Uterine Stroma-Derived Tumors and the Extracellular Matrix: A Comparative Review of Benign and Malignant Pathologies
Simple Summary
Abstract
1. Introduction
2. Classification and Histopathology
2.1. Classification and Histopathology of Endometrial Stromal Nodules (ESNs)
2.2. Classification and Histopathology of Low-Grade Endometrial Stromal Sarcomas (LG-ESSs)
2.3. Classification and Histopathology of High-Grade Endometrial Stromal Sarcomas (HG-ESSs)
2.4. Classification and Histopathology of Undifferentiated Uterine Sarcomas (UUSs)
3. Immunohistochemical and Molecular Features
3.1. Immunohistochemical and Molecular Features of Endometrial Stromal Nodules (ESNs)
3.2. Immunohistochemical and Molecular Features of Low-Grade Endometrial Stromal Sarcomas (LG-ESSs)
3.3. Immunohistochemical and Molecular Features of High-Grade Endometrial Stromal Sarcomas (HG-ESSs)
3.4. Immunohistochemical and Molecular Features of Undifferentiated Uterine Sarcomas (UUSs)
4. Extracellular Matrix
4.1. Introduction
4.2. Cellular Components of Endometrial Cancer Microenvironment
4.3. ECM of Endometrial Cancer
4.3.1. GAGs
4.3.2. PGs
4.3.3. Osteopontin (OPN)
4.3.4. Collagens
4.3.5. MMPs-TIMPs
4.3.6. Other ECM Molecules
5. Clinical Presentation and Diagnostic Approach
6. Treatment Options
6.1. Surgical Treatment
6.2. Adjuvant/Neo-Adjuvant Treatment
6.2.1. Uterine Leiomyosarcoma
6.2.2. Low-Grade Endometrial Stromal Sarcoma (LG-ESS)
6.2.3. High-Grade Endometrial Stroma Sarcoma/Undifferentiated Uterine Sarcoma
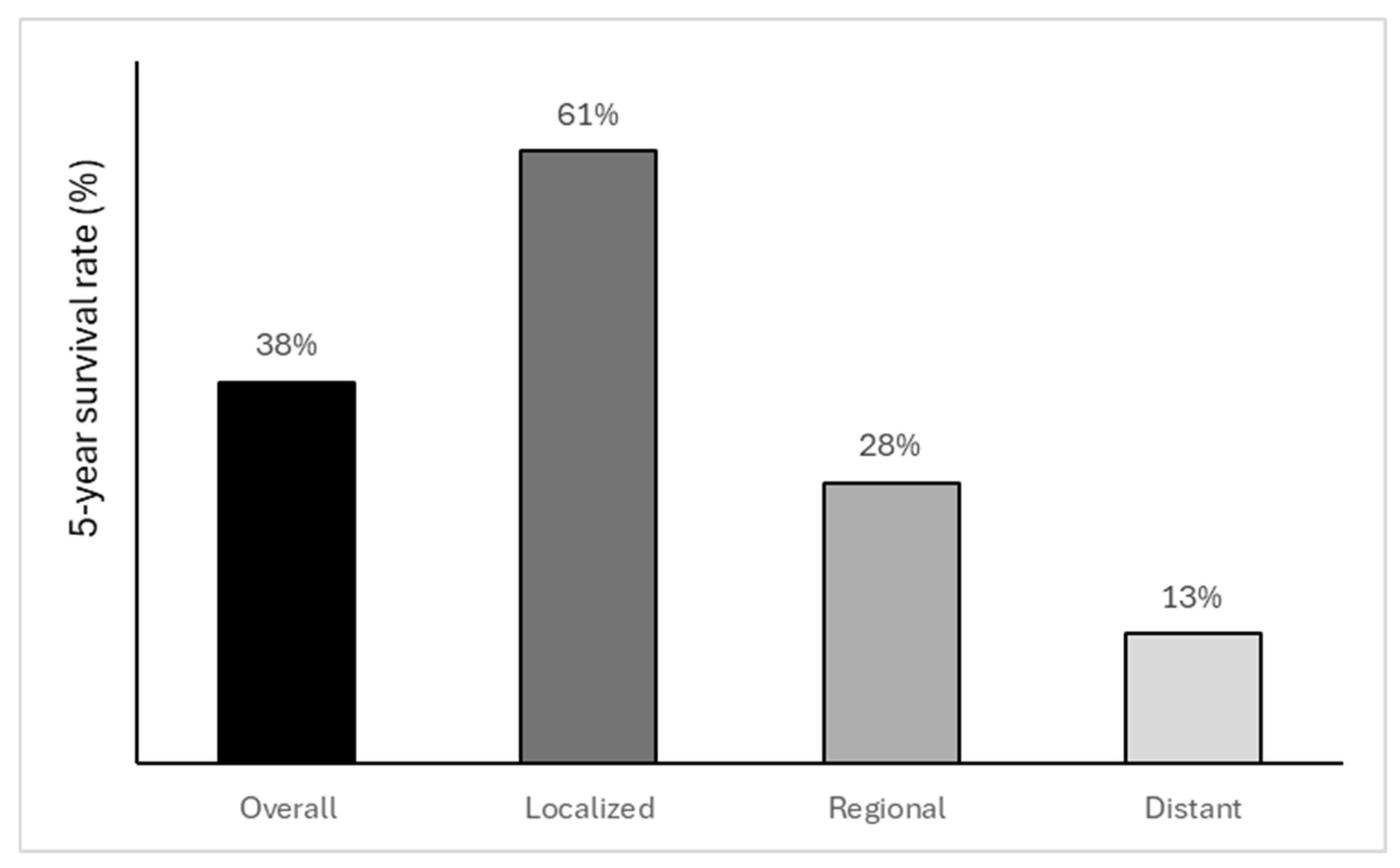
7. Prognosis
8. Emerging Therapies and Future Directions
8.1. Tumors’ Microenvironment and ECM-Targeted Therapies
8.2. Receptor Tyrosine Kinase Inhibitors
8.3. Epigenetic Modulators
8.4. Immune Checkpoint Inhibitors
8.5. Personalized Medicine—Genetic Profiling
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| αSMA | alpha-smooth muscle actin |
| bFGF | basic fibroblast growth factor |
| CAF | carcinoma-associated fibroblasts |
| CCL5 or RANTES | Chemokine ligand 5 |
| CD44 | Cluster of Differentiation 44 |
| CS | chondroitin sulfate |
| CSF-1 | colony-stimulating factor 1 |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| DS | dermatan sulfate |
| ECM | extracellular matrix |
| EEC | endometrial endometrioid carcinoma |
| EMT | epithelial-to-mesenchymal transition |
| ESNs | Stromal Nodules |
| ESTs | Endometrial stromal tumors |
| FIGO | Federation of Gynecology and Obstetrics |
| GAGs | glycosaminoglycans |
| HA | Hyaluronan |
| HDACs | histone deacetylases |
| HG-ESSs | High-Grade Endometrial Stromal Sarcomas |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| HS | heparan sulfate |
| ICI | Immune Checkpoint Inhibitors |
| IL | interleukin |
| IL-1b | interleukin 1 beta |
| IL-6 | interleukin 6 |
| KS | keratan sulfate |
| LG-ESSs | Low-Grade Endometrial Stromal Sarcomas |
| LOX | lysyl oxidase |
| LRP6 | Low-density lipoprotein receptor-related protein 6 |
| MCP-1 or CCL2 | monocyte chemotactic protein- 1 |
| MMPs | Matrix metalloproteinases |
| NK | natural killer |
| OPN | osteopontin |
| PD-1 | Programmed cell Death protein 1 |
| PDGFR | Platelet-Derived Growth Factor receptor |
| PGs | proteoglycans |
| PR | progesterone receptor |
| ER | estrogen receptor |
| RHAMM | receptor for hyaluronic acid-mediated motility |
| RTKs | Receptor tyrosine kinases |
| SLRPs | small leucine-rich proteoglycans |
| SMA | smooth muscle actin |
| TADCs | tumor-associated dendritic cells |
| TAM | tumor-associated macrophages |
| TIMPs | inhibitors of metalloproteinases |
| TKIs | tyrosine kinase inhibitors |
| TME | tumor microenvironment |
| TNF-a | tumor necrosis factor-alpha |
| UCEC | uterine corpus endometrial carcinoma |
| UUSs | Undifferentiated Uterine Sarcomas |
| VEGF | vascular endothelial growth factor |
References
- Mahdy, H.; Vadakekut, E.S.; Crotzer, D. Endometrial Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gadducci, A.; Multinu, F.; De Vitis, L.A.; Cosio, S.; Carinelli, S.; Aletti, G.D. Endometrial Stromal Tumors of the Uterus: Epidemiology, Pathological and Biological Features, Treatment Options and Clinical Outcomes. Gynecol. Oncol. 2023, 171, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Zheng, W. Endometrial Stromal Tumors: Diagnostic Updates and Challenges. Semin. Diagn. Pathol. 2022, 39, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Akaev, I.; Yeoh, C.C.; Rahimi, S. Update on Endometrial Stromal Tumours of the Uterus. Diagnostics 2021, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Ricotta, G.; Russo, S.A.; Fagotti, A.; Martinez, A.; Gauroy, E.; Del, M.; Thibaud, V.; Guillaume, B.; Ferron, G. Endometrial Stromal Sarcoma: An Update. Cancers 2025, 17, 1893. [Google Scholar] [CrossRef]
- Wang, C.; Liang, D.; Kuang, W.; Sun, H.; Kou, Y.; Wang, W.; Zeng, J. Risk Factors, Survival Analysis, and Nomograms for High-Grade Endometrial Stromal Sarcoma Patients with Distant Metastasis: A Population-Based Study (2010–2019). Front. Oncol. 2025, 15, 1567195. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, T.; Li, N.; Yao, H. Undifferentiated Uterine Sarcoma: Experience of a Single Center. World J. Surg. Oncol. 2024, 22, 325. [Google Scholar] [CrossRef]
- Hoang, L.; Chiang, S.; Lee, C.-H. Endometrial Stromal Sarcomas and Related Neoplasms: New Developments and Diagnostic Considerations. Pathology 2018, 50, 162–177. [Google Scholar] [CrossRef]
- Ali, R.H.; Rouzbahman, M. Endometrial Stromal Tumours Revisited: An Update Based on the 2014 WHO Classification. J. Clin. Pathol. 2015, 68, 325–332. [Google Scholar] [CrossRef]
- Oliva, E. Practical Issues in Uterine Pathology from Banal to Bewildering: The Remarkable Spectrum of Smooth Muscle Neoplasia. Mod. Pathol. 2016, 29, S104–S120. [Google Scholar] [CrossRef]
- Guo, W.; Chen, G.; Zhu, C.; Wang, H. Expression of Matrix Metalloproteinase-2, 9 and It’s Tissue Inhibitor-1, 2 in Endometrial Carcinoma. Zhonghua Fu Chan Ke Za Zhi 2002, 37, 604–607. [Google Scholar]
- Pautier, P.; Nam, E.J.; Provencher, D.M.; Hamilton, A.L.; Mangili, G.; Siddiqui, N.A.; Westermann, A.M.; Reed, N.S.; Harter, P.; Ray-Coquard, I. Gynecologic Cancer InterGroup (GCIG) Consensus Review for High-Grade Undifferentiated Sarcomas of the Uterus. Int. J. Gynecol. Cancer 2014, 24, S73–S77. [Google Scholar] [CrossRef]
- Subbaraya, S.; Murthy, S.S.; Devi, G.S. Immunohistochemical and Molecular Characterization of Endometrial Stromal Sarcomas. Clin. Pathol. 2020, 13, 2632010–20916736. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, Y.; Fu, F.; Ren, W.; Wang, T.; Wang, S.; Li, Y. Advancement in Multi-Omics Approaches for Uterine Sarcoma. Biomark. Res. 2024, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Berdiaki, A.; Neagu, M.; Tzanakakis, P.; Spyridaki, I.; Pérez, S.; Nikitovic, D. Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease. Biomolecules 2024, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yang, H.; Zhang, X.; Xu, W. The Emerging Roles of Exosomes in Tumor-Stroma Interaction. J. Cancer Res. Clin. Oncol. 2016, 142, 1897–1907. [Google Scholar] [CrossRef]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological Hallmarks of Stromal Cells in the Tumour Microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef]
- Casas-Arozamena, C.; Abal, M. Endometrial Tumour Microenvironment. Adv. Exp. Med. Biol. 2020, 1296, 215–225. [Google Scholar] [CrossRef]
- Li, M.; Xin, X.Y.; Jin, Z.S.; Hua, T.; Wang, H.B.; Wang, H.B. Transcriptomic Analysis of Stromal Cells from Patients with Endometrial Carcinoma. Int. J. Clin. Exp. Pathol. 2017, 10, 9853–9857. [Google Scholar]
- Tzanakakis, G.; Giatagana, E.-M.; Kuskov, A.; Berdiaki, A.; Tsatsakis, A.M.; Neagu, M.; Nikitovic, D. Proteoglycans in the Pathogenesis of Hormone-Dependent Cancers: Mediators and Effectors. Cancers 2020, 12, 2401. [Google Scholar] [CrossRef]
- Dun, E.C.; Hanley, K.; Wieser, F.; Bohman, S.; Yu, J.; Taylor, R.N. Infiltration of Tumor-Associated Macrophages Is Increased in the Epithelial and Stromal Compartments of Endometrial Carcinomas. Int. J. Gynecol. Pathol. 2013, 32, 576–584. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-Associated Macrophages in Tumor Metastasis: Biological Roles and Clinical Therapeutic Applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef]
- Krishnan, V.; Schaar, B.; Tallapragada, S.; Dorigo, O. Tumor Associated Macrophages in Gynecologic Cancers. Gynecol. Oncol. 2018, 149, 205–213. [Google Scholar] [CrossRef]
- Najafi, M.; Goradel, N.H.; Farhood, B.; Salehi, E.; Solhjoo, S.; Toolee, H.; Kharazinejad, E.; Mortezaee, K. Tumor Microenvironment: Interactions and Therapy. J. Cell Physiol. 2019, 234, 5700–5721. [Google Scholar] [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-Associated Fibroblasts: Orchestrating the Composition of Malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Zhang, X.D.; Hondermarck, H.; Tanwar, P.S. The Emerging Role of the Microenvironment in Endometrial Cancer. Cancers 2018, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.S.; Kim, H.-J.; Yoon, J.-H.; Yoo, S.-C.; Jo, H.; Lee, S.Y.; Min, C.K.; Ryu, H.-S. Endometrial Cancer Invasion Depends on Cancer-Derived Tumor Necrosis Factor-Alpha and Stromal Derived Hepatocyte Growth Factor. Int. J. Cancer 2009, 124, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Tian, W.Y.; Wang, Y.M.; Zhang, Y.F.; Guo, F.; Zhao, J.; Gao, C.; Xue, F.X. Cancer-Associated Fibroblasts Promote the Progression of Endometrial Cancer via the SDF-1/CXCR4 Axis. J. Hematol. Oncol. 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, S.; Mangoni, M.; Castiglione, F.; Beltrami, C.; Pieralli, A.; Andersson, K.L.; Fambrini, M.; Taddei, G.L.; Serio, M.; Orlando, C. The CXCR4/CXCL12 Axis in Endometrial Cancer. Clin. Exp. Metastasis 2009, 26, 261–268. [Google Scholar] [CrossRef]
- Subramaniam, K.S.; Omar, I.S.; Kwong, S.C.; Mohamed, Z.; Woo, Y.L.; Mat Adenan, N.A.; Chung, I. Cancer-Associated Fibroblasts Promote Endometrial Cancer Growth via Activation of Interleukin-6/STAT-3/c-Myc Pathway. Am. J. Cancer Res. 2016, 6, 200–213. [Google Scholar]
- Tzanakakis, G.; Kavasi, R.-M.; Voudouri, K.; Berdiaki, A.; Spyridaki, I.; Tsatsakis, A.; Nikitovic, D. Role of the Extracellular Matrix in Cancer-Associated Epithelial to Mesenchymal Transition Phenomenon. Dev. Dyn. 2018, 247, 368–381. [Google Scholar] [CrossRef]
- Felix, A.S.; Weissfeld, J.; Edwards, R.; Linkov, F. Future Directions in the Field of Endometrial Cancer Research: The Need to Investigate the Tumor Microenvironment. Eur. J. Gynaecol. Oncol. 2010, 31, 139–144. [Google Scholar] [PubMed]
- Kamat, A.A.; Merritt, W.M.; Coffey, D.; Lin, Y.G.; Patel, P.R.; Broaddus, R.; Nugent, E.; Han, L.Y.; Landen, C.N.; Spannuth, W.A.; et al. Clinical and Biological Significance of Vascular Endothelial Growth Factor in Endometrial Cancer. Clin. Cancer Res. 2007, 13, 7487–7495. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Nakagawara, A.; Oosaki, T.; Hayashi, Y.; Hirono, M.; Yoshihara, T. Expression of Vascular Endothelial Growth Factors (VEGF-A/VEGF-1 and VEGF-C/VEGF-2) in Postmenopausal Uterine Endometrial Carcinoma. Gynecol. Oncol. 2001, 80, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dyke, J.M.; Crook, M.L.; Platten, M.; Stewart, C.J.R. Extravascular Migratory Metastasis in Gynaecological Carcinosarcoma. Histopathology 2014, 65, 363–370. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Keely, P.J. Mechanical Signaling through the Cytoskeleton Regulates Cell Proliferation by Coordinated Focal Adhesion and Rho GTPase Signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef]
- Leppert, P.C.; Jayes, F.L.; Segars, J.H. The Extracellular Matrix Contributes to Mechanotransduction in Uterine Fibroids. Obstet. Gynecol. Int. 2014, 2014, 783289. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Giatagana, E.-M.; Kuskov, A.; Tsatsakis, A.M.; Tzanakakis, G.N.; Nikitovic, D. Glycosaminoglycans: Carriers and Targets for Tailored Anti-Cancer Therapy. Biomolecules 2021, 11, 395. [Google Scholar] [CrossRef]
- Iozzo, R.V. Proteoglycans: Structure, Biology, and Molecular Interactions; Experientia Supplementum; Marcel Dekker: New York, NY, USA, 2000; ISBN 9786610105328. [Google Scholar]
- Aplin, J.D.; Charlton, A.K.; Ayad, S. An Immunohistochemical Study of Human Endometrial Extracellular Matrix during the Menstrual Cycle and First Trimester of Pregnancy. Cell Tissue Res. 1988, 253, 231–240. [Google Scholar] [CrossRef]
- Kelly, F.D.; Tawia, S.A.; Rogers, P.A. Immunohistochemical Characterization of Human Endometrial Microvascular Basement Membrane Components during the Normal Menstrual Cycle. Hum. Reprod. 1995, 10, 268–276. [Google Scholar] [CrossRef]
- Hoadley, M.E.; Seif, M.W.; Aplin, J.D. Menstrual-Cycle-Dependent Expression of Keratan Sulphate in Human Endometrium. Biochem. J. 1990, 266, 757–763. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Dermatan Sulfate Proteoglycan Biglycan as a Potential Selectin L/CD44 Ligand Involved in Selective Recruitment of Peripheral Blood CD16(-) Natural Killer Cells into Human Endometrium. J. Leukoc. Biol. 2009, 85, 391–400. [Google Scholar] [CrossRef]
- Nasciutti, L.E.; Ferrari, R.; Berardo, P.T.; Souza, M.L.; Takiya, C.M.; Borojevic, R.; Abrao, M.S.; Silva, L.C. Distribution of Chondroitin Sulfate in Human Endometrium. Micron 2006, 37, 544–550. [Google Scholar] [CrossRef]
- Hosokawa, S.; Norimatsu, Y.; Shinagawa, A.; Kurokawa, T.; Yoshida, Y.; Nishikawa, T.; Suzuki, H.; Irino, S.; Kobayashi, T.K. Assessment of the Localization of Chondroitin Sulfate in Various Types of Endometrial Carcinoma. PLoS ONE 2024, 19, e0304420. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Hu, M.; Huang, K.; Lin, S.; Du, H. Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression. Int. J. Mol. Sci. 2020, 21, 5983. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Mambetsariev, N.; Mirzapoiazova, T.; Mambetsariev, B.; Sammani, S.; Lennon, F.E.; Garcia, J.G.; Singleton, P.A. Hyaluronic Acid Binding Protein 2 Is a Novel Regulator of Vascular Integrity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 483–490. [Google Scholar] [CrossRef]
- Nikitovic, D.; Tzardi, M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N. Cancer Microenvironment and Inflammation: Role of Hyaluronan. Front. Immunol. 2015, 6, 169. [Google Scholar] [CrossRef]
- Kuang, D.M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.M.; Zheng, L. Tumor-Derived Hyaluronan Induces Formation of Immunosuppressive Macrophages through Transient Early Activation of Monocytes. Blood 2007, 110, 587–595. [Google Scholar] [CrossRef]
- Kobayashi, N.; Miyoshi, S.; Mikami, T.; Koyama, H.; Kitazawa, M.; Takeoka, M.; Sano, K.; Amano, J.; Isogai, Z.; Niida, S.; et al. Hyaluronan Deficiency in Tumor Stroma Impairs Macrophage Trafficking and Tumor Neovascularization. Cancer Res. 2010, 70, 7073–7083. [Google Scholar] [CrossRef]
- Nykopp, T.K.; Rilla, K.; Tammi, M.I.; Tammi, R.H.; Sironen, R.; Hamalainen, K.; Kosma, V.M.; Heinonen, S.; Anttila, M. Hyaluronan Synthases (HAS1-3) and Hyaluronidases (HYAL1-2) in the Accumulation of Hyaluronan in Endometrioid Endometrial Carcinoma. BMC Cancer 2010, 10, 512. [Google Scholar] [CrossRef]
- Kouvidi, K.; Nikitovic, D.; Berdiaki, A.; Tzanakakis, G.N. Hyaluronan/RHAMM Interactions in Mesenchymal Tumor Pathogenesis: Role of Growth Factors. Adv. Cancer Res. 2014, 123, 319–349. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.T.; Roehrig, K.; Schondorf, T.; Lazar, A.; Fleisch, M.; Niederacher, D.; Bender, H.G.; Dall, P. Expression of the Hyaluronan Receptor RHAMM in Endometrial Carcinomas Suggests a Role in Tumour Progression and Metastasis. J. Cancer Res. Clin. Oncol. 2003, 129, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Agren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.M. Hyaluronan in Peritumoral Stroma and Malignant Cells Associates with Breast Cancer Spreading and Predicts Survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Anttila, M.A.; Tammi, R.H.; Tammi, M.I.; Syrjänen, K.J.; Saarikoski, S.V.; Kosma, V.M. High Levels of Stromal Hyaluronan Predict Poor Disease Outcome in Epithelial Ovarian Cancer. Cancer Res. 2000, 60, 150–155. [Google Scholar]
- Berdiaki, A.; Giatagana, E.-M.; Tzanakakis, G.; Nikitovic, D. The Landscape of Small Leucine-Rich Proteoglycan Impact on Cancer Pathogenesis with a Focus on Biglycan and Lumican. Cancers 2023, 15, 3549. [Google Scholar] [CrossRef]
- Kitaya, K.; Tada, Y.; Hayashi, T.; Taguchi, S.; Funabiki, M.; Nakamura, Y.; Yasuo, T. Diverse Functions of Uterine Proteoglycans in Human Reproduction (Review). Mol. Med. Rep. 2012, 5, 1375–1381. [Google Scholar] [CrossRef]
- Lucariello, A.; Trabucco, E.; Boccia, O.; Perna, A.; Sellitto, C.; Castaldi, M.A.; De Falco, M.; De Luca, A.; Cobellis, L. Small Leucine Rich Proteoglycans Are Differently Distributed in Normal and Pathological Endometrium. In Vivo 2015, 29, 217–222. [Google Scholar]
- Halari, C.D.; Zheng, M.; Lala, P.K. Roles of Two Small Leucine-Rich Proteoglycans Decorin and Biglycan in Pregnancy and Pregnancy-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 10584. [Google Scholar] [CrossRef]
- Appunni, S.; Anand, V.; Khandelwal, M.; Gupta, N.; Rubens, M.; Sharma, A. Small Leucine Rich Proteoglycans (Decorin, Biglycan and Lumican) in Cancer. Clin. Chim. Acta 2019, 491, 1–7. [Google Scholar] [CrossRef]
- Schaefer, L.; Iozzo, R.V. Biological Functions of the Small Leucine-Rich Proteoglycans: From Genetics to Signal Transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycans in Health and Disease: Novel Regulatory Signaling Mechanisms Evoked by the Small Leucine-Rich Proteoglycans. FEBS J. 2010, 277, 3864–3875. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin: A Guardian from the Matrix. Am. J. Pathol. 2012, 181, 380–387. [Google Scholar] [CrossRef]
- Nikitovic, D.; Katonis, P.; Tsatsakis, A.; Karamanos, N.K.; Tzanakakis, G.N. Lumican, a Small Leucine-Rich Proteoglycan. IUBMB Life 2008, 60, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Levens, E.; Luo, X.; Ding, L.; Williams, R.S.; Chegini, N. Fibromodulin Is Expressed in Leiomyoma and Myometrium and Regulated by Gonadotropin-Releasing Hormone Analogue Therapy and TGF-Beta through Smad and MAPK-Mediated Signalling. Mol. Hum. Reprod. 2005, 11, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Luo, X.; Chegini, N. Genomic and Proteomic Profiling I: Leiomyomas in African Americans and Caucasians. Reprod. Biol. Endocrinol. 2007, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Catherino, W.H. Novel Method to Characterize Primary Cultures of Leiomyoma and Myometrium with the Use of Confirmatory Biomarker Gene Arrays. Fertil. Steril. 2007, 87, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.G.; Sampaio, L.O.; Franco, C.R.; Cesar, R.M.; Michelacci, Y.M. A Comparative Analysis of Structure and Spatial Distribution of Decorin in Human Leiomyoma and Normal Myometrium. Biochim. Biophys. Acta 2003, 1619, 98–112. [Google Scholar] [CrossRef]
- San Martin, S.; Zorn, T.M. The Small Proteoglycan Biglycan Is Associated with Thick Collagen Fibrils in the Mouse Decidua. Cell. Mol. Biol. 2003, 49, 673–678. [Google Scholar]
- Sanches, J.C.; Jones, C.J.; Aplin, J.D.; Iozzo, R.V.; Zorn, T.M.; Oliveira, S.F. Collagen Fibril Organization in the Pregnant Endometrium of Decorin-Deficient Mice. J. Anat. 2010, 216, 144–155. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Sakko, A.J.; Ween, M.P.; Russell, D.L.; Horsfall, D.J. The Biological Role and Regulation of Versican Levels in Cancer. Cancer Metastasis Rev. 2009, 28, 233–245. [Google Scholar] [CrossRef]
- Kodama, J.; Hasengaowa; Kusumoto, T.; Seki, N.; Matsuo, T.; Ojima, Y.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Prognostic Significance of Stromal Versican Expression in Human Endometrial Cancer. Ann. Oncol. 2007, 18, 269–274. [Google Scholar] [CrossRef]
- Onyeisi, J.O.S.; Lopes, C.C.; Götte, M. Syndecan-4 as a Pathogenesis Factor and Therapeutic Target in Cancer. Biomolecules 2021, 11, 503. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, J.-H.; Ryu, H.-S.; Kim, H.C.; Han, J.H.; Lee, J.S.; Min, C.K. Syndecan-1, a Key Regulator of Cell Viability in Endometrial Cancer. Int. J. Cancer 2007, 121, 741–750. [Google Scholar] [CrossRef]
- Young, M.F.; Kerr, J.M.; Termine, J.D.; Wewer, U.M.; Wang, M.G.; McBride, O.W.; Fisher, L.W. cDNA Cloning, mRNA Distribution and Heterogeneity, Chromosomal Location, and RFLP Analysis of Human Osteopontin (OPN). Genomics 1990, 7, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Figula, J.; Baran, R.M.; Jach, R. Osteopontin as a Marker of Endometriosis—The Current State of Knowledge. Ginekol. Pol. 2024, 95, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huan, X.; Xiao, G.H.; Yu, W.H.; Li, T.F.; Gao, X.D.; Zhang, Y.C. Osteopontin and Its Downstream Carcinogenic Molecules: Regulatory Mechanisms and Prognostic Value in Cancer Progression. Neoplasma 2022, 69, 1253–1269. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Bulbule, A.; Kundu, G.C. Osteopontin Stimulates Tumor Growth and Activation of Promatrix Metalloproteinase-2 through Nuclear Factor-Kappa B-Mediated Induction of Membrane Type 1 Matrix Metalloproteinase in Murine Melanoma Cells. J. Biol. Chem. 2001, 276, 44926–44935. [Google Scholar] [CrossRef]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. S1), S64–S74. [Google Scholar] [CrossRef]
- Fu, X.; Yao, M.; Ye, C.; Fang, T.; Wu, R. Osteopontin Regulates Endometrial Stromal Cell Migration in Endometriosis through the PI3K Pathway: Osteopontin Regulates Endometrial Cell Migration in Endometriosis. Reprod. Sci. 2021, 28, 435–446. [Google Scholar] [CrossRef]
- Ho, N.-T.; Lin, S.-W.; Lee, Y.-R.; Tzeng, C.-R.; Kao, S.-H. Osteopontin Splicing Isoforms Contribute to Endometriotic Proliferation, Migration, and Epithelial-Mesenchymal Transition in Endometrial Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 15328. [Google Scholar] [CrossRef]
- Castello, L.M.; Raineri, D.; Salmi, L.; Clemente, N.; Vaschetto, R.; Quaglia, M.; Garzaro, M.; Gentilli, S.; Navalesi, P.; Cantaluppi, V.; et al. Osteopontin at the Crossroads of Inflammation and Tumor Progression. Mediat. Inflamm. 2017, 2017, 4049098. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The Multiple Functions and Mechanisms of Osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Giachelli, C.M.; Schwartz, S.M.; Vracko, R. Macrophages Express Osteopontin during Repair of Myocardial Necrosis. Am. J. Pathol. 1994, 145, 1450–1462. [Google Scholar] [PubMed]
- Kunii, Y.; Niwa, S.; Hagiwara, Y.; Maeda, M.; Seitoh, T.; Suzuki, T. The Immunohistochemical Expression Profile of Osteopontin in Normal Human Tissues Using Two Site-Specific Antibodies Reveals a Wide Distribution of Positive Cells and Extensive Expression in the Central and Peripheral Nervous Systems. Med. Mol. Morphol. 2009, 42, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin—A Promising Biomarker for Cancer Therapy. J. Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef]
- Cho, H.; Kang, E.S.; Kim, Y.T.; Kim, J.H. Diagnostic and Prognostic Impact of Osteopontin Expression in Endometrial Cancer. Cancer Investig. 2009, 27, 313–323. [Google Scholar] [CrossRef]
- Kardelen, E.; Atakul, T.; Yüksel, H. In Vitro Effects of Osteopontin on Endometrial Cancer Cells and Signaling Pathways in Epithelial-Mesenchymal Transition. Eur. J. Gynaecol. Oncol. 2024, 45, 152–161. Available online: https://www.ejgo.net/articles/10.22514/ejgo.2024.103 (accessed on 16 September 2025). [CrossRef]
- Li, Y.; Xie, Y.; Cui, D.; Ma, Y.; Sui, L.; Zhu, C.; Kong, H.; Kong, Y. Osteopontin Promotes Invasion, Migration and Epithelial-Mesenchymal Transition of Human Endometrial Carcinoma Cell HEC-1A Through AKT and ERK1/2 Signaling. Cell Physiol. Biochem. 2015, 37, 1503–1512. [Google Scholar] [CrossRef]
- Weber, G.F. The Cancer Biomarker Osteopontin: Combination with Other Markers. Cancer Genom. Proteom. 2011, 8, 263–288. [Google Scholar]
- Al Maghrabi, H.; Gomaa, W.; Al-Maghrabi, J. Increased Osteopontin Expression in Endometrial Carcinoma Is Associated with Better Survival Outcome. Ginekol. Pol. 2020, 91, 73–78. [Google Scholar] [CrossRef]
- Gu, Y.; Muller, W.J. The Multifaceted Role of Osteopontin in Modulating the Tumor Microenvironment. Cancer Res. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Ruggiero, F. The Collagen Superfamily: From the Extracellular Matrix to the Cell Membrane. Pathol. Biol. 2005, 53, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen Density Promotes Mammary Tumor Initiation and Progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, N.; Bissell, M.J. Extracellular Matrix Signaling: Integration of Form and Function in Normal and Malignant Cells. Curr. Opin. Cell Biol. 1998, 10, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.G.; Oba, S.M.; Michelacci, Y.M.; Sampaio, L.O. Galactosaminoglycans from Normal Myometrium and Leiomyoma. Braz. J. Med. Biol. Res. 2001, 34, 633–637. [Google Scholar] [CrossRef]
- Leppert, P.C.; Baginski, T.; Prupas, C.; Catherino, W.H.; Pletcher, S.; Segars, J.H. Comparative Ultrastructure of Collagen Fibrils in Uterine Leiomyomas and Normal Myometrium. Fertil. Steril. 2004, 82 (Suppl. S3), 1182–1187. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Bellingham, C.M.; Davis, E.C.; Hubmacher, D.; Reinhardt, D.P.; Mecham, R.P.; Keeley, F.W. Fibrillins, Fibulins, and Matrix-Associated Glycoprotein Modulate the Kinetics and Morphology of In Vitro Self-Assembly of a Recombinant Elastin-like Polypeptide. Biochemistry 2008, 47, 12601–12613. [Google Scholar] [CrossRef]
- Leppert, P.C.; Yu, S.Y. Three-Dimensional Structures of Uterine Elastic Fibers: Scanning Electron Microscopic Studies. Connect. Tissue Res. 1991, 27, 15–31. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of Fibronectin Extracellular Matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef]
- Hocking, D.C.; Titus, P.A.; Sumagin, R.; Sarelius, I.H. Extracellular Matrix Fibronectin Mechanically Couples Skeletal Muscle Contraction with Local Vasodilation. Circ. Res. 2008, 102, 372–379. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Bazellieres, E.; Allen, M.D.; Andreu, I.; Oria, R.; Sunyer, R.; Gomm, J.J.; Marshall, J.F.; Jones, J.L.; Trepat, X.; et al. Rigidity Sensing and Adaptation through Regulation of Integrin Types. Nat. Mater. 2014, 13, 631–637. [Google Scholar] [CrossRef]
- Israeli-Rosenberg, S.; Manso, A.M.; Okada, H.; Ross, R.S. Integrins and Integrin-Associated Proteins in the Cardiac Myocyte. Circ. Res. 2014, 114, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.V.; Letarte, M.; Lye, S.J. The Expression of Integrins and Cadherins in Normal Human Uterus and Uterine Leiomyomas. Am. J. Obstet. Gynecol. 1996, 175, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Peavey, M.; Salleh, N.; Leppert, P. Collagen-Binding Alpha11 Integrin Expression in Human Myometrium and Fibroids Utilizing a Novel RNA in Situ Probe. Reprod. Sci. 2014, 21, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Segars, J.; Catherino, W.H. Integrin β1 Regulates Leiomyoma Cytoskeletal Integrity and Growth. Matrix Biol. 2012, 31, 389–397. [Google Scholar] [CrossRef]
- Catherino, W.H.; Leppert, P.C.; Stenmark, M.H.; Payson, M.; Potlog-Nahari, C.; Nieman, L.K.; Segars, J.H. Reduced Dermatopontin Expression Is a Molecular Link between Uterine Leiomyomas and Keloids. Genes Chromosomes Cancer 2004, 40, 204–217. [Google Scholar] [CrossRef]
- Sampaio, L.O.; Dietrich, C.P.; Filho, O.G. Changes in Sulfated Mucopolysaccharide Composition of Mammalian Tissues during Growth and in Cancer Tissues. Biochim. Biophys. Acta 1977, 498, 123–131. [Google Scholar] [CrossRef]
- Barker, H.E.; Cox, T.R.; Erler, J.T. The Rationale for Targeting the LOX Family in Cancer. Nat. Rev. Cancer 2012, 12, 540–552. [Google Scholar] [CrossRef]
- Jussila, T.; Kauppila, S.; Bode, M.; Tapanainen, J.; Risteli, J.; Risteli, L.; Kauppila, A.; Stenbäck, F. Synthesis and Maturation of Type I and Type III Collagens in Endometrial Adenocarcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 66–74. [Google Scholar] [CrossRef]
- Zhang, Q.; An, Z.-Y.; Jiang, W.; Jin, W.-L.; He, X.-Y. Collagen Code in Tumor Microenvironment: Functions, Molecular Mechanisms, and Therapeutic Implications. Biomed. Pharmacother. 2023, 166, 115390. [Google Scholar] [CrossRef]
- Fata, J.E.; Ho, A.T.; Leco, K.J.; Moorehead, R.A.; Khokha, R. Cellular Turnover and Extracellular Matrix Remodeling in Female Reproductive Tissues: Functions of Metalloproteinases and Their Inhibitors. Cell. Mol. Life Sci. CMLS 2000, 57, 77–95. [Google Scholar] [CrossRef]
- Norris, H.J.; Taylor, H.B. Mesenchymal Tumors of the Uterus. I. A Clinical and Pathological Study of 53 Endometrial Stromal Tumors. Cancer 1966, 19, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Honkavuori, M.; Talvensaari-Mattila, A.; Soini, Y.; Turpeenniemi-Hujanen, T.; Santala, M. MMP-2 Expression Associates with CA 125 and Clinical Course in Endometrial Carcinoma. Gynecol. Oncol. 2007, 104, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Talvensaari-Mattila, A.; Santala, M.; Soini, Y.; Turpeenniemi-Hujanen, T. Prognostic Value of Matrix Metalloproteinase-2 (MMP-2) Expression in Endometrial Endometrioid Adenocarcinoma. Anticancer Res. 2005, 25, 4101–4105. [Google Scholar] [PubMed]
- Graesslin, O.; Cortez, A.; Fauvet, R.; Lorenzato, M.; Birembaut, P.; Darai, E. Metalloproteinase-2, -7 and -9 and Tissue Inhibitor of Metalloproteinase-1 and -2 Expression in Normal, Hyperplastic and Neoplastic Endometrium: A Clinical-Pathological Correlation Study. Ann. Oncol. 2006, 17, 637–645. [Google Scholar] [CrossRef]
- Tongtawee, T.; Kaewpitoon, S.J.; Loyd, R.; Chanvitan, S.; Leelawat, K.; Praditpol, N.; Jujinda, S.; Kaewpitoon, N. High Expression of Matrix Metalloproteinase-11 Indicates Poor Prognosis in Human Cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 3697–3701. [Google Scholar] [CrossRef][Green Version]
- Liokumovich, P.; Goldberg, I.; Davidson, B.; Gotlieb, W.H.; Zahavi, T.; Ben-Baruch, G.; Reder, I.; Kopolovic, J. Expression of Metalloproteinases Endometrial Stromal Sarcoma: Immunohistochemical Study Using Image Analysis. J. Clin. Pathol. 1999, 52, 198–202. [Google Scholar] [CrossRef]
- Di Nezza, L.A.; Misajon, A.; Zhang, J.; Jobling, T.; Quinn, M.A.; Ostor, A.G.; Nie, G.; Lopata, A.; Salamonsen, L.A. Presence of Active Gelatinases in Endometrial Carcinoma and Correlation of Matrix Metalloproteinase Expression with Increasing Tumor Grade and Invasion. Cancer 2002, 94, 1466–1475. [Google Scholar] [CrossRef]
- Gomez-Macias, G.S.; Garza-Rodriguez, M.L.; Garza-Guajardo, R.; Monsivais-Ovalle, D.; Ancer-Rodriguez, J.; Barrera-Saldana, H.A.; Barboza-Quintana, O. Overexpression of the Matrix Metalloproteinase 11 Gene Is a Potential Biomarker for Type 1 Endometrial Cancer. Oncol. Lett. 2018, 16, 1073–1078. [Google Scholar] [CrossRef]
- Murphy, G.; Nagase, H. Progress in Matrix Metalloproteinase Research. Mol. Asp. Med. 2008, 29, 290–308. [Google Scholar] [CrossRef]
- Obokata, A.; Watanabe, J.; Nishimura, Y.; Arai, T.; Kawaguchi, M.; Kuramoto, H. Significance of Matrix Metalloproteinase-7 [Correction of Matrix Metalloproteinase-2], -11 and Tissue Inhibitor of Metalloproteinase-1 Expression in Normal, Hyperplastic and Neoplastic Endometrium. Anticancer Res. 2007, 27, 95–105. [Google Scholar] [PubMed]
- Docherty, A.J.; Lyons, A.; Smith, B.J.; Wright, E.M.; Stephens, P.E.; Harris, T.J.; Murphy, G.; Reynolds, J.J. Sequence of Human Tissue Inhibitor of Metalloproteinases and Its Identity to Erythroid-Potentiating Activity. Nature 1985, 318, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Hampton, A.L.; Salamonsen, L.A. Expression of Messenger Ribonucleic Acid Encoding Matrix Metalloproteinases and Their Tissue Inhibitors Is Related to Menstruation. J. Endocrinol. 1994, 141, R1–R3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Fan, Y.; Lang, F.; Fu, F.; Liu, Q. MMP11 and MMP17 Are Potential Biomarkers for Uterine Corpus Endometrial Carcinoma Prognosis. Clin. Transl. Oncol. 2024, 26, 653–663. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Zhang, M.; Li, N.; Chen, Y.; Su, W.; Liu, Y.; Lu, D.; Li, S.; Yang, Y.; et al. Legumain: A Biomarker for Diagnosis and Prognosis of Human Ovarian Cancer. J. Cell Biochem. 2012, 113, 2679–2686. [Google Scholar] [CrossRef]
- Bayramoglu, Z.; Kilinc, A.N.U.; Omeroglu, E.; Yilmaz, F.; Bayramoglu, D.; Unlu, Y.; Aydin, H.A. Expression of Extracellular Matrix Proteins Nidogen-1 and Legumain in Endometrial Carcinomas. J. Obstet. Gynaecol. Res. 2022, 48, 1019–1025. [Google Scholar] [CrossRef]
- Lebar, V.; Čelebić, A.; Calleja-Agius, J.; Jakimovska Stefanovska, M.; Drusany Staric, K. Advancements in Uterine Sarcoma Management: A Review. Eur. J. Surg. Oncol. 2025, 51, 109646. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Casali, P.G.; Croce, S.; Fennessy, F.M.; Fischerova, D.; Jones, R.; Sanfilippo, R.; Zapardiel, I.; Amant, F.; Blay, J.Y.; et al. ESGO/EURACAN/GCIG Guidelines for the Management of Patients with Uterine Sarcomas. Int. J. Gynecol. Cancer 2024, 34, 1499–1521. [Google Scholar] [CrossRef]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in Gynecological Disease (15): Clinical and Ultrasound Characteristics of Uterine Sarcoma. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, J.P.; Steiner, A.; Bay, C.; Eisenhauer, E.; Muto, M.G.; George, S.; Fennessy, F.M. Differentiating Leiomyosarcoma from Leiomyoma: In Support of an MR Imaging Predictive Scoring System. Abdom. Radiol. 2021, 46, 4927–4935. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Zawaideh, J.P.; Sahin, H.; Freeman, S.; Bolton, H.; Addley, H.C. Differentiating Uterine Sarcoma from Leiomyoma: BET(1)T(2)ER Check! Br. J. Radiol. 2021, 94, 20201332. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Bonaffini, P.A.; Nougaret, S.; Fournier, L.; Dohan, A.; Chong, J.; Smith, J.; Addley, H.; Reinhold, C. How to Differentiate Uterine Leiomyosarcoma from Leiomyoma with Imaging. Diagn. Interv. Imaging 2019, 100, 619–634. [Google Scholar] [CrossRef]
- Chura, J.C.; Truskinovsky, A.M.; Judson, P.L.; Johnson, L.; Geller, M.A.; Downs, L.S. Positron Emission Tomography and Leiomyomas: Clinicopathologic Analysis of 3 Cases of PET Scan-Positive Leiomyomas and Literature Review. Gynecol. Oncol. 2007, 104, 247–252. [Google Scholar] [CrossRef]
- Kitajima, K.; Murakami, K.; Yamasaki, E.; Kaji, Y.; Sugimura, K. Standardized Uptake Values of Uterine Leiomyoma with 18F-FDG PET/CT: Variation with Age, Size, Degeneration, and Contrast Enhancement on MRI. Ann. Nucl. Med. 2008, 22, 505–512. [Google Scholar] [CrossRef]
- Berger-Richardson, D.; Swallow, C.J. Needle Tract Seeding after Percutaneous Biopsy of Sarcoma: Risk/Benefit Considerations. Cancer 2017, 123, 560–567. [Google Scholar] [CrossRef]
- Mbatani, N.; Olawaiye, A.B.; Prat, J. Uterine Sarcomas. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 51–58. [Google Scholar] [CrossRef]
- Benito, V.; Lubrano, A.; León, L.; Molano, F.; Pinar, B. Does Iatrogenic Tumor Rupture during Surgery Have Prognostic Implications for the Outcome of Uterine Sarcomas? Int. J. Gynecol. Cancer 2020, 30, 1726–1732. [Google Scholar] [CrossRef]
- D’Angelo, E.; Prat, J. Uterine Sarcomas: A Review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef]
- Yang, Q.; Madueke-Laveaux, O.S.; Cun, H.; Wlodarczyk, M.; Garcia, N.; Carvalho, K.C.; Al-Hendy, A. Comprehensive Review of Uterine Leiomyosarcoma: Pathogenesis, Diagnosis, Prognosis, and Targeted Therapy. Cells 2024, 13, 1106. [Google Scholar] [CrossRef]
- Lewis, D.; Liang, A.; Mason, T.; Ferriss, J.S. Current Treatment Options: Uterine Sarcoma. Curr. Treat. Options Oncol. 2024, 25, 829–853. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Ko, E.M.; Kolovos, G.; Vagios, S.; Kalliouris, D.; Giuntoli, R.L. Ovarian Preservation for Low-Grade Endometrial Stromal Sarcoma: A Systematic Review of the Literature and Meta-Analysis. Int. J. Gynecol. Cancer 2019, 29, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Foresta, A.; Giudice, M.; Reino, A.; La Verde, M.; Della Corte, L.; Bifulco, G.; Franciscis, P.; Cianci, S.; Capozzi, V.A. Is Adnexectomy Mandatory at the Time of Hysterectomy for Uterine Sarcomas? A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1140. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, E.; Rumpf, J.; Ratan, R.; Fleming, N.D.; Jazaeri, A.; Fellman, B.; Meyer, L.; Soliman, P. Hormone Receptor Status and the Role of Oophorectomy in Uterine Leiomyosarcoma. Gynecol. Oncol. 2022, 167, 490–495. [Google Scholar] [CrossRef]
- Li, Y.; Gong, Q.; Peng, J.; Liu, Y.; Jiang, Y.; Zhang, S. Prognostic Significance of Lymphadenectomy in Uterine Leiomyosarcomas and Endometrial Stromal Sarcomas: Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 279, 94–101. [Google Scholar] [CrossRef]
- Nasioudis, D.; Mastroyannis, S.A.; Latif, N.A.; Ko, E.M.; Haggerty, A.F.; Kim, S.H.; Morgan, M.A.; Giuntoli, R.L. 2nd. Role of Lymphadenectomy for Apparent Early Stage Uterine Sarcoma; a Comprehensive Analysis of the National Cancer Database. Surg. Oncol. 2021, 38, 101589. Available online: https://pubmed.ncbi.nlm.nih.gov/33957499/ (accessed on 22 October 2025).
- Matsuzaki, S.; Matsuzaki, S.; Chang, E.J.; Yasukawa, M.; Roman, L.D.; Matsuo, K. Surgical and Oncologic Outcomes of Hyperthermic Intraperitoneal Chemotherapy for Uterine Leiomyosarcoma: A Systematic Review of Literature. Gynecol. Oncol. 2021, 161, 70–77. [Google Scholar] [CrossRef]
- Olivier, T.; Pop, D.; Chouiter Djebaili, A.; Falk, A.T.; Iannessi, A.; Saada, E.; Nettekoven, W.; Blay, J.Y.; Baque, P.; Cupissol, D.; et al. Treating Metastatic Sarcomas Locally: A Paradoxe, a Rationale, an Evidence? Crit. Rev. Oncol./Hematol. 2015, 95, 62–77. [Google Scholar] [CrossRef]
- Zang, Y.; Dong, M.; Zhang, K.; Gao, C.; Guo, F.; Wang, Y.; Xue, F. Hormonal Therapy in Uterine Sarcomas. Cancer Med. 2019, 8, 1339–1349. [Google Scholar] [CrossRef]
- Costales, A.B.; Radeva, M.; Ricci, S. Characterizing the Efficacy and Trends of Adjuvant Therapy versus Observation in Women with Early Stage (Uterine Confined) Leiomyosarcoma: A National Cancer Database Study. J. Gynecol. Oncol. 2020, 31, e21. [Google Scholar] [CrossRef]
- Hensley, M.L.; Enserro, D.; Hatcher, H.; Ottevanger, P.B.; Krarup-Hansen, A.; Blay, J.Y.; Fisher, C.; Moxley, K.M.; Lele, S.B.; Lea, J.S.; et al. Adjuvant Gemcitabine Plus Docetaxel Followed by Doxorubicin Versus Observation for High-Grade Uterine Leiomyosarcoma: A Phase III NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2018, 36, Jco1800454. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.S.; Mangioni, C.; Malmström, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.J.; Coens, C.; et al. Phase III Randomised Study to Evaluate the Role of Adjuvant Pelvic Radiotherapy in the Treatment of Uterine Sarcomas Stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (Protocol 55874). Eur. J. Cancer 2008, 44, 808–818. [Google Scholar] [CrossRef]
- Seagle, B.L.; Sobecki-Rausch, J.; Strohl, A.E.; Shilpi, A.; Grace, A.; Shahabi, S. Prognosis and Treatment of Uterine Leiomyosarcoma: A National Cancer Database Study. Gynecol. Oncol. 2017, 145, 61–70. [Google Scholar] [CrossRef]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and Docetaxel versus Doxorubicin as First-Line Treatment in Previously Untreated Advanced Unresectable or Metastatic Soft-Tissue Sarcomas (GeDDiS): A Randomised Controlled Phase 3 Trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, L.; Touati, N.; Blay, J.Y.; Grignani, G.; Flippot, R.; Czarnecka, A.M.; Piperno-Neumann, S.; Martin-Broto, J.; Sanfilippo, R.; Katz, D.; et al. Doxorubicin plus Dacarbazine, Doxorubicin plus Ifosfamide, or Doxorubicin Alone as a First-Line Treatment for Advanced Leiomyosarcoma: A Propensity Score Matching Analysis from the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer 2020, 126, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Balleyguier, C.; Lebrun-Ly, V.; et al. Doxorubicin Alone versus Doxorubicin with Trabectedin Followed by Trabectedin Alone as First-Line Therapy for Metastatic or Unresectable Leiomyosarcoma (LMS-04): A Randomised, Multicentre, Open-Label Phase 3 Trial. Lancet Oncol. 2022, 23, 1044–1054. [Google Scholar] [CrossRef]
- Hwang, W.Y.; Kim, J.H.; Noh, J.J.; Baek, M.H.; Choi, M.C.; Lee, Y.J.; Lee, M.; Suh, D.H.; Kim, Y.B.; Kim, D.Y. Clinical Practice Guidelines for Uterine Corpus Cancer: An Update to the Korean Society of Gynecologic Oncology Guidelines. J. Gynecol. Oncol. 2025, 36, e71. [Google Scholar] [CrossRef]
- Benson, C.; Ray-Coquard, I.; Sleijfer, S.; Litière, S.; Blay, J.Y.; Le Cesne, A.; Papai, Z.; Judson, I.; Schöffski, P.; Chawla, S.; et al. Outcome of Uterine Sarcoma Patients Treated with Pazopanib: A Retrospective Analysis Based on Two European Organisation for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group (STBSG) Clinical Trials 62043 and 62072. Gynecol. Oncol. 2016, 142, 89–94. [Google Scholar] [CrossRef]
- Ijaz, I.; Shahzad, M.N.; Hosseinifard, H.; Liu, S.; Sefidan, M.O.; Kahloon, L.E.; Imani, S.; Hua, Z.; Zhang, Y.Q. Evaluation of the Efficacy of Systemic Therapy for Advanced Uterine Leiomyosarcoma: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Cancer Med. 2023, 12, 13894–13911. [Google Scholar] [CrossRef]
- Sanfilippo, R.; Sbaraglia, M.; Fabbroni, C.; Croce, S.; Ray-Coquard, I.; Guermazi, F.; Paolini, B.; Blanc-Durand, F.; Lecesne, A.; Chiappa, V.; et al. Low-Grade Uterine Leiomyosarcoma Is Highly Sensitive to Hormonal Treatment. Clin. Cancer Res. 2023, 29, 4679–4684. [Google Scholar] [CrossRef]
- Bai, H.; Yang, J.; Cao, D.; Huang, H.; Xiang, Y.; Wu, M.; Cui, Q.; Chen, J.; Lang, J.; Shen, K. Ovary and Uterus-Sparing Procedures for Low-Grade Endometrial Stromal Sarcoma: A Retrospective Study of 153 Cases. Gynecol. Oncol. 2014, 132, 654–660. [Google Scholar] [CrossRef]
- Morimoto, A.; Tsubamoto, H.; Inoue, K.; Ikeda, Y.; Hirota, S. Fatal Case of Multiple Recurrences of Endometrial Stromal Sarcoma after Fertility-Sparing Management. J. Obstet. Gynaecol. Res. 2015, 41, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Cao, D.; Yang, J.; Jiang, X.; Shen, K.; Pan, L.; Huang, H.; Lang, J.; You, Y.; Chen, J. Fertility-Sparing Surgery for Patients with Low-Grade Endometrial Stromal Sarcoma. Oncotarget 2017, 8, 10602–10608. [Google Scholar] [CrossRef] [PubMed]
- Trozzi, R.; Tuyaerts, S.; Annibali, D.; Herreros Pomares, A.; Boog, L.; Van Dam, P.; Leunen, K.; Deroose, C.; Trum, H.; Amant, F. An Open-Label, Single-Arm, Prospective, Multi-Center, Tandem Two-Stage Designed Phase II Study to Evaluate the Efficacy of Fulvestrant in Women with Recurrent/Metastatic Estrogen Receptor-Positive Gynecological Malignancies (FUCHSia Study). Int. J. Gynecol. Cancer 2024, 34, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Dessources, K.; Miller, K.M.; Kertowidjojo, E.; Da Cruz Paula, A.; Zou, Y.; Selenica, P.; da Silva, E.M.; Benayed, R.; Ashley, C.W.; Abu-Rustum, N.R.; et al. ESR1 Hotspot Mutations in Endometrial Stromal Sarcoma with High-Grade Transformation and Endocrine Treatment. Mod. Pathol. 2022, 35, 972–978. [Google Scholar] [CrossRef]
- Meurer, M.; Floquet, A.; Ray-Coquard, I.; Bertucci, F.; Auriche, M.; Cordoba, A.; Piperno-Neumann, S.; Salas, S.; Delannes, M.; Chevalier, T.; et al. Localized High Grade Endometrial Stromal Sarcoma and Localized Undifferentiated Uterine Sarcoma: A Retrospective Series of the French Sarcoma Group. Int. J. Gynecol. Cancer 2019, 29, 691–698. [Google Scholar] [CrossRef]
- Seagle, B.L.; Shilpi, A.; Buchanan, S.; Goodman, C.; Shahabi, S. Low-Grade and High-Grade Endometrial Stromal Sarcoma: A National Cancer Database Study. Gynecol. Oncol. 2017, 146, 254–262. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin Alone versus Intensified Doxorubicin plus Ifosfamide for First-Line Treatment of Advanced or Metastatic Soft-Tissue Sarcoma: A Randomised Controlled Phase 3 Trial. Lancet. Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Costa, A.; Astolfi, A.; Gozzellino, L.; Nannini, M.; Pasquinelli, G.; Pantaleo, M.A. Molecular Insights in Endometrial Stromal Sarcomas: Exploring New Targets for Novel Therapeutic Approaches. Biomolecules 2025, 15, 265. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Seligson, N.D.; Hays, J.L.; Miles, W.O.; Chen, J.L. Clinical Utility of CDK4/6 Inhibitors in Sarcoma: Successes and Future Challenges. JCO Precis. Oncol. 2022, 6, e2100211. Available online: https://pubmed.ncbi.nlm.nih.gov/35108033/ (accessed on 22 October 2025).
- Ortega, Á.; Vera, I.; Diaz, M.P.; Navarro, C.; Rojas, M.; Torres, W.; Parra, H.; Salazar, J.; De Sanctis, J.B.; Bermúdez, V. The YAP/TAZ Signaling Pathway in the Tumor Microenvironment and Carcinogenesis: Current Knowledge and Therapeutic Promises. Int. J. Mol. Sci. 2021, 23, 430. [Google Scholar] [CrossRef]
- Li, C.; Wang, C. LG-ESSs and HG-ESSs: Underlying Molecular Alterations and Potential Therapeutic Strategies. J. Zhejiang Univ. Sci. B 2021, 22, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Dermawan, J.K.; Haller, F.; Semrau, S.; Meidenbauer, N.; Stoehr, R.; Lax, S.; Hartmann, A.; Zou, Y.S.; Xing, D.; et al. ERBB2/ERBB3-Mutated S100/SOX10-Positive Unclassified High-Grade Uterine Sarcoma: First Detailed Description of a Novel Entity. Virchows Arch. 2024, 485, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Zannoni, G.F. Uterine Smooth Muscle Tumors of Unknown Malignant Potential: A Challenging Question. Gynecol. Oncol. 2019, 154, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Major, F.J.; Blessing, J.A.; Silverberg, S.G.; Morrow, C.P.; Creasman, W.T.; Currie, J.L.; Yordan, E.; Brady, M.F. Prognostic Factors in Early-Stage Uterine Sarcoma. A Gynecologic Oncology Group Study. Cancer 1993, 71, 1702–1709. [Google Scholar] [CrossRef]
- American Cancer Society. Uterine Sarcoma Early Detection Diagnosis and Staging. Available online: https://www.cancer.org/cancer/types/uterine-sarcoma/detection-diagnosis-staging/survival-rates.html (accessed on 24 October 2025).
- Ferrandina, G.; Aristei, C.; Biondetti, P.R.; Cananzi, F.C.M.; Casali, P.; Ciccarone, F.; Colombo, N.; Comandone, A.; Corvo, R.; De Iaco, P.; et al. Italian Consensus Conference on Management of Uterine Sarcomas on Behalf of S.I.G.O. (Societa’ Italiana Di Ginecologia E Ostetricia). Eur. J. Cancer 2020, 139, 149–168. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.; Qin, M.; Cai, Y.; Jin, Y.; Pan, L.Y. High-Grade Endometrial Stromal Sarcoma: A Retrospective Study of Factors Influencing Prognosis. Cancer Manag. Res. 2019, 11, 831–837. [Google Scholar] [CrossRef]
- Zapardiel, I.; Gracia Segovia, M.; Macuks, R.; Mancari, R.; Achimas-Cadariu, P.; Corrado, G.; Bartusevicius, A.; Sukhin, V.; Muruzabal, J.C.; Coronado Martín, P.J.; et al. Prognostic Factors in Patients with Uterine Sarcoma: The SARCUT Study. Int. J. Gynecol. Cancer 2023, 33, 897–904. [Google Scholar] [CrossRef]
- Keire, P.A.; Bressler, S.L.; Lemire, J.M.; Edris, B.; Rubin, B.P.; Rahmani, M.; McManus, B.M.; van de Rijn, M.; Wight, T.N. A Role for Versican in the Development of Leiomyosarcoma. J. Biol. Chem. 2014, 289, 34089–34103. [Google Scholar] [CrossRef]
- Gultekin, O.; Gonzalez-Molina, J.; Hardell, E.; Moyano-Galceran, L.; Mitsios, N.; Mulder, J.; Kokaraki, G.; Isaksson, A.; Sarhan, D.; Lehti, K.; et al. FOXP3+ T Cells in Uterine Sarcomas Are Associated with Favorable Prognosis, Low Extracellular Matrix Expression and Reduced YAP Activation. npj Precis. Oncol. 2021, 5, 97. [Google Scholar] [CrossRef]
- Pankova, V.; Krasny, L.; Kerrison, W.; Tam, Y.B.; Chadha, M.; Burns, J.; Wilding, C.P.; Chen, L.; Chowdhury, A.; Perkins, E.; et al. Clinical Implications and Molecular Features of Extracellular Matrix Networks in Soft Tissue Sarcomas. Clin. Cancer Res. 2024, 30, 3229–3242. [Google Scholar] [CrossRef]
- Sun, H.; Ma, L.; Chen, J. Hyaluronan-Mediated Motility Receptor Expression Functions as a Prognostic Biomarker in Uterine Carcinosarcoma Based on Bioinformatics Analysis. J. Int. Med. Res. 2021, 49, 3000605211021043. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.E.; Hjelmeland, M.E.; Berg, H.F.; Gold, R.M.; Woie, K.; Akslen, L.A.; Haldorsen, I.S.; Krakstad, C. Multiplex Single-Cell Profiling of Putative Cancer Stem Cell Markers ALDH1, SOX9, SOX2, CD44, CD133 and CD15 in Endometrial Cancer. Mol. Oncol. 2025, 19, 1651–1667. [Google Scholar] [CrossRef] [PubMed]
- Basarir, Z.O.; Caydere, M.; Karabulut, S.; Kotanoglu, M.S.; Arslanca, T.; Uçar, Y.Ö.; Üstün, Y. The Prognostic Value of Tumor Microenvironment in Endometrioid Type Endometrial Cancer: Effect of CD44 on Oncologic Outcome. Int. J. Gynaecol. Obstet. 2025, 169, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, C.; Walker, F.; Madelenat, P.; Bringuier, A.F.; Scoazec, J.Y.; Feldmann, G.; Darai, E. Expression of CD44 Standard and Isoforms V3 and V6 in Uterine Smooth Muscle Tumors: A Possible Diagnostic Tool for the Diagnosis of Leiomyosarcoma. Hum. Pathol. 2001, 32, 1190–1196. [Google Scholar] [CrossRef]
- Kansu-Celik, H.; Gungor, M.; Ortac, F.; Kankaya, D.; Ensari, A. Expression of CD44 Variant 6 and Its Prognostic Value in Benign and Malignant Endometrial Tissue. Arch. Gynecol. Obstet. 2017, 296, 313–318. [Google Scholar] [CrossRef]
- Solmaz, O.A. The Role of Syndecan-1 during Endometrial Carcinoma Progression. J. Cancer Res. Ther. 2019, 15, 1265–1269. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Li, X.; Tai, Y.; Lü, Q.; Yang, N.; Jiang, J. Expression and Significance of Biglycan in Endometrial Cancer. Arch. Gynecol. Obstet. 2014, 289, 649–655. [Google Scholar] [CrossRef]
- Nikitovic, D.; Berdiaki, A.; Spyridaki, I.; Krasanakis, T.; Tsatsakis, A.; Tzanakakis, G.N. Proteoglycans-Biomarkers and Targets in Cancer Therapy. Front. Endocrinol. 2018, 9, 69. [Google Scholar] [CrossRef]
- Aquino, C.I.; Venkatesan, S.; Ligori, A.; Tinelli, R.; Grossini, E.; Surico, D. Osteopontin Expression and Its Role in Endometrial Cancer: A Systematic Review. Cancers 2025, 17, 2245. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Hu, X.; Tang, B.; Deng, F. Role of Osteopontin in Cancer Development and Treatment. Heliyon 2023, 9, e21055. [Google Scholar] [CrossRef] [PubMed]
- Kruger, T.E.; Miller, A.H.; Godwin, A.K.; Wang, J. Bone Sialoprotein and Osteopontin in Bone Metastasis of Osteotropic Cancers. Crit. Rev. Oncol. Hematol. 2014, 89, 330–341. [Google Scholar] [CrossRef]
- Jayes, F.L.; Liu, B.; Moutos, F.T.; Kuchibhatla, M.; Guilak, F.; Leppert, P.C. Loss of Stiffness in Collagen-Rich Uterine Fibroids after Digestion with Purified Collagenase Clostridium Histolyticum. Am. J. Obstet. Gynecol. 2016, 215, e1–e596. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B. Mechanisms of Action of Novel Drugs Targeting Angiogenesis-Promoting Matrix Metalloproteinases. Front. Immunol. 2019, 10, 1278. [Google Scholar] [CrossRef]
- Ooki, A.; Satoh, T.; Muro, K.; Takashima, A.; Kadowaki, S.; Sakai, D.; Ichimura, T.; Mitani, S.; Kudo, T.; Chin, K.; et al. A Phase 1b Study of Andecaliximab in Combination with S-1 plus Platinum in Japanese Patients with Gastric Adenocarcinoma. Sci. Rep. 2022, 12, 11007. [Google Scholar] [CrossRef]
- Tanaka, N.; Sakamoto, T. MT1-MMP as a Key Regulator of Metastasis. Cells 2023, 12, 2187. [Google Scholar] [CrossRef]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef]
- Li, S.; Sampson, C.; Liu, C.; Piao, H.; Liu, H.-X. Integrin Signaling in Cancer: Bidirectional Mechanisms and Therapeutic Opportunities. Cell Commun. Signal. 2023, 21, 266. [Google Scholar] [CrossRef]
- Bergonzini, C.; Kroese, K.; Zweemer, A.J.M.; Danen, E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front. Cell Dev. Biol. 2022, 10, 863850. [Google Scholar] [CrossRef]
- Kumar, S.; Weaver, V.M. Mechanics, Malignancy, and Metastasis: The Force Journey of a Tumor Cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Sanderson, R.D. Proteoglycans in Cancer Biology, Tumour Microenvironment and Angiogenesis. J. Cell. Mol. Med. 2011, 15, 1013–1031. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H.M. Bevacizumab. Oncologist 2010, 15, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Green, A.K.; Wenham, R.M.; Mutch, D.; Davidson, B.; Miller, D.S. New Therapies for Advanced, Recurrent, and Metastatic Endometrial Cancers. Gynecol. Oncol. Res. Pract. 2017, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-Approved Small Molecule Protein Kinase Inhibitors: A 2024 Update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef]
- Cuppens, T.; Tuyaerts, S.; Amant, F. Potential Therapeutic Targets in Uterine Sarcomas. Sarcoma 2015, 2015, 243298. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, K.-R.; Nam, J.-H. Immunohistochemical Analysis for Therapeutic Targets and Prognostic Markers in Low-Grade Endometrial Stromal Sarcoma. Int. J. Gynecol. Cancer 2013, 23, 81–89. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of Platelet-Derived Growth Factors in Physiology and Medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H. Targeting the PDGF Signaling Pathway in Tumor Treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The Biology and Function of Fibroblasts in Cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Morandi, P.; Krishnamurthy, S.; Reuben, J.M.; Lee, B.-N.; Francis, D.; Booser, D.J.; Green, M.C.; Arun, B.K.; Pusztai, L.; et al. Imatinib Mesylate (Gleevec®) in Advanced Breast Cancer-Expressing C-Kit or PDGFR-β: Clinical Activity and Biological Correlations. Ann. Oncol. 2008, 19, 1713–1719. [Google Scholar] [CrossRef]
- Roskoski, R. Sunitinib: A VEGF and PDGF Receptor Protein Kinase and Angiogenesis Inhibitor. Biochem. Biophys. Res. Commun. 2007, 356, 323–328. [Google Scholar] [CrossRef]
- Zhao, H.-L.; Yang, F.; Huang, X.; Zhou, Q.-H. Overview of Fundamental Study of Pazopanib in Cancer. Thorac. Cancer 2014, 5, 487–493. [Google Scholar] [CrossRef][Green Version]
- Dickerson, E.B.; Marley, K.; Edris, W.; Tyner, J.W.; Schalk, V.; MacDonald, V.; Loriaux, M.; Druker, B.J.; Helfand, S.C. Imatinib and Dasatinib Inhibit Hemangiosarcoma and Implicate PDGFR-β and Src in Tumor Growth. Transl. Oncol. 2013, 6, 158-IN7. [Google Scholar] [CrossRef]
- Wu, C.-P.; Lusvarghi, S.; Wang, J.-C.; Hsiao, S.-H.; Huang, Y.-H.; Hung, T.-H.; Ambudkar, S.V. Avapritinib: A Selective Inhibitor of KIT and PDGFRα That Reverses ABCB1 and ABCG2-Mediated Multidrug Resistance in Cancer Cell Lines. Mol. Pharm. 2019, 16, 3040–3052. [Google Scholar] [CrossRef]
- Bose, S.; Schwartz, G.K.; Ingham, M. Novel Therapeutics in the Treatment of Uterine Sarcoma. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 900–909. [Google Scholar] [CrossRef]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt Signaling Pathways in Biology and Disease: Mechanisms and Therapeutic Advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Aggelidakis, J.; Berdiaki, A.; Nikitovic, D.; Papoutsidakis, A.; Papachristou, D.J.; Tsatsakis, A.M.; Tzanakakis, G.N. Biglycan Regulates MG63 Osteosarcoma Cell Growth Through a LPR6/β-Catenin/IGFR-IR Signaling Axis. Front. Oncol. 2018, 8, 470. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Wang, J.; Ma, C.X.; Gao, X.; Patriub, V.; Sklar, J.L. The JAZF1-SUZ12 Fusion Protein Disrupts PRC2 Complexes and Impairs Chromatin Repression during Human Endometrial Stromal Tumorogenesis. Oncotarget 2016, 8, 4062–4078. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, B.C.; dos Anjos, L.G.; Dobroff, A.S.; Baracat, E.C.; Yang, Q.; Al-Hendy, A.; Carvalho, K.C. Epigenetic Features in Uterine Leiomyosarcoma and Endometrial Stromal Sarcomas: An Overview of the Literature. Biomedicines 2022, 10, 2567. [Google Scholar] [CrossRef]
- Wang, F.; Malnassy, G.; Qiu, W. The Epigenetic Regulation of Microenvironment in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 653037. [Google Scholar] [CrossRef]
- Van Beneden, K.; Mannaerts, I.; Pauwels, M.; Van den Branden, C.; van Grunsven, L.A. HDAC Inhibitors in Experimental Liver and Kidney Fibrosis. Fibrogenesis Tissue Repair 2013, 6, 1. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Hossen, M.M.; Ma, Y.; Yin, Z.; Xia, Y.; Du, J.; Huang, J.Y.; Huang, J.J.; Zou, L.; Ye, Z.; Huang, Z. Current Understanding of CTLA-4: From Mechanism to Autoimmune Diseases. Front. Immunol. 2023, 14, 1198365. [Google Scholar] [CrossRef]
- Bejar, J.F.G.; Galende, E.Y.; Zeng, Q.; Genestie, C.; Rouleau, E.; de Bruyn, M.; Klein, C.; Formal, A.L.; Edmond, E.; Moreau, M.; et al. Immune Predictors of Response to Immune Checkpoint Inhibitors in Mismatch Repair-Deficient Endometrial Cancer. J. Immunother. Cancer 2024, 12, e009143. [Google Scholar] [CrossRef]
- Kang, N.; Zhang, Y.; Guo, S.; Chen, R.; Kong, F.; Wang, S.; Yuan, M.; Chen, R.; Shen, D.; Wang, J. Genomic and Transcriptomic Characterization Revealed the High Sensitivity of Targeted Therapy and Immunotherapy in a Subset of Endometrial Stromal Sarcoma. Cancer Res. Treat. 2023, 55, 978–991. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Kyriakopoulou, K.; Koutsakis, C.; Mastronikolis, S.; Karamanos, N.K. Key Matrix Remodeling Enzymes: Functions and Targeting in Cancer. Cancers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of Matrix Metalloproteinases in Cancer Progression and Their Pharmacological Targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Neill, T.; Iozzo, R.V. Matrix Modeling and Remodeling: A Biological Interplay Regulating Tissue Homeostasis and Diseases. Matrix Biol. 2019, 75–76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The Extracellular Matrix: Tools and Insights for the “Omics” Era. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, C.; Wei, H.; Ding, S.; Li, H.; Hao, Y. Directed Evolution of Proteoglycan-Modifying Enzymes: Functional Applications in Cervical Cancer Therapy. Int. J. Biol. Macromol. 2025, 304, 140659. [Google Scholar] [CrossRef]

| EST Type | Tumor’s Nature | Invasion | Cell Morphology | Mitotic Rate |
|---|---|---|---|---|
| ESN | Benign [4,9,10] | None | Small cells, oval nuclei, scant cytoplasm | Low |
| LG-ESS | Malignant [3,4,8,9] | Tong-like myometrial Possible lymphovascular | Small cells, oval nuclei, scant cytoplasm [3,9] | Low |
| HG-ESS | Malignant [4,8,9] | Tong-like myometrial Lymphovascular | Large, high-grade round cells Low-grade spindle cells | High |
| UUS | Malignant [4,8] | Destructive myometrial Lymphovascular | Epithelioid and spindled cells, multiple and unconventional nuclei | High |
| EST Type | ESN [4,13] | LG-ESS [4,9] | HG-ESS [4,9] | UUS [4,8] |
|---|---|---|---|---|
| Chromosomal rearrangement | JAZF1 SUZ12 fusion | JAZF1 SUZ12, JAZF1-PHF1, EPC1-PHF1, MEAF6-PHF1, ZC3H7-164 BCOR62, MBTD1-CXorf67 | YWHAE rearrangements | Variable. JAZF1-SUZ12, YWHAE |
| CD10 | Positive | Positive | Low or negative in round cells Occasionally positive in spindle cells | Variable |
| ER/PR | ERα and PR focally | ERα positive ERβ negative PR normal | Low or negative Occasionally positive in spindle cells | Variable. Positive in uniform neoplasms |
| WT1/β-catenin | Negative | WT1 positive β-catenin variable | WT1 low or negative | Variable |
| P53 | Normal | Normal | Normal | Aberrant |
| Other molecules | SMA, vimentin, desmin positive | AR, SMA, Desmin | Cyclin D1 overexpression SMA, desmin negative | SMA, desmin variable |
| Proteoglycan (PG) | Normal Endometrium | Fibroids/ Leiomyomas | Hyperplasia/ Polyps | Carcinoma (EEC/ESC) |
|---|---|---|---|---|
| Decorin [59,67,68,69,70,71] | Present, regulates collagen fibrils | Overexpressed; longer GAG chains | Absent | Altered; loss linked to invasion/metastasis |
| Biglycan [37,59,60,61,70] | Present, contributes to fibrillogenesis | Highly expressed; thick collagen fibrils | Low expression | Upregulated in stroma, linked to EMT & inflammation |
| Lumican [59] | Present, low expression | Variable expression | Low expression | Reduced in polyps/ hyperplasia, variable in carcinoma |
| Fibromodulin [59] | Present | Highly expressed (proliferative phase) | Absent | Reduced/Absent |
| Versican [67,72,73] | Baseline expression | Overexpressed | Altered; associated with proliferation | Upregulated in tumor stroma; supports proliferation |
| Syndecans [67,74,75] | Baseline expression | Overexpressed | Altered expression | Overexpressed; linked to invasion and viability of cells |
| ECM protein-2 [67] | Baseline expression | Overexpressed | ND | Upregulated |
| MRI Sequence | Features |
|---|---|
| T2-weighted imaging [132] | Heterogeneity of the solid-enhancing component Hyperintensity of the solid enhancing component |
| T1-weighted imaging (pre-contrast) [132] | Intra-tumoral hemorrhage |
| T1-weighted imaging (post-contrast) [132] | Heterogeneous enhancement Enhancing finger-like projections Ill-defined borders with the myometrium Central necrosis |
| Diffusion-weighted imaging (DWI) [132] | Restricted diffusion (apparent diffusion coefficient value < 0.9) |
| Agent (Example) | Primary Mechanism of Action | Efficacy | Main Side Effects |
|---|---|---|---|
| Aromatase Inhibitors (AIs), Letrozole Anastrozole | Block estrogen production from peripheral tissues. | Highly effective for advanced/recurrent disease; reduces recurrence risk. | Hot flashes, joint pain, risk of bone loss. |
| Progestins, Megestrol Acetate (MA), Medroxyprogesterone Acetate (MPA) | Suppress endometrial growth; exert anti-estrogenic effects. | Effective as primary or adjuvant therapy; reduces recurrence. | Weight gain, fluid retention, metabolic changes. |
| GnRH Agonists Goserelin, Leuprolide | Inhibit ovarian function | Used mainly in premenopausal women to lower estrogen levels. | Hot flashes, risk of bone loss. |
| Selective Estrogen Receptor Degraders (SERDs) Fulvestrant | Bind to and degrade the Estrogen Receptor (ER). | Emerging option, particularly for tumors resistant to AIs. | Injection site pain, nausea, hot flashes. |
| Stage | Definition |
|---|---|
| I | Tumor limited to uterus [140] |
| IA | Less than 5 cm [140] |
| IB | More than 5 cm [140] |
| II | Tumor extends beyond the uterus, within the pelvis [140] |
| IIA | Adnexal involvement [140] |
| IIB | Involvement of other pelvic tissues [140] |
| III | Tumor invades abdominal tissues (not just protruding into the abdomen) [140] |
| IIIA | One site [140] |
| IIIB | More than one site [140] |
| IIIC | Metastasis to pelvic and/or para-aortic lymph nodes [140] |
| IVA | Tumor invades bladder and/or rectum [140] |
| IVB | Distant metastasis [140] |
| Target/Molecular Pathway | Agent | Rationale |
|---|---|---|
| Angiogenesis/ VEGF | Tyrosine Kinase Inhibitors (TKI) | HG-ESS and other uterine sarcomas are often highly vascular |
| BCOR Gene Alterations | CDK4/6 Inhibitors MDM2 Inhibitors | HG-ESS with BCOR rearrangements frequently show co-occurring molecular changes, such as alterations in the CDK4 pathway or MDM2 amplification [173] |
| YWHAE::NUMT2A/B gene fusion | RAF/MEK/MA PK inhibitors Hippo/YAP- TAZ inhibitors Cyclin D1 inhibitors | Knockdown of YWHAE::NUTM2 leads to down-regulation of cell proliferation [172] YAP activation is associated with poor prognosis [174] |
| c-kit | c-kit inhibitors | Pazopanib and Imatinib mesylate show promising results in treating c-kit-expressing HG-ESS [172] |
| Immune Checkpoint | Immune Checkpoint Inhibitors (ICI) | HG-ESS is often characterized by a high degree of immune cell infiltration and can express positive predictors of immunotherapy efficacy |
| PI3K/AKT/mTOR | PI3K/AKT/mT OR Pathway Inhibitors | This pathway is frequently involved in cell growth and is mutated/activated in a subset of HG-ESS [175] |
| HER2/ERBB2 | Antibody-Drug Conjugates | HER2 overexpression is reported in a small subset of mesenchymal uterine tumors [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmara, M.; Vrekoussis, T.; Makrygiannakis, F.; Nikitovic, D.; Berdiaki, A. Uterine Stroma-Derived Tumors and the Extracellular Matrix: A Comparative Review of Benign and Malignant Pathologies. Cancers 2025, 17, 3501. https://doi.org/10.3390/cancers17213501
Marmara M, Vrekoussis T, Makrygiannakis F, Nikitovic D, Berdiaki A. Uterine Stroma-Derived Tumors and the Extracellular Matrix: A Comparative Review of Benign and Malignant Pathologies. Cancers. 2025; 17(21):3501. https://doi.org/10.3390/cancers17213501
Chicago/Turabian StyleMarmara, Maria, Thomas Vrekoussis, Fanourios Makrygiannakis, Dragana Nikitovic, and Aikaterini Berdiaki. 2025. "Uterine Stroma-Derived Tumors and the Extracellular Matrix: A Comparative Review of Benign and Malignant Pathologies" Cancers 17, no. 21: 3501. https://doi.org/10.3390/cancers17213501
APA StyleMarmara, M., Vrekoussis, T., Makrygiannakis, F., Nikitovic, D., & Berdiaki, A. (2025). Uterine Stroma-Derived Tumors and the Extracellular Matrix: A Comparative Review of Benign and Malignant Pathologies. Cancers, 17(21), 3501. https://doi.org/10.3390/cancers17213501







