generation
2.2. Cell Viability by MTT Assay
2.3. Liv e/Dead Staining by Confocal Microscopy
2.4. Gene Expression Analysis by qRTPCR
2.5. Immunofluorescence Analysis
2.6. Flow Cytometric Analysis
2.7. Fourier-Transform Infrared Spectrscopy (FTIR) Analysis
2.8. Statistical Analysis
3. Results
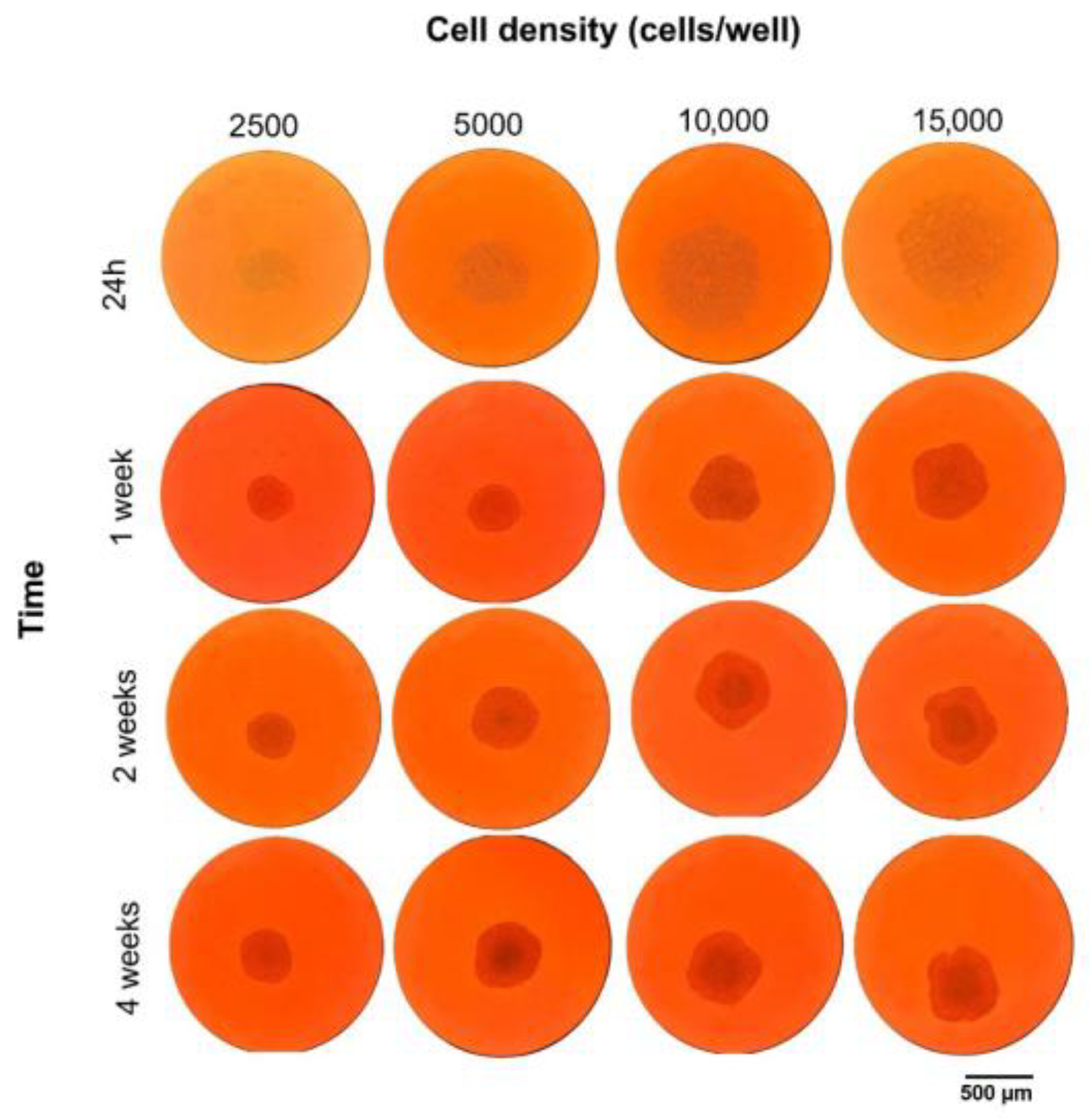
3.1. Three-Dimensional HCC Model’s Morphological and Structural Evolution
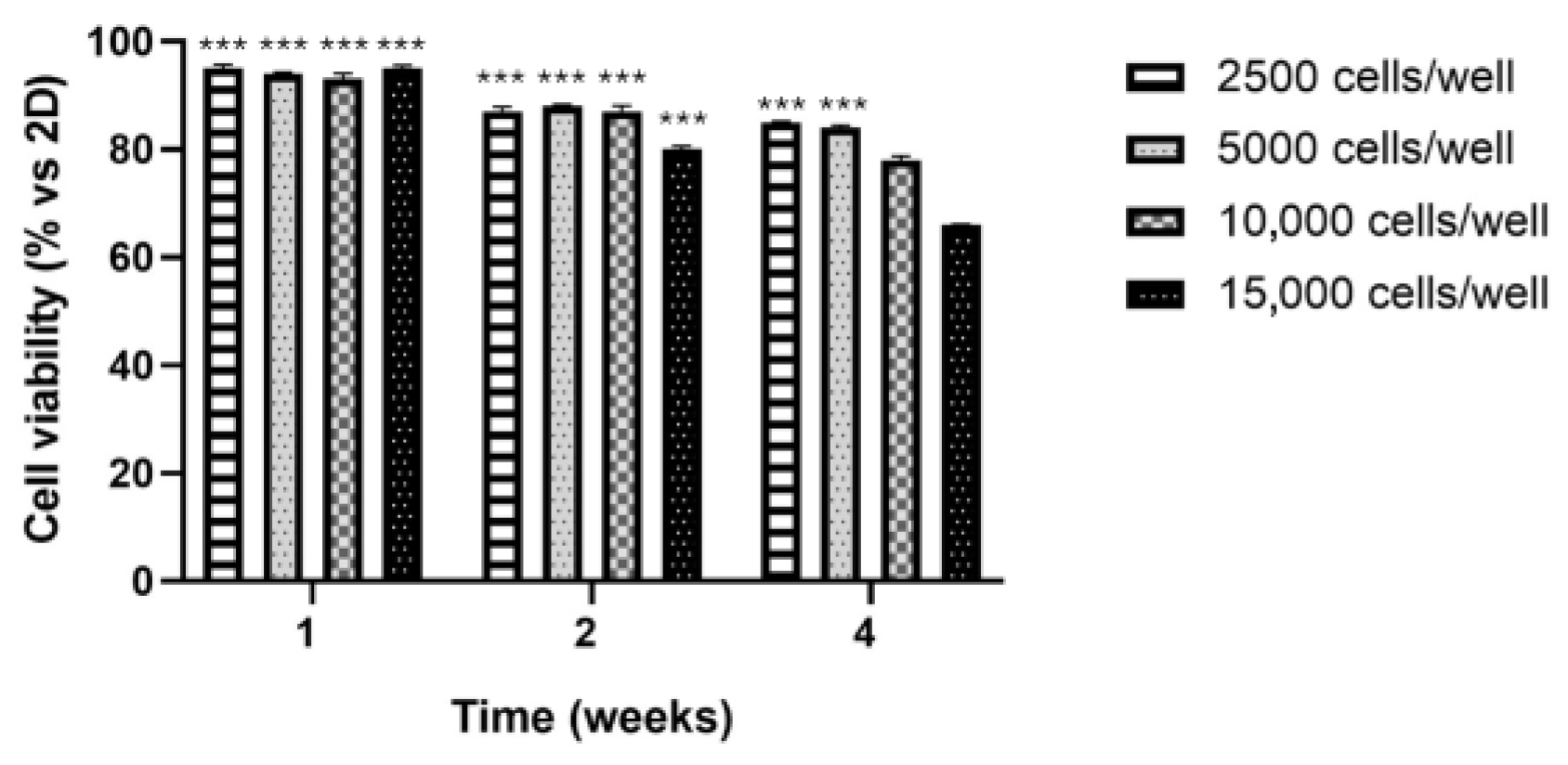
3.2. Cell Viability by MTT Assay
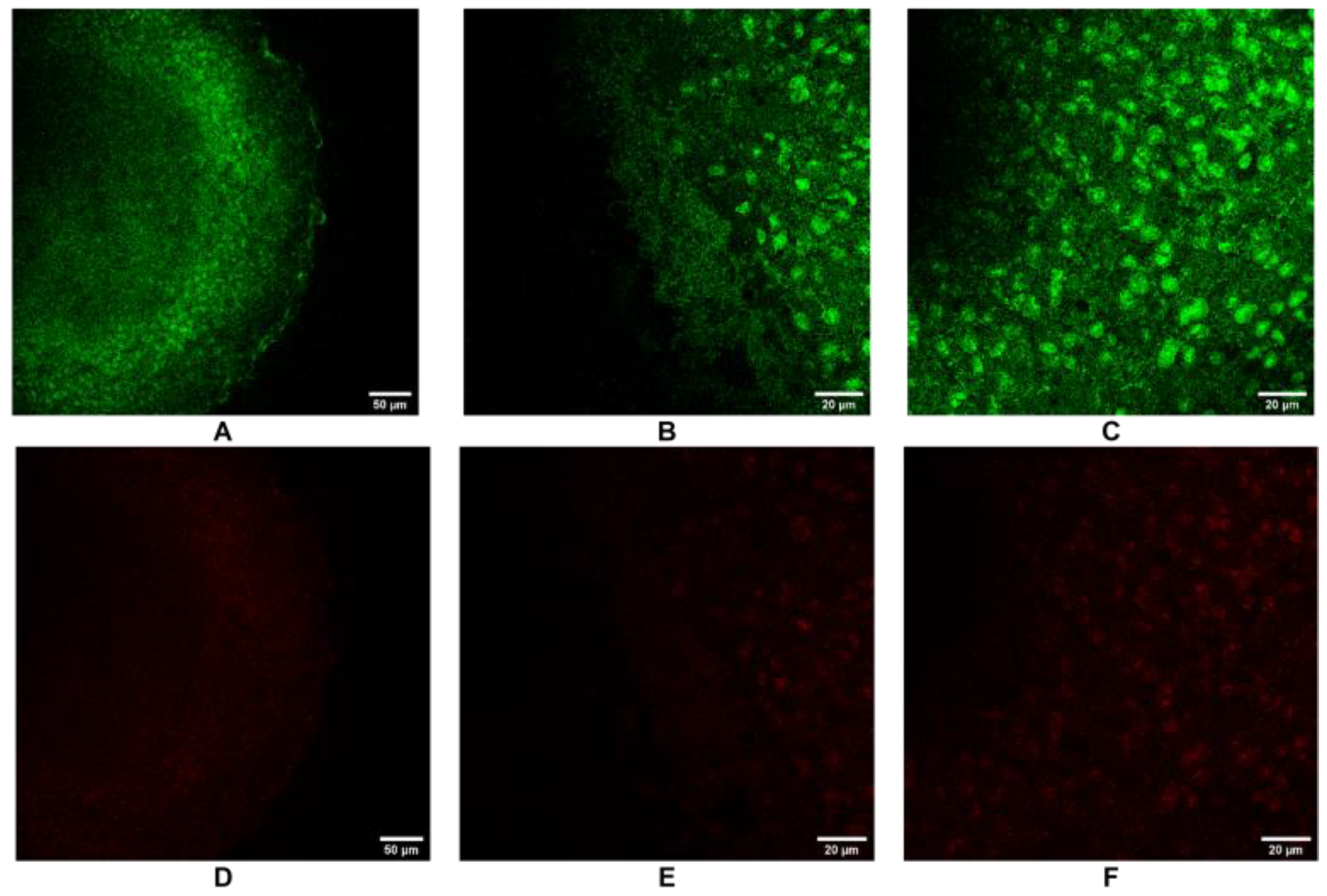
3.3. Live/Dead Staining by Confocal Microscopy
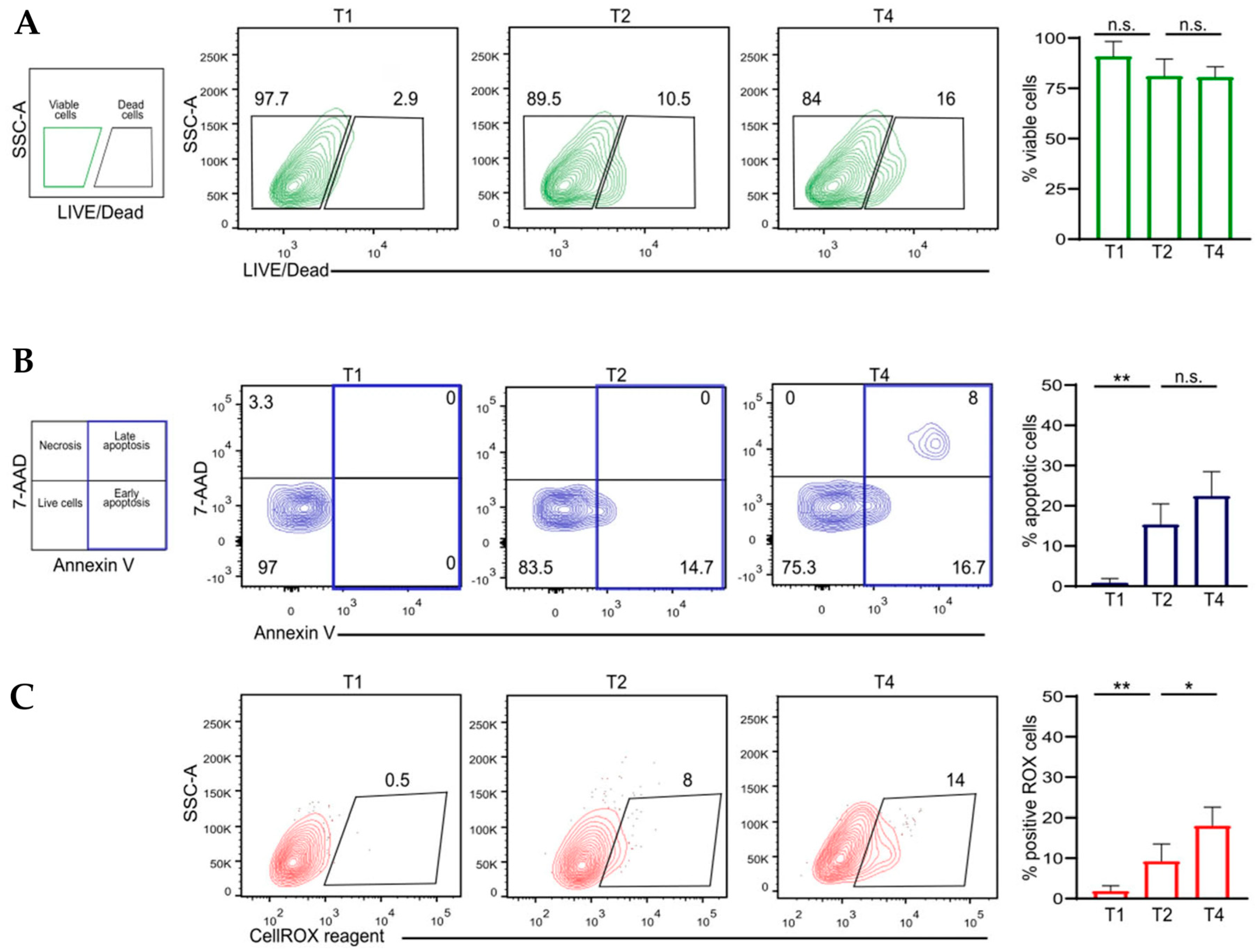
3.4. Flow Cytometric Analysis
3.5. Gene Expression Analysis by qRTPCR
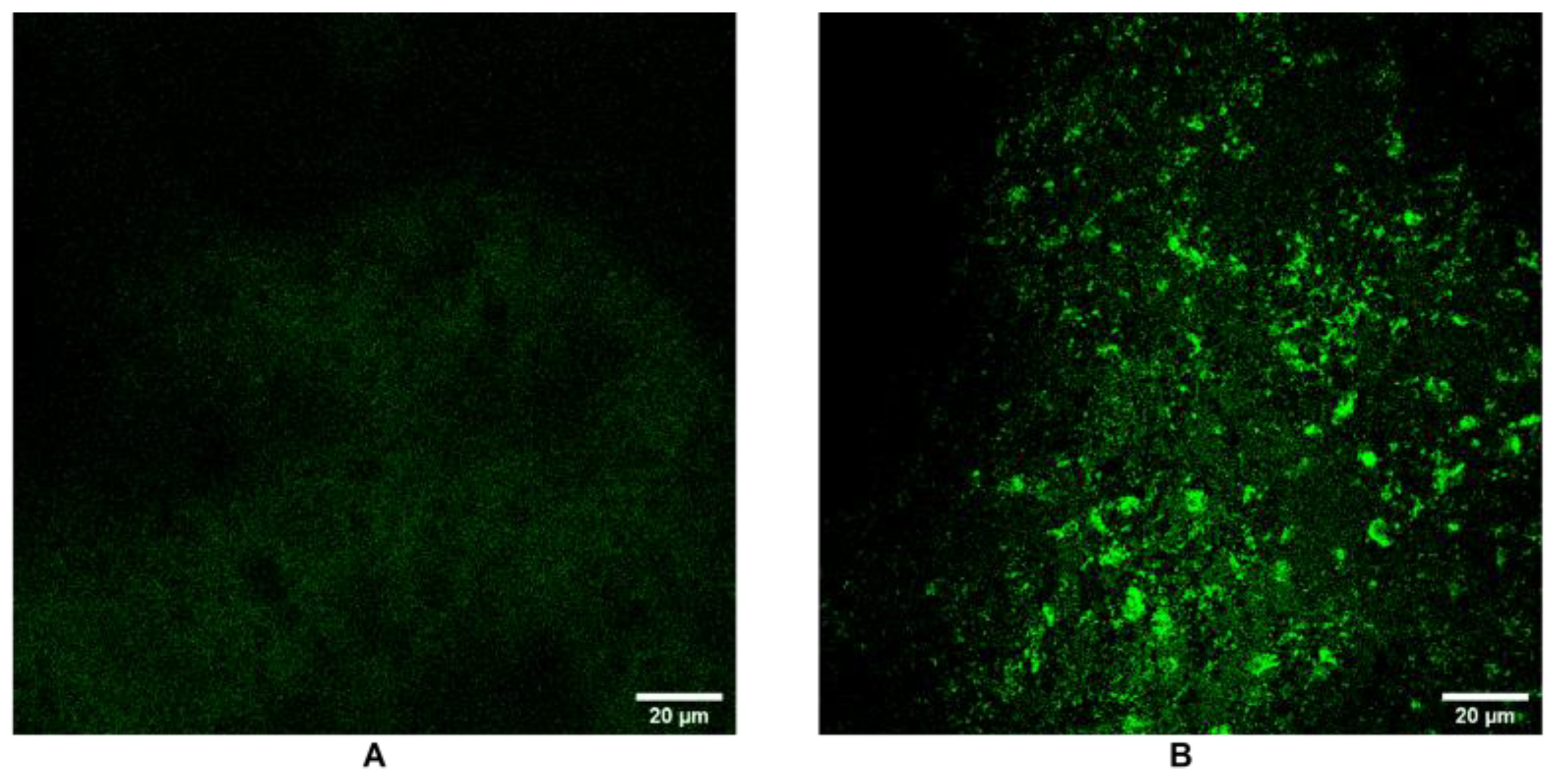
3.6. SPARC Expression and Extracellular Matrix Remodeling by Confocal Microscopy
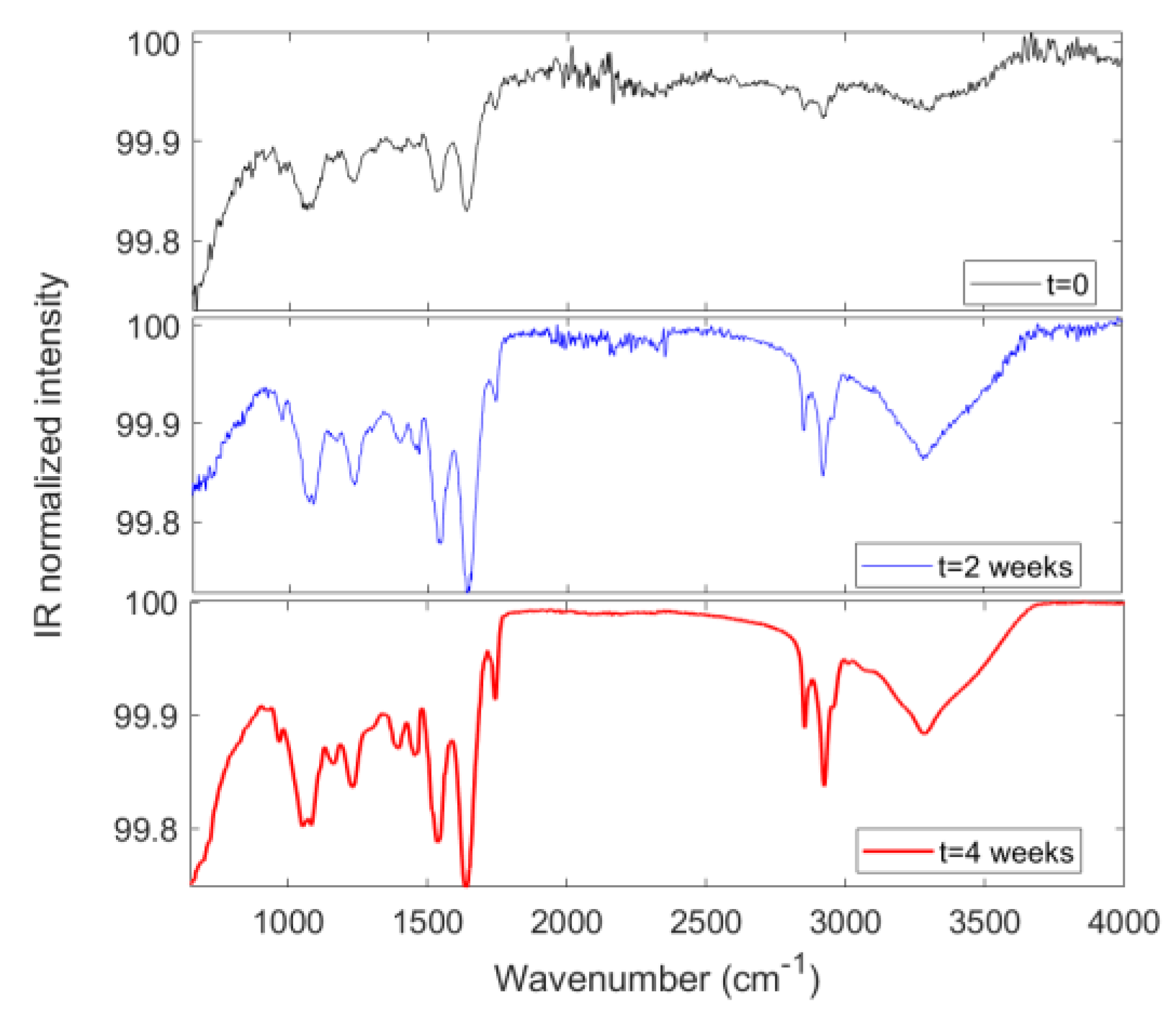
3.7. FTIR Spectral Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and Surveillance for Hepatocellular Carcinoma: New Trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Boix, L.; Sala, M.; Llovet, J.M. Focus on Hepatocellular Carcinoma. Cancer Cell 2004, 5, 215–219. [Google Scholar] [CrossRef]
- Sedda, F.; Caddeo, A.; Sasidharan, K.; Perra, G.; Pal, R.; Lai, N.; Kowalik, M.A.; Perra, A. Galectin-3 Inhibition Ameliorates Hepatic Steatosis in a Multilineage 3D Spheroid Model. PLoS ONE 2025, 20, e0326373. [Google Scholar] [CrossRef]
- Chu, Y.-J.; Yang, H.-I.; Wu, H.-C.; Lee, M.-H.; Liu, J.; Wang, L.-Y.; Lu, S.-N.; Jen, C.-L.; You, S.-L.; Santella, R.M.; et al. Aflatoxin B1 Exposure Increases the Risk of Hepatocellular Carcinoma Associated with Hepatitis C Virus Infection or Alcohol Consumption. Eur. J. Cancer 2018, 94, 37–46. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global Epidemiology of Hepatocellular Carcinoma: An Emphasis on Demographic and Regional Variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human Primary Liver Cancer-Derived Organoid Cultures for Disease Modeling and Drug Screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, E.; Naranjo-Alcazar, R.; Tort-Ausina, I.; Donato, M.T.; Salmeron-Sanchez, M.; Tolosa, L.; Gallego-Ferrer, G. Injectable Cell-Laden Gelatin-Chondroitin Sulphate Hydrogels for Liver in Vitro Models. Int. J. Biol. Macromol. 2025, 288, 138693. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef]
- Yang, S.; Ooka, M.; Margolis, R.J.; Xia, M. Liver Three-Dimensional Cellular Models for High-Throughput Chemical Testing. Cell Rep. Methods 2023, 3, 100432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Morganti, D.; Smeriglio, A.; Sciuto, E.L.; Spata, M.O.; Trombetta, D.; Fazio, B.; Guglielmino, S.P.P.; Conoci, S. Formation of 3D Human Osteoblast Spheroids Incorporating Extracellular Matrix-Mimetic Phage Peptides as a Surrogate Bone Tissue Model. Int. J. Mol. Sci. 2025, 26, 8482. [Google Scholar]
- Yu, Y.; Zhong, H.; Niu, Q.; Zhao, M.; Li, B.; Zhou, X. Establishment and Validation of an in Vitro Liver Model Based Extracellular Matrix for Hepatotoxicity Prediction. Hum. Exp. Toxicol. 2025, 44, 09603271251350797. [Google Scholar] [CrossRef]
- Caygill, C.H.; Alqurashi, S.O.; Adolfi, A.; Carson, J.; Sturm, A.; Evans, D.S.; Jinks, J.B.; Dechering, K.J.; Reimer, L.; Pennington, S.H.; et al. An Accessible 3D HepG2/C3A Liver Spheroid Model Supporting the Complete Intrahepatocytic Lifecycle of Plasmodium falciparum. Parasitology 2025, 1–8. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, C.; Liu, S.; Yang, L.; Han, P.; Cui, T.; Zhang, Y. Advancements and Application Prospects of Three-Dimensional Models for Primary Liver Cancer: A Comprehensive Review. Front. Bioeng. Biotechnol. 2023, 11, 1343177. [Google Scholar] [CrossRef]
- Chen, L.; Ma, H.; Li, K.; Song, X.; Zeng, X. Liver Extracellular Matrix Hydrogel-Based Three-Dimensional Culture System of HepG2 Cells to Enhance Cancer Stem Cell Properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112119. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Cordaro, M.; Morganti, D.; Gugliandolo, E.; Fusco, R.; Marino, Y.; Franco, G.A.; Cuzzocrea, S.; Di Paola, R.; Conoci, S. Osteogenic Activity and Bone Matrix Mineralization Induced by Vitis Vinifera Leaves Extract in Human Osteoblastic Cells. Food Sci. Nutr. 2025, 13, e70785. [Google Scholar] [CrossRef]
- Lo Furno, D.; Romano, I.R.; Russo, V.; Rizzo, M.G.; Mannino, G.; Calabrese, G.; Giuffrida, R.; D’Aprile, S.; Salvatorelli, L.; Magro, G.; et al. Hydroxyapatite Scaffold and Bioactive Factor Combination as a Tool to Improve Osteogenesis, In Vitro and In Vivo Experiments Using Phage Display Technology. Int. J. Mol. Sci. 2025, 26, 7040. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of Hypoxia in the Tumor Microenvironment and Targeted Therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef] [PubMed]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of In Vitro 3D Cell Model Developed from Human Hepatocellular Carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in Cancer: Significance and Impact on Clinical Outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Codony, V.L.; Tavassoli, M. Hypoxia-Induced Therapy Resistance: Available Hypoxia-Targeting Strategies and Current Advances in Head and Neck Cancer. Transl. Oncol. 2021, 14, 101017. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Multisanti, C.R.; Faggio, C.; Impellitteri, F. Cell-Based Evaluation of Anti-Inflammatory Activity from the Combination of Natural Compounds in LPS-Stimulated U937 Monocytes. Cytotechnology 2025, 77, 117. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered Metabolism in Cancer: Insights into Energy Pathways and Therapeutic Targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef]
- Khedr, M.A.; Mohamed, Z.; El-Derby, A.M.; Soliman, M.M.; Edris, A.A.F.; Badr, E.; El-Badri, N. Development of Hepatocellular Carcinoma Organoid Model Recapitulating HIF-1A Metabolic Signature. Clin. Exp. Med. 2024, 25, 9. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, Z.; Yang, L.; Gao, Y.; Zhu, Q.; Hu, L.; Huang, D.; Xu, Q. Hypoxia-inducible Factors in Hepatocellular Carcinoma (Review). Oncol. Rep. 2020, 43, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, M.; Xia, S.; Zeng, F.; Liu, Q. Systematic and Comprehensive Insights into HIF-1 Stabilization under Normoxic Conditions: Implications for Cellular Adaptation and Therapeutic Strategies in Cancer. Cell. Mol. Biol. Lett. 2025, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.N.; Ciriolo, M.R.; Ciccarone, F. Hypoxia-Induced Reactive Oxygen Species: Their Role in Cancer Resistance and Emerging Therapies to Overcome It. Antioxidants 2025, 14, 94. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.-F.H. Tumor Glycolysis as a Target for Cancer Therapy: Progress and Prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef]
- Latham, T.; Mackay, L.; Sproul, D.; Karim, M.; Culley, J.; Harrison, D.J.; Hayward, L.; Langridge-Smith, P.; Gilbert, N.; Ramsahoye, B.H. Lactate, a Product of Glycolytic Metabolism, Inhibits Histone Deacetylase Activity and Promotes Changes in Gene Expression. Nucleic Acids Res. 2012, 40, 4794–4803. [Google Scholar] [CrossRef]
- Yu, X.; Yang, J.; Xu, J.; Pan, H.; Wang, W.; Yu, X.; Shi, S. Histone Lactylation: From Tumor Lactate Metabolism to Epigenetic Regulation. Int. J. Biol. Sci. 2024, 20, 1833. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, C.; Yang, L.; He, T.; Wu, X.; Zhang, H.; Dong, K. SPARC Overexpression Promotes Liver Cancer Cell Proliferation and Tumor Growth. Front. Mol. Biosci. 2021, 8, 775743. [Google Scholar] [CrossRef]
- Gao, J.; Senthil, M.; Ren, B.; Yan, J.; Xing, Q.; Yu, J.; Zhang, L.; Yim, J.H. IRF-1 Transcriptionally Upregulates PUMA, Which Mediates the Mitochondrial Apoptotic Pathway in IRF-1-Induced Apoptosis in Cancer Cells. Cell Death Differ. 2010, 17, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shen, W.; Peng, H.; Li, Y.; Chen, F.; Zheng, L.; Xu, J.; Jia, L. Fibronectin 1 Promotes Melanoma Proliferation and Metastasis by Inhibiting Apoptosis and Regulating EMT. OncoTargets Ther. 2019, 12, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, F.; Wang, Y.; Gao, J. Secreted Protein Acidic and Rich in Cysteine Promotes Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma Cells and Acquisition of Cancerstem Cell Phenotypes. J. Gastroenterol. Hepatol. 2019, 34, 1860–1868. [Google Scholar] [CrossRef]
- Conway, G.E.; Chavanel, B.; Virard, F.; Shah, U.-K.; Burgum, M.J.; Evans, S.J.; Korenjak, M.; Thomas, L.E.; Jenkins, G.J.; Zavadil, J.; et al. Harnessing the Power of an Advanced in Vitro 3D Liver Model and Error-Corrected Duplex Sequencing for the Detection of Mutational Signatures. Mutagenesis 2025, geaf015. [Google Scholar] [CrossRef]
- Moon, Y.-W.; Dobroski, T.; Willson, K.; Jeong, J.-O.; Bishop, C.; Atala, A.; Yoo, J.J.; Lee, S.J. 3D Bioprinted Thick Hepatic Constructs with Vascular Network as a Physiologically Relevant in Vitro Organ Model. Mater. Today Bio 2025, 32, 101786. [Google Scholar] [CrossRef]
- Duan, K.; Hu, J.; Zhao, Y.; Lin, M.; Xu, M.; Li, T.; Lee, B.H. Human Serum Albumin-Based Cryogels as a Biomimetic Platform for 3D Co-Culture and Drug-Induced Liver Injury Prediction. Int. J. Biol. Macromol. 2025, 321, 146376. [Google Scholar] [CrossRef]
- Dalsbecker, P.; Suominen, S.; Faridi, M.A.; Mahdavi, R.; Johansson, J.; Blomqvist, C.H.; Goksör, M.; Aalto-Setälä, K.; Viiri, L.E.; Adiels, C.B. An in Vivo Mimetic Liver-Lobule-Chip (LLoC) for Stem Cell Maturation, and Zonation of Hepatocyte-like Cells on Chip. Lab Chip 2025, 25, 4328–4344. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Yadav, N.; Morganti, D.; Calorenni, P.; Sciuto, E.L.; Nicolò, M.; Fazio, B.; Guglielmino, S.; Lorenzelli, L.; Conoci, S. Conoci, S., Di Natale, C., Prodi, L., Valenti, G., Eds.; 3D MicroOrganoSpheres Formation of Hepatocellular Carcinoma on Chip as a Novel in Vitro Model for Physiological and Therapeutic Studies. In Sensors and Microsystems, Proceedings of the AISEM 2024, Bologna, Italy, 7–9 February 2024; Lecture Notes in Electrical Engineering; Springer: Cham, Switzerland, 2025; Volume 1334, pp. 9–18. [Google Scholar]
- Rizzo, M.G.; Zammuto, V.; Spanò, A.; Gugliandolo, C.; Calabrese, G.; Guglielmino, S. Anti-Inflammatory Effects in LPS-Induced Macrophages and Antibiofilm Activity of the Mannose-Rich Exopolysaccharide Produced by Bacillus Licheniformis B3-15. Heliyon 2024, 10, e38367. [Google Scholar] [CrossRef]
- Rizzo, C.; Zammuto, V.; Lo Giudice, A.; Rizzo, M.G.; Spanò, A.; Laganà, P.; Martinez, M.; Guglielmino, S.; Gugliandolo, C. Antibiofilm Activity of Antarctic Sponge-Associated Bacteria against Pseudomonas aeruginosa and Staphylococcus aureus. J. Mar. Sci. Eng. 2021, 9, 243. [Google Scholar] [CrossRef]
- Apicella, C.; Ruano, C.S.M.; Jacques, S.; Gascoin, G.; Méhats, C.; Vaiman, D.; Miralles, F. Urothelial Cancer Associated 1 (UCA1) and miR-193 Are Two Non-Coding RNAs Involved in Trophoblast Fusion and Placental Diseases. Front. Cell Dev. Biol. 2021, 9, 633937. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, X.; Liu, X.; Jiang, C.; Ren, W.; Huang, W.; Jiang, Y.; Wang, D. Squalene Monooxygenase Facilitates Bladder Cancer Development in Part by Regulating PCNA. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119681. [Google Scholar] [CrossRef]
- Huang, S.-W.; Kao, J.-K.; Wu, C.-Y.; Wang, S.-T.; Lee, H.-C.; Liang, S.-M.; Chen, Y.-J.; Shieh, J.-J. Targeting Aerobic Glycolysis and HIF-1alpha Expression Enhance Imiquimod-Induced Apoptosis in Cancer Cells. Oncotarget 2014, 5, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Vousden, K.H. PUMA, a Novel Proapoptotic Gene, Is Induced by P53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Han, J.; Flemington, C.; Houghton, A.B.; Gu, Z.; Zambetti, G.P.; Lutz, R.J.; Zhu, L.; Chittenden, T. Expression of Bbc3, a pro-Apoptotic BH3-Only Gene, Is Regulated by Diverse Cell Death and Survival Signals. Proc. Natl. Acad. Sci. USA 2001, 98, 11318–11323. [Google Scholar] [CrossRef] [PubMed]
- Plano, L.M.D.; Franco, D.; Rizzo, M.G.; Zammuto, V.; Gugliandolo, C.; Silipigni, L.; Torrisi, L.; Guglielmino, S.P.P. Role of Phage Capsid in the Resistance to UV-C Radiations. Int. J. Mol. Sci. 2021, 22, 3408. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, S.M.; Dai, Q.; Zhang, J.; Zamilpa, R.; Ramirez, T.A.; Dayah, T.; Nguyen, N.; Jin, Y.-F.; Bradshaw, A.D.; Lindsey, M.L. SPARC Mediates Early Extracellular Matrix Remodeling Following Myocardial Infarction. Am. J. Physiol.—Heart Circ. Physiol. 2011, 301, H497–H505. [Google Scholar] [CrossRef]
- Strzalka, W.; Ziemienowicz, A. Proliferating Cell Nuclear Antigen (PCNA): A Key Factor in DNA Replication and Cell Cycle Regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef]
- Abu El Makarem, M. An Overview of Biomarkers for the Diagnosis of Hepatocellular Carcinoma. Hepat. Mon. 2012, 12, e6122. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1α): An Essential Regulator in Cellular Metabolic Control. Cureus 2024, 16, e63852. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wang, L.-T.; Hung, T.I.; Hong, Y.-R.; Chen, C.-H.; Ho, C.-J.; Wang, C. Severe Cellular Stress Drives Apoptosis through a Dual Control Mechanism Independently of P53. Cell Death Discov. 2022, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, C.; Chen, C.; Xiong, H.; Xie, B.; Zhou, J.; Chen, Y.; Zheng, S.; Wang, L. Glucose Transporter GLUT1 Expression and Clinical Outcome in Solid Tumors: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 16875–16886. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Spada, S.; Tocci, A.; Di Modugno, F.; Nisticò, P. Fibronectin as a Multiregulatory Molecule Crucial in Tumor Matrisome: From Structural and Functional Features to Clinical Practice in Oncology. J. Exp. Clin. Cancer Res. 2021, 40, 102. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Thomas, A.; Zhuo, G.-Y.; Mazumder, N.; Jain, S.; Thomas, A.; Zhuo, G.-Y.; Mazumder, N. Fourier Transform Infrared (FTIR) Spectroscopy of Biomolecules. In Recent Advances in Infrared Spectroscopy and Its Applications in Biotechnology; IntechOpen: London, UK, 2025; ISBN 978-0-85014-920-3. [Google Scholar]
- Movasaghi, Z.; Rehman, S.; Ur Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Živković, Z.; Opačak-Bernardi, T. An Overview on Spheroid and Organoid Models in Applied Studies. Sci 2025, 7, 27. [Google Scholar] [CrossRef]
- Xiong, T.; Wang, K. Reconstructing the Hepatocellular Carcinoma Microenvironment: The Current Status and Challenges of 3D Culture Technology. Discov. Oncol. 2025, 16, 506. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized Extracellular Matrix Biomaterials for Regenerative Therapies: Advances, Challenges and Clinical Prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Di Santo, R.; Vaccaro, M.; Romanò, S.; Di Giacinto, F.; Papi, M.; Rapaccini, G.L.; De Spirito, M.; Miele, L.; Basile, U.; Ciasca, G. Machine Learning-Assisted FTIR Analysis of Circulating Extracellular Vesicles for Cancer Liquid Biopsy. J. Pers. Med. 2022, 12, 949. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Srisongkram, T.; Weerapreeyakul, N.; Thumanu, K. Evaluation of Melanoma (SK-MEL-2) Cell Growth between Three-Dimensional (3D) and Two-Dimensional (2D) Cell Cultures with Fourier Transform Infrared (FTIR) Microspectroscopy. Int. J. Mol. Sci. 2020, 21, 4141. [Google Scholar] [CrossRef]
- Portaccio, M.; Faramarzi, B.; Lepore, M. Probing Biochemical Differences in Lipid Components of Human Cells by Means of ATR-FTIR Spectroscopy. Biophysica 2023, 3, 524–538. [Google Scholar] [CrossRef]
- Wu, L.; Vllasaliu, D.; Cui, Q.; Raimi-Abraham, B.T. In Situ Self-Assembling Liver Spheroids with Synthetic Nanoscaffolds for Preclinical Drug Screening Applications. ACS Appl. Mater. Interfaces 2024, 16, 25610–25621. [Google Scholar] [CrossRef] [PubMed]
- Junhom, C.; Weerapreeyakul, N.; Tanthanuch, W.; Thumanu, K. FTIR Microspectroscopy Defines Early Drug Resistant Human Hepatocellular Carcinoma (HepG2) Cells. Exp. Cell Res. 2016, 340, 71–80. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Corsaro, C.; Marrara, S.; Crupi, V.; Conoci, S.; Neri, F.; Fazio, E. Raman Spectral Analyses to Investigate the Physiological and Metabolic Development of a 3D Hepatocellular Carcinoma Model. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 343, 126564. [Google Scholar] [CrossRef]
- Yin, Z.-F.; Wang, C.-H. Research advances on alpha-fetoprotein physiological function and clinical potential. Ai Zheng Chin. J. Cancer 2003, 22, 108–111. [Google Scholar]
- Tian, H.; Zhu, X.; Lv, Y.; Jiao, Y.; Wang, G. Glucometabolic Reprogramming in the Hepatocellular Carcinoma Microenvironment: Cause and Effect. Cancer Manag. Res. 2020, 12, 5957–5974. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Xing, Z.; Lou, C.; Fang, J.; Wang, Z.; Li, M.; He, H.; Bai, H. Fibronectin 1 Derived from Tumor-Associated Macrophages and Fibroblasts Promotes Metastasis through the JUN Pathway in Hepatocellular Carcinoma. Int. Immunopharmacol. 2022, 113, 109420. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W.; Guo, C. Emerging Roles and the Regulation of Aerobic Glycolysis in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 126. [Google Scholar] [CrossRef] [PubMed]

| Protein Name | Target Gene | Forward | Reverse |
|---|---|---|---|
| Glyceraldehyde3-phosphate dehydrogenase | GAPDH | AACAGCGACACCCACTCCTC | CATACCAGGAAATGAGCTTGACAA |
| Proliferating cell nuclear antigen | PCNA | CAAGTAATGTCGATAAAGAGGAGG | GTGTCACCGTTGAAGAGAGTGG |
| Proliferation marker protein KI-67 | KI-67 | GAAAGAGTGGCAACCTGCCTTC | GCACCAAGTTTTACTACATCTGCC |
| Alpha-fetoprotein | AFP | GCAGAGGAGATGTGCTGGATTG | CGTGGTCAGTTTGCAGCATTCTG |
| Hypoxia-inducible factor 1-alpha | HIF1α | TATGAGCCAGAAGAACTTTTAGGC | GATGGCAGTAGCTGCGCTGATA |
| Bcl-2-binding component 3 | BBC3 | ACGACCTCAACGCACAGTACGA | GCAGGAGTCCCATGATGAGATTGT |
| Solute Carrier Family 2, Member 1 | SLC2A1 (GLUT1) | TTGCAGGCTTCTCCAACTGGAC | CAGAACCAGGAGCACAGTGAAG |
| Lactate Dehyfrogenase A | LDHA | GGATCTCCAACATGGCAGCCTT | AGACGGCTTTCTCCCTCTTGCT |
| Secreted Protein Acidic and Cysteine-Rich | SPARC | TGCCTGATGAGACAGAGGTGGT | CTTCGGTTTCCTCTGCACCATC |
| Fibronectin 1 | FN1 | ACAACACCGAGGTGACTGAGAC | GGACACAACGATGCTTCCTGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, M.G.; Fazio, E.; De Pasquale, C.; Sciuto, E.L.; Cannatà, G.; Multisanti, C.R.; Impellitteri, F.; D’Agostino, F.G.; Guglielmino, S.P.P.; Faggio, C.; et al. Physiopathological Features in a Three-Dimensional In Vitro Model of Hepatocellular Carcinoma: Hypoxia-Driven Oxidative Stress and ECM Remodeling. Cancers 2025, 17, 3082. https://doi.org/10.3390/cancers17183082
Rizzo MG, Fazio E, De Pasquale C, Sciuto EL, Cannatà G, Multisanti CR, Impellitteri F, D’Agostino FG, Guglielmino SPP, Faggio C, et al. Physiopathological Features in a Three-Dimensional In Vitro Model of Hepatocellular Carcinoma: Hypoxia-Driven Oxidative Stress and ECM Remodeling. Cancers. 2025; 17(18):3082. https://doi.org/10.3390/cancers17183082
Chicago/Turabian StyleRizzo, Maria Giovanna, Enza Fazio, Claudia De Pasquale, Emanuele Luigi Sciuto, Giorgia Cannatà, Cristiana Roberta Multisanti, Federica Impellitteri, Federica Gilda D’Agostino, Salvatore Pietro Paolo Guglielmino, Caterina Faggio, and et al. 2025. "Physiopathological Features in a Three-Dimensional In Vitro Model of Hepatocellular Carcinoma: Hypoxia-Driven Oxidative Stress and ECM Remodeling" Cancers 17, no. 18: 3082. https://doi.org/10.3390/cancers17183082
APA StyleRizzo, M. G., Fazio, E., De Pasquale, C., Sciuto, E. L., Cannatà, G., Multisanti, C. R., Impellitteri, F., D’Agostino, F. G., Guglielmino, S. P. P., Faggio, C., & Conoci, S. (2025). Physiopathological Features in a Three-Dimensional In Vitro Model of Hepatocellular Carcinoma: Hypoxia-Driven Oxidative Stress and ECM Remodeling. Cancers, 17(18), 3082. https://doi.org/10.3390/cancers17183082










