The Usefulness of Indocyanine Green in Modern Gynecological Oncology—Analysis, Literature Review, and Future Perspectives
Simple Summary
Abstract
1. Introduction
2. Physical Characteristics
3. Lymphadenectomy and Compartmental Surgery in Ovarian Cancer
4. Vulvar Cancer
5. Cervical Cancer
6. Endometrial Cancer
7. Ureteral Visualization
8. Nerve Visualization
9. Lymphography and Lymph Node Transfer
10. Bowel Surgery
11. Therapeutic Applications
12. Discussion and Future Perspectives
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, G.E.; Peyton, W.T.; French, L.A.; Walker, W.W. The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J. Neurosurg. 1948, 5, 392–398. [Google Scholar] [CrossRef]
- DeLong, J.C.; Hoffman, R.M.; Bouvet, M. Current status and future perspectives of fluorescence-guided surgery for cancer. Expert Rev. Anticancer. Ther. 2015, 16, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, Ó.G.; Murphy, R.; Chadwick, V.S. Physicochemical studies of indocyanine green (ICG): Absorbance/concentration relationship, pH tolerance and assay precision in various solvents. Experientia 1982, 38, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.W. Injection technique for indocyanine green and sodium fluorescein dye angiography of the eye. Investig. Ophthalmol. 1973, 12, 881–895. [Google Scholar]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef]
- Kochubey, V.I.; Kulyabina, T.V.; Tuchin, V.V.; Altshuler, G.B. Spectral characteristics of indocyanine Green upon its interaction with biological tissues. Opt. Spectrosc. 2005, 99, 560–566. [Google Scholar] [CrossRef]
- Engel, E.; Schraml, R.; Maisch, T.; Kobuch, K.; König, B.; Szeimies, R.M.; Hillenkamp, J.; Bäumler, W.; Vasold, R. Light-Induced Decomposition of Indocyanine Green. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Ciamberlini, C.; Guarnieri, V.; Longobardi, G.; Poggi, P.; Donati, M.C.; Panzardi, G. Indocyanine green videoangiography using cooled charge-coupled devices in central serous choroidopathy. J. Biomed. Opt. 1997, 2, 218–225. [Google Scholar] [CrossRef]
- Paumgartner, G. The handling of indocyanine green by the liver. Schweiz. Med. Wochenschr. 1975, 105 (Suppl. S17), 1–30. [Google Scholar]
- Jacques, S.L. Corrigendum: Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 5007–5008. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Angioli, R.; Bailey, A.E.; Broach, V.; Buda, A.; Coriddi, M.R.; Dayan, J.H.; Frumovitz, M.; Kim, Y.M.; Kimmig, R.; et al. IGCS Intraoperative Technology Taskforce. Update on near infrared imaging technology: Beyond white light and the naked eye, indocyanine green and near infrared technology in the treatment of gynecologic cancers. Int. J. Gynecol. Cancer 2020, 30, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.; Stanton, A.; Azarbod, P.; Sherman, M.D.; Levick, J.; Mortimer, P. Enhanced Cutaneous Lymphatic Network in the Forearms of Women with Postmastectomy Oedema. J. Vasc. Res. 2000, 37, 501–512. [Google Scholar] [CrossRef]
- Pruimboom, T.; Schols, R.M.; Kuijk, S.M.V.; Van der Hulst, R.R.; Qiu, S.S. Indocyanine Green Angiography for Preventing Postoperative Mastectomy Skin Flap Necrosis in Immediate Breast Reconstruction; Cochrane Library: London, UK, 2020; Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013280.pub2/full (accessed on 20 November 2024).
- Solass, W.; Horvath, P.; Struller, F.; Königsrainer, I.; Beckert, S.; Königsrainer, A.; Weinreich, F.-J.; Schenk, M. Functional vascular anatomy of the peritoneum in health and disease. Pleura Peritoneum 2019, 1, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. Available online: https://jnccn.org/configurable/content/journals$002fjnccn$002f17$002f1$002farticle-p64.xml?t:ac=journals%24002fjnccn%24002f17%24002f1%24002farticle-p64.xml (accessed on 30 November 2024). [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Planchamp, F.; Chiva, L.; Fotopoulou, C.; Barton, D.; Cibula, D.; Aletti, G.; Carinelli, S.; Creutzberg, C.; Davidson, B.; et al. European Society of Gynaecological Oncology (ESGO) Guidelines for Ovarian Cancer Surgery. Int. J. Gynecol. Cancer 2017, 27, 1534–1542. [Google Scholar] [CrossRef]
- Kleppe, M.; Kraima, A.C.; Kruitwagen, R.F.; Van Gorp, T.; Smit, N.N.; van Munsteren, J.C.; DeRuiter, M.C. Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 1405–1414. [Google Scholar] [CrossRef]
- Panici, P.B.; Angioli, R. Role of lymphadenectomy in ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2002, 16, 529–551. [Google Scholar] [CrossRef]
- Uccella, S.; Garzon, S.; Bosco, M.; Porcari, I.; Lanzo, G.; Laganà, A.S.; Chiantera, V.; Cliby, W.A.; Mariani, A.; Franchi, M.; et al. Cervical versus Utero-Ovarian Ligament Injection of the Tracer for the Pelvic Sentinel Lymph Node Mapping in Gynecologic Oncology: A Prospective Observational Study. Gynecol. Obstet. Investig. 2022, 87, 242–247. [Google Scholar] [CrossRef]
- Angelucci, M.; Corrado, G.; Mancini, E. Laparoscopic Indocyanine green sentinel lymph node mapping in early ovarian cancer. A pilot study and review of the literature. Ital. J. Gynaecol. Obstet. 2016, 28, 23–28. [Google Scholar] [CrossRef]
- Buda, A.; Passoni, P.; Corrado, G.; Bussi, B.; Cutillo, G.; Magni, S.; Vizza, E. Near-infrared Fluorescence-guided Sentinel Node Mapping of the Ovary With Indocyanine Green in a Minimally Invasive Setting: A Feasible Study. J. Minim. Invasive Gynecol. 2017, 24, 165–170. [Google Scholar] [CrossRef]
- Agusti, N.; Viveros-Carreño, D.; Grillo-Ardila, C.; Izquierdo, N.; Paredes, P.; Vidal-Sicart, S.; Torne, A.; Díaz-Feijoo, B. Sentinel lymph node detection in early-stage ovarian cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023, 33, 1493–1501. [Google Scholar] [CrossRef]
- Kimmig, R.; Buderath, P.; Rusch, P.; Mach, P.; Aktas, B. Early ovarian cancer surgery with indocyanine-green-guided targeted compartmental lymphadenectomy (TCL, pelvic part). J. Gynecol. Oncol. 2017, 28, e68. [Google Scholar] [CrossRef]
- Kimmig, R.; Aktas, B.; Buderath, P.; Rusch, P.; Heubner, M. Intraoperative navigation in robotically assisted compartmental surgery of uterine cancer by visualisation of embryologically derived lymphatic networks with indocyanine-green (ICG). J. Surg. Oncol. 2016, 113, 554–559. [Google Scholar] [CrossRef]
- Kimmig, R.; Buderath, P.; Mach, P.; Rusch, P.; Aktas, B. Surgical treatment of early ovarian cancer with compartmental resection of regional lymphatic network and indocyanine-green-guided targeted compartmental lymphadenectomy (TCL, paraaortic part). J. Gynecol. Oncol. 2017, 28, e41. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M. Morphogenetic fields of embryonic development in locoregional cancer spread. Lancet Oncol. 2015, 16, e148–e151. [Google Scholar] [CrossRef]
- Way, S. The anatomy of the lymphatic drainage of the vulva and its influence on the radical operation for carcinoma. Ann. R. Coll. Surg. Engl. 1948, 3, 187–209. [Google Scholar]
- Way, S. Carcinoma of the vulva. Am. J. Obstet. Gynecol. 1960, 79, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Taussig, F.J. Cancer of the Vulva: AN ANALYSIS of 155 CASES (1911–1940). Am. J. Obstet. Gynecol. 1940, 40, 764–779. [Google Scholar] [CrossRef]
- Oonk, M.H.; Planchamp, F.; Baldwin, P.; Mahner, S.; Mirza, M.R.; Fischerová, D.; Creutzberg, C.L.; Guillot, E.; Garganese, G.; Lax, S.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 1023–1043. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, A.G.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Dorst, E.B.; et al. Sentinel Node Dissection Is Safe in the Treatment of Early-Stage Vulvar Cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Deken, M.M.; van Doorn, H.C.; Verver, D.; Boogerd, L.S.F.; de Valk, K.S.; Rietbergen, D.D.D.; van Poelgeest, M.I.E.; de Kroon, C.D.; Beltman, J.J.; van Leeuwen, F.W.B.; et al. Near-infrared fluorescence imaging compared to standard sentinel lymph node detection with blue dye in patients with vulvar cancer—A randomized controlled trial. Gynecol. Oncol. 2020, 159, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Di Donna, M.C.; Quartuccio, N.; Giallombardo, V.; Sturiale, L.; Arnone, A.; Ricapito, R.; Sozzi, G.; Arnone, G.; Chiantera, V. Detection of sentinel lymph node in vulvar cancer using 99mTc-labeled colloid lymphoscintigraphy, blue dye, and indocyanine-green fluorescence: A meta-analysis of studies published in 2010–2020. Arch. Gynecol. Obstet. 2022, 307, 1677–1686. [Google Scholar] [CrossRef]
- Broach, V.; Abu-Rustum, N.R.; Sonoda, Y.; Brown, C.L.; Jewell, E.; Gardner, G.; Chi, D.S.; Zivanovic, O.; Leitao, M.M., Jr. Evolution and outcomes of sentinel lymph node mapping in vulvar cancer. Int. J. Gynecol. Cancer 2020, 30, 383–386. [Google Scholar] [CrossRef]
- Prader, S.; du Bois, A.; Harter, P.; Breit, E.; Schneider, S.; Baert, T.; Heitz, F.; Traut, A.; Ehmann, S.; Pauly, N.; et al. Sentinel lymph node mapping with fluorescent and radioactive tracers in vulvar cancer patients. Arch. Gynecol. Obstet. 2020, 301, 729–736. [Google Scholar] [CrossRef]
- Holm, C.; Mayr, M.; Höfter, E.; Becker, A.; Pfeiffer, U.; Mühlbauer, W. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br. J. Plast. Surg. 2002, 55, 635–644. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Monfardini, L.; Sozzi, G.; Armano, G.; Rosati, A.; Alletti, S.G.; Cosentino, F.; Ercoli, A.; Cianci, S.; Berretta, R. Subcutaneous Vulvar Flap Viability Evaluation With Near-Infrared Probe and Indocyanine Green for Vulvar Cancer Reconstructive Surgery: A Feasible Technique. Front. Surg. 2021, 8, 721770. [Google Scholar] [CrossRef]
- Castro, C.C.; Marina, T.; Glickman, A.; Carreras-Dieguez, N.; Luzarraga, A.; Feijoo, B.D.; Torné, A.; Fusté, P. 1215 Indocyanine green-based fluorescence imaging for assessment of flap perfusion in vulvar cancer reconstruction. In Proceedings of the ESGO 2024 Congress, Barcelona, Spain, 7–10 March 2024; pp. A543.3–A544. [Google Scholar]
- Benedetti-Panici, P.; Maneschi, F.; D’Andrea, G.; Cutillo, G.; Rabitti, C.; Congiu, M.; Coronetta, F.; Capelli, A. Early cervical carcinoma. Cancer 2000, 88, 2267–2274. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Richard, S.D.; Krivak, T.C.; Castleberry, A.; Beriwal, S.; Kelley, J.L.; Edwards, R.P.; Sukumvanich, P. Survival for stage IB cervical cancer with positive lymph node involvement: A comparison of completed vs. abandoned radical hysterectomy. Gynecol. Oncol. 2008, 109, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.P.; Morley, G.W. Radical hysterectomy versus radiation therapy for stage ib squamous cell cancer of the cervix. Cancer 1991, 68, 272–277. [Google Scholar] [CrossRef]
- Rocha, A.; Domínguez, A.M.; Lécuru, F.; Bourdel, N. Indocyanine green and infrared fluorescence in detection of sentinel lymph nodes in endometrial and cervical cancer staging—A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jewell, E.L.; Huang, J.J.; Abu-Rustum, N.R.; Gardner, G.J.; Brown, C.L.; Sonoda, Y.; Barakat, R.R.; Levine, D.A.; Leitao, M.M., Jr. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol. Oncol. 2014, 133, 274–277. [Google Scholar] [CrossRef]
- Ruscito, I.; Gasparri, M.L.; Braicu, E.I.; Bellati, F.; Raio, L.; Sehouli, J.; Mueller, M.D.; Panici, P.B.; Papadia, A. Sentinel Node Mapping in Cervical and Endometrial Cancer: Indocyanine Green Versus Other Conventional Dyes—A Meta-Analysis. Ann. Surg. Oncol. 2016, 23, 3749–3756. [Google Scholar] [CrossRef]
- Diab, Y. Sentinel Lymph Nodes Mapping in Cervical Cancer a Comprehensive Review. Int. J. Gynecol. Cancer 2017, 27, 154–158. [Google Scholar] [CrossRef]
- Ulain, Q.; Han, L.; Wu, Q.; Zhao, L.; Wang, Q.; Tuo, X.; Wang, Y.; Wang, Q.; Ma, S.; Sun, C.; et al. Indocyanine green can stand alone in detecting sentinel lymph nodes in cervical cancer. J. Int. Med. Res. 2018, 46, 4885–4897. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Buda, A.; Papadia, A.; Zapardiel, I.; Vizza, E.; Ghezzi, F.; De Ponti, E.; Lissoni, A.A.; Imboden, S.; Diestro, M.D.; Verri, D.; et al. From Conventional Radiotracer Tc-99m with Blue Dye to Indocyanine Green Fluorescence: A Comparison of Methods Towards Optimization of Sentinel Lymph Node Mapping in Early Stage Cervical Cancer for a Laparoscopic Approach. Ann. Surg. Oncol. 2016, 23, 2959–2965. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Robledo, K.P.; Frumovitz, M.; Pareja, R.; Ribeiro, R.; Lopez, A.; Yan, X.; Isla, D.; Moretti, R.; Bernardini, M.Q.; et al. LACC Trial: Final Analysis on Overall Survival Comparing Open Versus Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. J. Clin. Oncol. 2024, 42, 2741–2746. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Kang, S.; Yoo, H.J.; Hwang, J.H.; Lim, M.-C.; Seo, S.-S.; Park, S.-Y. Sentinel lymph node biopsy in endometrial cancer: Meta-analysis of 26 studies. Gynecol. Oncol. 2011, 123, 522–527. [Google Scholar] [CrossRef]
- Leitao, M.M., Jr. Sentinel Lymph Node Mapping in Patients with Endometrial Carcinoma: Less Can Be More. Curr. Obstet. Gynecol. Rep. 2016, 5, 279–285. [Google Scholar] [CrossRef]
- Rozenholc, A.; Samouelian, V.; Warkus, T.; Gauthier, P.; Provencher, D.; Sauthier, P.; Gauthier, F.; Drakopoulos, P.; Cormier, B. Green versus blue: Randomized controlled trial comparing indocyanine green with methylene blue for sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2019, 153, 500–504. [Google Scholar] [CrossRef]
- Eriksson, A.G.Z.; Montovano, M.; Beavis, A.; Soslow, R.A.; Zhou, Q.; Abu-Rustum, N.R.; Gardner, G.J.; Zivanovic, O.; Barakat, R.R.; Brown, C.L.; et al. Impact of Obesity on Sentinel Lymph Node Mapping in Patients with Newly Diagnosed Uterine Cancer Undergoing Robotic Surgery. Ann. Surg. Oncol. 2016, 23, 2522–2528. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; Glaser, G.E.; Zivanovic, O.; Sonoda, Y.; Levine, D.A.; Chi, D.S.; Gemignani, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Improving sentinel lymph node detection rates in endometrial cancer: How many cases are needed? Gynecol. Oncol. 2009, 115, 453–455. [Google Scholar] [CrossRef]
- Accorsi, G.; de Paiva, L.L.; Schmidt, R.; Vieira, M.A.; Reis, R.; Andrade, C.E. Sentinel Lymph Node Mapping VS Systematic Lymphadenectomy for Endometrial Cancer: Surgical Morbidity and Lymphatic Complications. J. Minim. Invasive Gynecol. 2020, 27, 938–945.e2. [Google Scholar] [CrossRef] [PubMed]
- Nahshon, C.; Kadan, Y.; Lavie, O.; Ostrovsky, L.; Segev, Y. Sentinel lymph node sampling versus full lymphadenectomy in endometrial cancer: A SEER database analysis. Int. J. Gynecol. Cancer 2023, 33, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.H.; Leitao, M.M.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef]
- Kimmig, R.; Iannaccone, A.; Aktas, B.; Buderath, P.; Heubner, M. Embryologically based radical hysterectomy as peritoneal mesometrial resection (PMMR) with pelvic and para-aortic lymphadenectomy for loco-regional tumor control in endometrial cancer: First evidence for efficacy. Arch. Gynecol. Obstet. 2015, 294, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, O.; Chatterjee, R.; Gaba, F.; Butler, J. SF025/#1065 Utilizing indocyanine green (ICG) for ureter identification in gynaecological oncology surgery. In Proceedings of the IGCS 2024 Annual Global Meeting, Dublin, Ireland, 16–18 October 2024; pp. A364.4–A365. [Google Scholar]
- Mandovra, P.; Kalikar, V.; Patankar, R.V. Real-Time Visualization of Ureters Using Indocyanine Green During Laparoscopic Surgeries: Can We Make Surgery Safer? Surg. Innov. 2019, 26, 464–468. [Google Scholar] [CrossRef]
- Lee, Z.; Simhan, J.; Parker, D.C.; Reilly, C.; Llukani, E.; Lee, D.I.; Mydlo, J.H.; Eun, D.D. Novel Use of Indocyanine Green for Intraoperative, Real-time Localization of Ureteral Stenosis During Robot-assisted Ureteroureterostomy. Urology 2013, 82, 729–733. [Google Scholar] [CrossRef]
- Cabanes, M.; Boria, F.; Gutiérrez, A.H.; Zapardiel, I. Intra-operative identification of ureters using indocyanine green for gynecological oncology procedures. Int. J. Gynecol. Cancer 2020, 30, 278. [Google Scholar] [CrossRef]
- Mccombe, B.O.; Paracha, K.; Russell, O.; Langleben, N. SF033/#1181 Instillation of ICG dye into ureters for intra-operative identification during robotic surgery for gynecologic malignancies. Int. J. Gynecol. Cancer 2024, 34, A367. [Google Scholar]
- Federico, A.; Gallotta, V.; Foschi, N.; Costantini, B.; Conte, C.; Pinto, F.; Ercoli, A.; Ferrandina, G.; Moro, F.D.; Bassi, P.; et al. Surgical outcomes of segmental ureteral resection with ureteroneocystostomy after major gynecologic surgery. Eur. J. Surg. Oncol. 2020, 46, 1366–1372. [Google Scholar] [CrossRef]
- Loverro, M.; Bizzarri, N.; Capomacchia, F.M.; Watrowski, R.; Querleu, D.; Gioè, A.; Naldini, A.; Santullo, F.; Foschi, N.; Fagotti, A.; et al. Indocyanine green fluorescence applied to gynecologic oncology: Beyond sentinel lymph node. Int. J. Surg. 2024, 110, 3641–3653. [Google Scholar] [CrossRef]
- van Gent, M.D.J.M.; Romijn, L.M.; van Santen, K.E.; Trimbos, J.B.M.Z.; de Kroon, C.D. Nerve-sparing radical hysterectomy versus conventional radical hysterectomy in early-stage cervical cancer. A systematic review and meta-analysis of survival and quality of life. Maturitas 2016, 94, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Moawad, G.N.; Wu, C.; Klebanoff, J.S.; Urbina, P.; Alkatout, I. Pelvic Neuroanatomy: An Overview of Commonly Encountered Pelvic Nerves in Gynecologic Surgery. J. Minim. Invasive Gynecol. 2021, 28, 178. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Liu, P.; Li, W.; Wang, L.; Liu, Y. The morbidity of sexual dysfunction of 125 Chinese women following different types of radical hysterectomy for gynaecological malignancies. Arch. Gynecol. Obstet. 2017, 297, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—A new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wei, W.; Jiang, P. Laparoscopic Nerve-Sparing Radical Hysterectomy for Cervical Carcinoma: Emphasis on Nerve Content in Removed Cardinal Ligaments. Int. J. Gynecol. Cancer 2016, 26, 192–198. [Google Scholar] [CrossRef]
- Walsh, E.M.; Cole, D.; Tipirneni, K.E.; Bland, K.I.; Udayakumar, N.; Kasten, B.B.; Bevans, S.L.; McGrew, B.M.; Kain, J.J.; Nguyen, Q.T.; et al. Fluorescence Imaging of Nerves During Surgery. Ann. Surg. 2019, 270, 69–76. [Google Scholar] [CrossRef]
- Glasgow, H.L.; Whitney, M.A.; Gross, L.A.; Friedman, B.; Adams, S.R.; Crisp, J.L.; Hussain, T.; Frei, A.P.; Novy, K.; Wollscheid, B.; et al. Laminin targeting of a peripheral nerve-highlighting peptide enables degenerated nerve visualization. Proc. Natl. Acad. Sci. USA 2016, 113, 12774–12779. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; van Driel, P.B.A.A.; de Jong, R.J.B.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Löwik, C.W.G.M.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021, 22, e186–e195. [Google Scholar] [CrossRef]
- He, K.; Li, P.; Zhang, Z.; Liu, J.; Liu, P.; Gong, S.; Chi, C.; Liu, P.; Chen, C.; Tian, J. Intraoperative near-infrared fluorescence imaging can identify pelvic nerves in patients with cervical cancer in real time during radical hysterectomy. Eur. J. Nucl. Med. 2022, 49, 2929–2937. [Google Scholar] [CrossRef]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef]
- Bakar, Y.; Tuğral, A. Lower Extremity Lymphedema Management after Gynecologic Cancer Surgery: A Review of Current Management Strategies. Ann. Vasc. Surg. 2017, 44, 442–450. [Google Scholar] [CrossRef]
- Brorson, H. Liposuction in Lymphedema Treatment. J. Reconstr. Microsurg. 2016, 32, 056–065. [Google Scholar] [CrossRef]
- Ozturk, C.N.; Ozturk, C.; Glasgow, M.; Platek, M.; Ashary, Z.; Kuhn, J.; Aronoff, N.; Lohman, R.; Djohan, R.; Gurunluoglu, R. Free vascularized lymph node transfer for treatment of lymphedema: A systematic evidence based review. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1234–1247. [Google Scholar] [CrossRef]
- Unno, N.; Inuzuka, K.; Suzuki, M.; Yamamoto, N.; Sagara, D.; Nishiyama, M.; Konno, H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J. Vasc. Surg. 2007, 45, 1016–1021. [Google Scholar] [CrossRef]
- Mehrara, B.J.; Greene, A.K. Lymphedema and Obesity: Is There a Link? Plast. Reconstr. Surg. 2014, 134, 154e–160e. [Google Scholar] [CrossRef]
- Gould, D.J.; Mehrara, B.J.; Neligan, P.; Cheng, M.; Patel, K.M. Lymph node transplantation for the treatment of lymphedema. J. Surg. Oncol. 2018, 118, 736–742. [Google Scholar] [CrossRef]
- Akita, S.; Mitsukawa, N.; Kuriyama, M.; Kubota, Y.; Hasegawa, M.; Tokumoto, H.; Ishigaki, T.; Togawa, T.; Kuyama, J.; Satoh, K. Comparison of Vascularized Supraclavicular Lymph Node Transfer and Lymphaticovenular Anastomosis for Advanced Stage Lower Extremity Lymphedema. Ann. Plast. Surg. 2015, 74, 573–579. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef]
- Peiretti, M.; Zanagnolo, V.; Aletti, G.D.; Bocciolone, L.; Colombo, N.; Landoni, F.; Minig, L.; Biffi, R.; Radice, D.; Maggioni, A. Role of maximal primary cytoreductive surgery in patients with advanced epithelial ovarian and tubal cancer: Surgical and oncological outcomes. Single institution experience. Gynecol. Oncol. 2010, 119, 259–264. [Google Scholar] [CrossRef]
- Grimm, C.; Harter, P.; Alesina, P.F.; Prader, S.; Schneider, S.; Ataseven, B.; Meier, B.; Brunkhorst, V.; Hinrichs, J.; Kurzeder, C.; et al. The impact of type and number of bowel resections on anastomotic leakage risk in advanced ovarian cancer surgery. Gynecol. Oncol. 2017, 146, 498–503. [Google Scholar] [CrossRef]
- Bartl, T.; Schwameis, R.; Stift, A.; Bachleitner-Hofmann, T.; Reinthaller, A.; Grimm, C.; Polterauer, S. Predictive and Prognostic Implication of Bowel Resections During Primary Cytoreductive Surgery in Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2018, 28, 1664–1671. [Google Scholar] [CrossRef]
- Fornasiero, M.; Geropoulos, G.; Kechagias, K.S.; Psarras, K.; Triantafyllidis, K.K.; Giannos, P.; Koimtzis, G.; Petrou, N.A.; Lucocq, J.; Kontovounisios, C.; et al. Anastomotic Leak in Ovarian Cancer Cytoreduction Surgery: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5464. [Google Scholar] [CrossRef]
- Costantini, B.; Vargiu, V.; Santullo, F.; Rosati, A.; Bruno, M.; Gallotta, V.; Lodoli, C.; Moroni, R.; Pacelli, F.; Scambia, G. Risk Factors for Anastomotic Leakage in Advanced Ovarian Cancer Surgery: A Large Single-Center Experience. Ann. Surg. Oncol. 2022, 29, 4791–4802. [Google Scholar] [CrossRef]
- Armstrong, G.; Croft, J.; Corrigan, N.; Brown, J.M.; Goh, V.; Quirke, P.; Hulme, C.; Tolan, D.; Kirby, A.; Cahill, R.; et al. IntAct: Intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: A randomized controlled trial. Color. Dis. 2018, 20, O226–O234. [Google Scholar] [CrossRef]
- Hussain, K.; Balamurugan, G.; Ravindra, C.; Kodali, R.; Hansalia, D.S.; Rengan, V. The impact of indocyanine green fluorescence angiography (ICG-FA) on anastomotic leak rates and postoperative outcomes in colorectal anastomoses: A systematic review. Surg. Endosc. 2025, 39, 749–765. [Google Scholar] [CrossRef]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion Assessment in Laparoscopic Left-Sided/Anterior Resection (PILLAR II): A Multi-Institutional Study. J. Am. Coll. Surg. 2015, 220, 82–92e1. [Google Scholar] [CrossRef]
- Jafari, M.D.; Pigazzi, A.; McLemore, E.C.; Mutch, M.G.; Haas, E.; Rasheid, S.H.; Wait, A.D.; Paquette, I.M.; Bardakcioglu, O.; Safar, B.; et al. Perfusion Assessment in Left-Sided/Low Anterior Resection (PILLAR III): A Randomized, Controlled, Parallel, Multicenter Study Assessing Perfusion Outcomes With PINPOINT Near-Infrared Fluorescence Imaging in Low Anterior Resection. Dis. Colon Rectum 2021, 64, 995–1002. [Google Scholar] [CrossRef]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Color. Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef]
- Nguyen, J.M.; Hogen, L.; Laframboise, S.; Bouchard-Fortier, G.; Ferguson, S.E.; Bernardini, M.Q.; May, T. The use of indocyanine green fluorescence angiography to assess anastomotic perfusion following bowel resection in surgery for gynecologic malignancies—A report of 100 consecutive anastomoses. Gynecol. Oncol. 2020, 158, 402–406. [Google Scholar] [CrossRef]
- Moukarzel, L.A.; Byrne, M.E.; Leiva, S.; Wu, M.; Zhou, Q.C.; Iasonos, A.; Abu-Rustum, N.R.; Sonoda, Y.; Gardner, G.; Leitao, M.M., Jr.; et al. The impact of near-infrared angiography and proctoscopy after rectosigmoid resection and anastomosis performed during surgeries for gynecologic malignancies. Gynecol Oncol. 2020, 158, 397–401. [Google Scholar] [CrossRef]
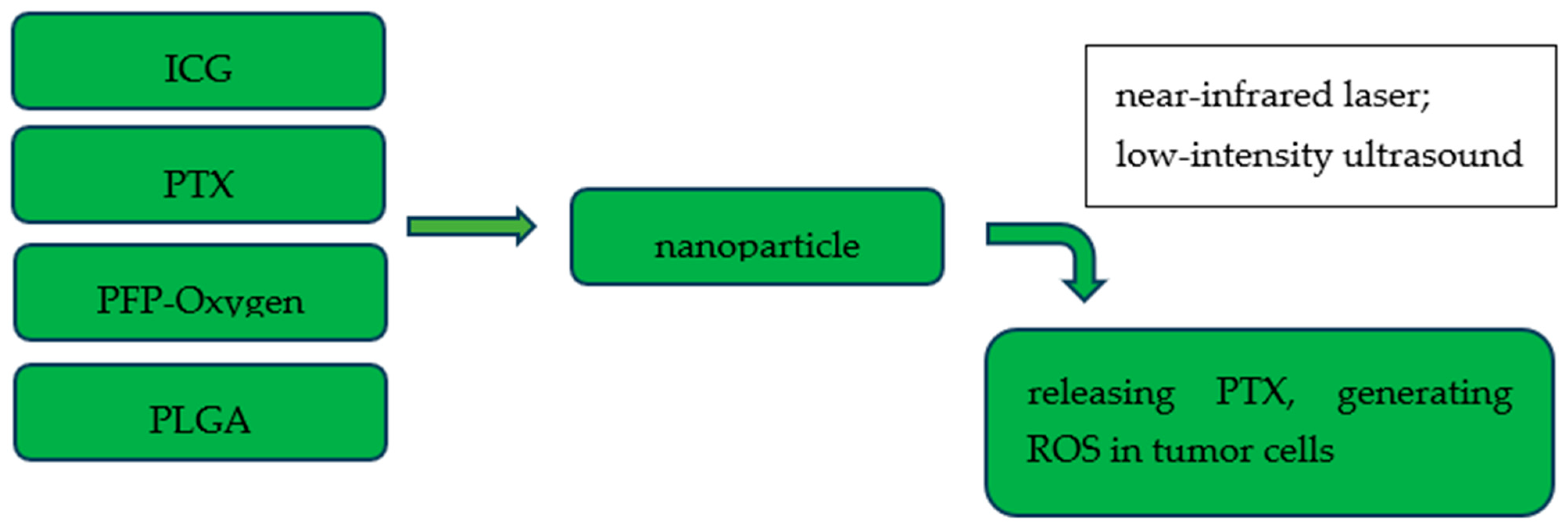
- Ma, R.; Alifu, N.; Zhong, D.; Chen, S.; Heng, Y.; Wang, J.; Zhu, L.; Ma, C.; Zhang, X. Indocyanine Green-Based Theranostic Nanoplatform for NIR Fluorescence Image-Guided Chemo/Photothermal Therapy of Cervical Cancer. Int. J. Nanomed. 2021, 16, 4847–4861. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, L.; Du, Z.; Ma, R.; Yan, T.; Alimu, G.; Zhang, X.; Alifu, N.; Ma, C. Polymer encapsulated clinical ICG nanoparticles for enhanced photothermal therapy and NIR fluorescence imaging in cervical cancer. RSC Adv. 2021, 11, 20850–20858. [Google Scholar] [CrossRef]
- Chen, C.; Sun, J.; Chen, S.; Liu, Y.; Zhu, S.; Wang, Z.; Chang, S. A multifunctional-targeted nanoagent for dual-mode image-guided therapeutic effects on ovarian cancer cells. Int. J. Nanomed. 2019, 14, 753–769. [Google Scholar] [CrossRef]
- Tang, Q.; Cui, J.; Tian, Z.; Sun, J.; Wang, Z.; Chang, S.; Zhu, S. Oxygen and indocyanine green loaded phase-transition nanoparticle-mediated photo-sonodynamic cytotoxic effects on rheumatoid arthritis fibroblast-like synoviocytes. Int. J. Nanomed. 2017, 12, 381–393. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, S.; Yang, B.; Chen, C.; Chen, S.; Liu, Y.; Nie, X.; Hao, L.; Wang, Z.; Sun, J.; et al. The Destruction Of Laser-Induced Phase-Transition Nanoparticles Triggered By Low-Intensity Ultrasound: An Innovative Modality To Enhance The Immunological Treatment Of Ovarian Cancer Cells. Int. J. Nanomed. 2019, 14, 9377–9393. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Zhu, S.; Chen, C.; Xie, W.; Xiao, L.; Zhu, Y.; Hao, L.; Wang, Z.; Sun, J.; et al. Dual-mode imaging and therapeutic effects of drug-loaded phase-transition nanoparticles combined with near-infrared laser and low-intensity ultrasound on ovarian cancer. Drug Deliv. 2018, 25, 1683–1693. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Du, Z.; Ma, R.; Chen, S.; Fan, H.; Heng, Y.; Yan, T.; Alimu, G.; Zhu, L.; Zhang, X.; Alifu, N.; et al. A highly efficient polydopamine encapsulated clinical ICG theranostic nanoplatform for enhanced photothermal therapy of cervical cancer. Nanoscale Adv. 2022, 4, 4016–4024. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, W.; Chen, Z.; Xiao, Y.; Wang, Y.; Gao, W.; Wang, Z.; Song, R.; Fang, Z.; Hu, W.; et al. Molecular Imaging of Ovarian Follicles and Tumors With Near-Infrared II Bioconjugates. Adv. Mater. 2024, 37, e2414129. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.-H.; Park, S.; Park, S.H.; Park, C.; Key, J. Indocyanine Green-Loaded PLGA Nanoparticles Conjugated with Hyaluronic Acid Improve Target Specificity in Cervical Cancer Tumors. Yonsei Med. J. 2021, 62, 1042–1051. [Google Scholar] [CrossRef]
- Hagen, B.; Valla, M.; Aune, G.; Ravlo, M.; Abusland, A.B.; Araya, E.; Sundset, M.; Tingulstad, S. Indocyanine green fluorescence imaging of lymph nodes during robotic-assisted laparoscopic operation for endometrial cancer. A prospective validation study using a sentinel lymph node surgical algorithm. Gynecol. Oncol. 2016, 143, 479–483. [Google Scholar] [CrossRef]
- Rossi, E.C.; Ivanova, A.; Boggess, J.F. Robotically assisted fluorescence-guided lymph node mapping with ICG for gynecologic malignancies: A feasibility study. Gynecol. Oncol. 2012, 124, 78–82. [Google Scholar] [CrossRef]
- Ha, M.; Eva, L. Imaging in Vulval Cancer. Cancers 2024, 16, 2269. [Google Scholar] [CrossRef]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.G.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef]
- Mc Entee, P.D.; Singaravelu, A.; Boland, P.A.; Moynihan, A.; Creavin, B.; Cahill, R.A. Impact of indocyanine green fluorescence angiography on surgeon action and anastomotic leak in colorectal resections. A systematic review and meta-analysis. Surg. Endosc. 2025, 39, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, A.; Mc Entee, P.D.M.; Hardy, N.P.; Khan, M.F.; Mulsow, J.; Shields, C.; Cahill, R.A. Clinical evaluation of real-time artificial intelligence provision of expert representation in indocyanine green fluorescence angiography during colorectal resections. Int. J. Surg. 2024, 110, 8246–8249. [Google Scholar] [CrossRef] [PubMed]

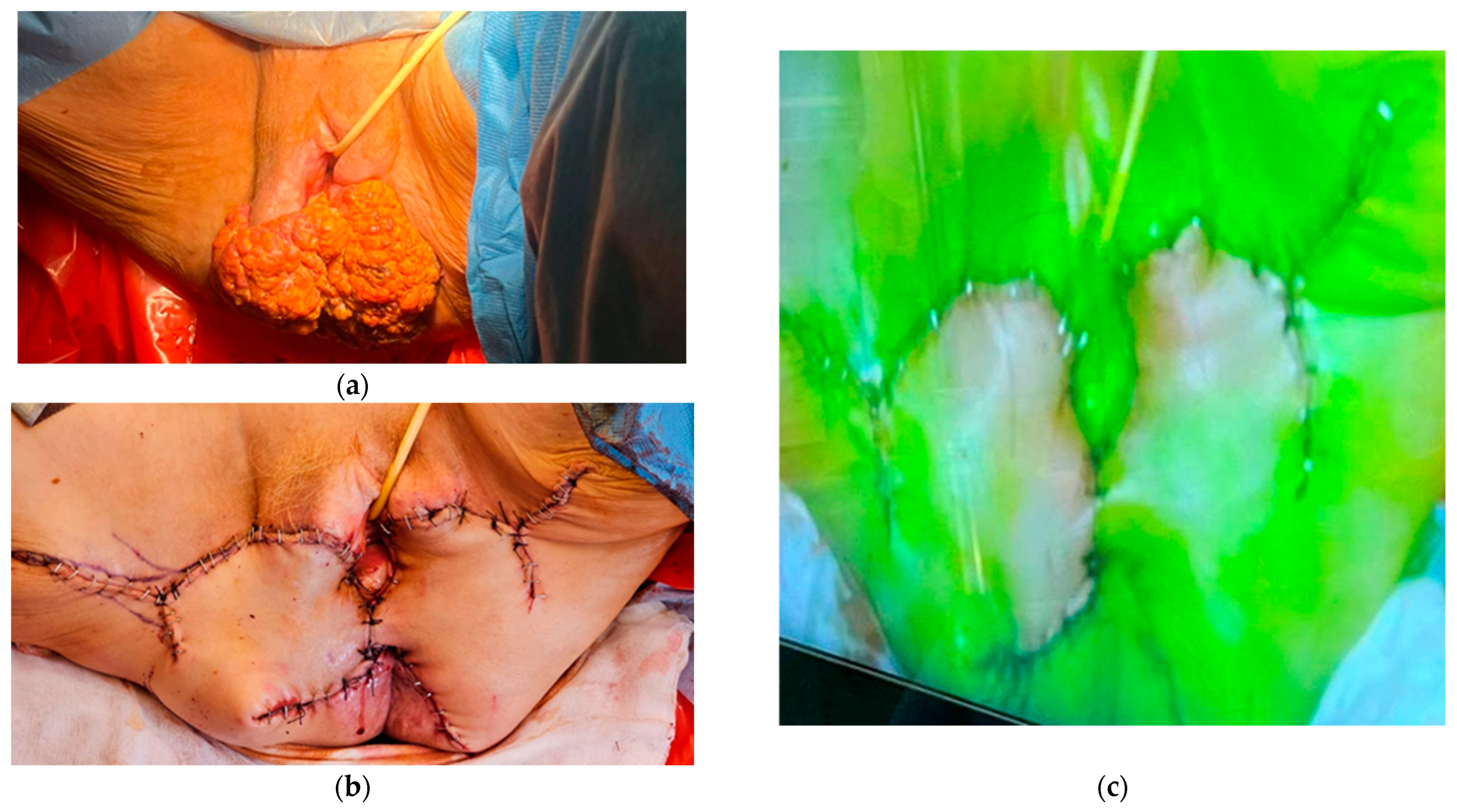
| Type of Cancer/Indications. | Applications of Indocyanine Green. |
|---|---|
| ovarian cancer | sentinel lymph node mapping; targeted compartmental lymphadenectomy (labeling ovarian lymphatic drainage) |
| vulvar cancer | sentinel lymph node mapping at the early stages of vulvar cancer |
| cervical cancer | sentinel lymph node mapping |
| endometrial cancer | sentinel lymph node staging; embryologically based compartmental surgery |
| lymphedema | lymphography; lymph node transfer |
| ureteral visualization | localization of ureters using near-infrared imaging; easier identification of ureters in patients with a high risk of complications |
| nerve visualization | pelvic nerve identification to lower the risk of nerve injuries |
| bowel surgery | indocyanine green fluorescence angiography colorectal perfusion assessment; reduction in the odds of anastomotic leakage |
| nanoparticles with indocyanine green (preclinical models) | photodynamic, sonodynamic therapy; immunotherapy, photothermal therapy, creating ICG particles to enhance the stability of ICG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzanowski, M.; Ziółkowski, G.; Mandes, A.; Panek, G.; Ciebiera, M.; Dąbrowski, F. The Usefulness of Indocyanine Green in Modern Gynecological Oncology—Analysis, Literature Review, and Future Perspectives. Cancers 2025, 17, 3081. https://doi.org/10.3390/cancers17183081
Kostrzanowski M, Ziółkowski G, Mandes A, Panek G, Ciebiera M, Dąbrowski F. The Usefulness of Indocyanine Green in Modern Gynecological Oncology—Analysis, Literature Review, and Future Perspectives. Cancers. 2025; 17(18):3081. https://doi.org/10.3390/cancers17183081
Chicago/Turabian StyleKostrzanowski, Michał, Grzegorz Ziółkowski, Agata Mandes, Grzegorz Panek, Michał Ciebiera, and Filip Dąbrowski. 2025. "The Usefulness of Indocyanine Green in Modern Gynecological Oncology—Analysis, Literature Review, and Future Perspectives" Cancers 17, no. 18: 3081. https://doi.org/10.3390/cancers17183081
APA StyleKostrzanowski, M., Ziółkowski, G., Mandes, A., Panek, G., Ciebiera, M., & Dąbrowski, F. (2025). The Usefulness of Indocyanine Green in Modern Gynecological Oncology—Analysis, Literature Review, and Future Perspectives. Cancers, 17(18), 3081. https://doi.org/10.3390/cancers17183081






