Older Age Does Not Predict Inadequate Pain Management in Cancer Patients: A Multicenter Prospective Analysis from Italian Radiotherapy Departments (ARISE-Study)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Aims
2.2. Study Design
2.3. Inclusion Criteria
2.4. End Points
2.5. Statistical Methods
3. Results
3.1. Selection of Variables
3.2. Model Performance
3.3. Model A: Predictive Model for Inadequate Pain Management in All Patients (Three Groups)
3.4. Model B: Predictive Model for Inadequate Pain Management in All Patients (Two Groups)
3.5. Model C: Predictive Model for Inadequate Pain Management (Only Patients Aged ≥ 65 Years Included)
3.6. Model D: Predictive Model for Inadequate Pain Management (Only Patients Aged 18–64 Years Included)
4. Discussion
4.1. Age-Specific Determinants of Pain Management Adequacy
4.2. Comparison with Previous Literature
4.3. Strengths and Limitations
4.4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patrick, D.L.; Ferketich, S.L.; Frame, P.S.; Harris, J.J.; Hendricks, C.B.; Levin, B.; Link, M.P.; Lustig, C.; McLaughlin, J.; Douglas Ried, L.; et al. National Institutes of Health State-of-the Science Conference statement: Symptom Management in Cancer: Pain, Depression, and Fatigue. J. Natl. Cancer Inst. 2003, 95, 1110–1117. [Google Scholar] [PubMed]
- Davies, A.N.; Dickman, A.; Reid, C.; Stevens, A.M.; Zeppetella, G. The management of cancer-related breakthrough pain: Recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur. J. Pain 2009, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Hamamoto, D.T.; Simone, D.A.; Wilcox, G.L. Mechanism of Cancer Pain. Mol. Interv. 2010, 10, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Viet, C.T.; Schmidt, B.L. Biologic Mechanisms of Oral Cancer Pain and Implications for Clinical Therapy. J. Dent. Res. 2012, 91, 447–453. [Google Scholar] [CrossRef]
- Connelly, S.T.; Schmidt, B.L. Evaluation of pain in patients with oral squamous cell carcinoma. J. Pain 2004, 5, 505–510. [Google Scholar] [CrossRef]
- Cuffari, L.; De Tesseroli, S.J.T.; Nemr, K.; Rapaport, A. Pain complaint as the first symptom of oral cancer: A descriptive study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 56–61. [Google Scholar] [CrossRef]
- Black, B.; Herr, K.; Fine, P.; Sanders, S.; Tang, X.; Bergen-Jackson, K.; Titler, M.; Forcucci, C. The relationships among pain, nonpain symptoms, and quality of life measures in older adults with cancer receiving hospice care. Pain Med. 2011, 12, 880–889. [Google Scholar] [CrossRef]
- Sherman, C.A.; Simonton, S.; Adams, D.C.; Vural, E.; Owens, B.; Hanna, E. Assessing quality of life in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 459–467. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Dow, K.H.; Grant, M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995, 4, 523–531. [Google Scholar] [CrossRef]
- Associazone Italiana di Oncologia Medica. Linee Guida AIOM: Terapia del Dolore in Oncologia, Edizione 2019; AIOM: Milan, Italy, 2019; Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Terapia_dolore.pdf (accessed on 31 October 2019).
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of Cancer Pain in Adult Patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv166–iv191. [Google Scholar] [CrossRef]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; De Conno, F.; Fallon, M.; Hanna, M.; et al. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol. 2012, 13, e58–e68. [Google Scholar] [CrossRef]
- Fairchild, A. Under-treatment of cancer pain. Curr. Opin. Support Palliat. Care 2010, 4, 11–15. [Google Scholar] [CrossRef]
- Deandrea, S.; Montanari, M.; Moja, L.; Apolone, G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann. Oncol. 2008, 19, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.F.; Wood Magee, L.; Fu, M.R.; Bernacki, R.; Bulls, H.; Merlin, J.; McTernan, M. The Contribution of Cancer-Specific Psychosocial Factors to the Pain Experience in Cancer Survivors. J. Hosp. Palliat. Nurs. 2023, 25, E85–E93. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Anderson, K.O.; Baker, T.A.; Campbell, L.C.; Decker, S.; Fillingim, R.B.; Kalauokalani, D.A.; Lasch, K.E.; Myers, C.; Tait, R.C.; et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med. 2003, 4, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Greenlee, H.; Bao, T.; Ismaila, N.; Bruera, E. Integrative Medicine for Pain Management in Oncology: Society for Integrative Oncology-ASCO Guideline Summary and Q&A. JCO Oncol. Pract. 2023, 19, 45–48. [Google Scholar]
- Hachem, G.E.; Rocha, F.O.; Pepersack, T.; Jounblat, Y.; Drowart, A.; Lago, L.D. Advances in pain management for older patients with cancer. Ecancermedicalscience 2019, 13, 980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delgado-Guay, M.O.; Bruera, E. Management of pain in the older person with cancer. Part 2: Treatment options. Oncology 2008, 22, 148–152; discussion 152, 155, 160 passim. [Google Scholar] [PubMed]
- Pysz-Waberski, D.T.; Sadurska, J.; Kędzierska-Jamróz, J.; Pietrzyński, Ł.; Lorek, A.; Jarosz, M.; Gisterek, I. Multidisciplinary management of chronic pain in elderly oncology patients. Contemp. Oncol. 2022, 26, 157–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paice, J.A.; Bohlke, K.; Barton, D.; Craig, D.S.; El-Jawahri, A.; Hershman, D.L.; Kong, L.R.; Kurita, G.P.; LeBlanc, T.W.; Mercadante, S.; et al. Use of opioids for adults with pain from cancer or cancer treatment: ASCO guideline. J. Clin. Oncol. 2023, 41, 914–930. [Google Scholar] [CrossRef]
- Arana-Chicas, E.; Culakova, E.; Mohamed, M.R.; Tylock, R.; Wells, M.; Flannery, M.; Mustian, K.M.; Cupertino, A.P.; Magnuson, A.; Mohile, S.G. Older adults with advanced cancer report pain not captured by clinician-graded Common Terminology Criteria for Adverse Events (CTCAE). J Geriatr. Oncol. 2023, 14, 101480. [Google Scholar] [CrossRef]
- Pickering, G.; Kotlińska-Lemieszek, A.; Krcevski Skvarc, N.; O’Mahony, D.; Monacelli, F.; Knaggs, R.; Morel, V.; Kocot-Kępska, M. Pharmacological pain treatment in older persons. Drugs Aging. 2024, 41, 959–976. [Google Scholar] [CrossRef]
- PDQ Supportive and Palliative Care Editorial Board. Cancer Pain (PDQ®)—Health Professional Version; National Cancer Institute (US): Bethesda, MD, USA, 2025. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/pain/pain-hp-pdq (accessed on 5 September 2025).
- Donati, C.M.; Nardi, E.; Zamagni, A.; Siepe, G.; Mammini, F.; Cellini, F.; Di Rito, A.; Portaluri, M.; De Tommaso, C.; Santacaterina, A.; et al. Adequacy of Pain Treatment in Radiotherapy Departments: Results of a Multicenter Study on 2104 Patients (Arise). Cancers 2022, 14, 4660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donati, C.M.; Maggiore, C.M.; Maltoni, M.; Rossi, R.; Nardi, E.; Zamagni, A.; Siepe, G.; Mammini, F.; Cellini, F.; Di Rito, A.; et al. Adequacy of Pain Management in Patients Referred for Radiation Therapy: A Subanalysis of the Multicenter ARISE-1 Study. Cancers 2023, 16, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donati, C.M.; Galietta, E.; Cellini, F.; Di Rito, A.; Portaluri, M.; De Tommaso, C.; Santacaterina, A.; Tamburella, C.; Mammini, F.; Di Franco, R.; et al. Further Clarification of Pain Management Complexity in Radiotherapy: Insights from Modern Statistical Approaches. Cancers 2024, 16, 1407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Atisook, R.; Euasobhon, P.; Saengsanon, A.; Jensen, M.P. Validity and utility of four pain intensity measures for use in international research. J Pain Res. 2021, 14, 1129–1139. [Google Scholar] [CrossRef]
- Anekar, A.A.; Hendrix, J.M.; Cascella, M.; Marinangeli, F.; Giarratano, A.; Cacciagrano, C.; Tizi, E.S.; Miceli, L.; Natoli, S.; Cuomo, A. WHO Analgesic Ladder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 23 April 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554435/ (accessed on 18 January 2025).
- Cascella, M.; Vittori, A.; Petrucci, E. Strengths and Weaknesses of Cancer Pain Management in Italy: Findings from a Nationwide SIAARTI Survey. Healthcare 2022, 10, 441. [Google Scholar] [CrossRef]
- Marinangeli, F.; Saetta, A.; Lugini, A. Current management of cancer pain in Italy: Expert opinion paper. Open Med. 2021, 17, 34–45. [Google Scholar] [CrossRef]
- Othman, W.M.; Al-Atiyyat, N.M. Knowledge, perceived barriers, and practices of oncology nurses regarding cancer pain management. Electron. J. Gen. Med. 2022, 19, em406. [Google Scholar] [CrossRef]
- Grassi, L.; Travado, L.; Moncayo, F.L.; Sabato, S.; Rossi, E.; SEPOS Group. Psychosocial morbidity and its correlates in cancer patients of the Mediterranean area: Findings from the Southern European Psycho-Oncology Study. J. Affect. Disord. 2004, 83, 243–248. [Google Scholar] [CrossRef]
- Shen, W.C.; Chen, J.S.; Shao, Y.Y.; Lee, K.D.; Chiou, T.J.; Sung, Y.C.; Rau, K.M.; Yen, C.J.; Liao, W.M.; Liu, T.C.; et al. Impact of Undertreatment of Cancer Pain with Analgesic Drugs on Patient Outcomes: A Nationwide Survey of Outpatient Cancer Patient Care in Taiwan. J. Pain Symptom Manag. 2017, 54, 55–65.e1. [Google Scholar] [CrossRef] [PubMed]
- Ilie, G.; Bradfield, J.; Moodie, L.; Lawen, T.; Ilie, A.; Lawen, Z.; Blackman, C.; Gainer, R.; Rutledge, R.D.H. The role of response-shift in studies assessing quality of life outcomes among cancer patients: A systematic review. Front. Oncol. 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S.; Gonin, R.; Hatfield, A.K.; Edmonson, J.H.; Blum, R.H.; Stewart, J.A.; Pandya, K. Pain and its treatment in outpatients with metastatic cancer. N. Engl. J. Med. 1994, 330, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Lukas, A.; Mayer, B.; Fialová, D.; Topinkova, E.; Gindin, J.; Onder, G.; Bernabei, R.; Nikolaus, T.; Denkinger, M.D. Treatment of pain in European nursing homes: Results from the Services and Health for Elderly in Long TERm Care (SHELTER) study. J. Am. Med. Dir. Assoc. 2013, 14, 821–831. [Google Scholar] [CrossRef]
- Bernabei, R.; Gambassi, G.; Lapane, K.; Landi, F.; Gatsonis, C.; Dunlop, R.; Lipsitz, L.; Steel, K.; Mor, V. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA 1998, 279, 1877–1882. [Google Scholar] [CrossRef]
- Torvik, K.; Hølen, J.; Kaasa, S.; Kirkevold, O.; Holtan, A.; Kongsgaard, U.; Rustøen, T. Pain in elderly hospitalized cancer patients with bone metastases in Norway. Int. J. Palliat. Nurs. 2008, 14, 238–245. [Google Scholar] [CrossRef]
- Eurostat. Statistic Explained. 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing&action=statexp-seat&lang=it (accessed on 20 June 2025).
- I Numeri del Cancro in Italia. Available online: https://www.avis.it/wp-content/uploads/2025/01/I-numeri-del-cancro-in-Italia-2024.pdf (accessed on 20 June 2025).
- Garavand, A.; Khodaveisi, T.; Aslani, N.; Hosseiniravandi, M.H.; Shams, R.; Behmanesh, A. Telemedicine in cancer care during COVID-19 pandemic: A systematic mapping study. Health Technol. 2023, 13, 665–678. [Google Scholar] [CrossRef]
- Piras, A.; Venuti, V.; D’Aviero, A.; Cusumano, D.; Pergolizzi, S.; Daidone, A.; Boldrini, L. Covid-19 and radiotherapy: A systematic review after 2 years of pandemic. Clin. Transl. Imaging 2022, 10, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Li, J.J.X.; Farahani, S.J.; Ioannidis, J.P.A. An umbrella review of systematic reviews on the impact of the COVID-19 pandemic on cancer prevention and management, and patient needs. eLife 2023, 12, e85679. [Google Scholar] [CrossRef] [PubMed]
- Thronæs, M.; Balstad, T.R.; Brunelli, C.; Torbjørn Løhre, E.; Klepstad, P.; Vagnildhaug, O.M.; Kaasa, S.; Knudsen, A.K.; Skeidsvoll, S.T. Pain management index (PMI)-does it reflect cancer patients’ wish for focus on pain? Support. Care Cancer 2020, 28, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Maltoni, M. Opioids, pain, and fear. Ann. Oncol. 2008, 19, 5–7. [Google Scholar] [CrossRef]
- Sakakibara, N.; Higashi, T.; Yamashita, I.; Yoshimoto, T.; Matoba, M. Negative pain management index scores do not necessarily indicate inadequate pain management: A cross-sectional study. BMC Palliat. Care 2018, 17, 102. [Google Scholar] [CrossRef]
- Sichetti, D.; Bandieri, E.; Romero, M.; Di Biagio, K.; Luppi, M.; Belfiglio, M.; Tognoni, G.; Ripamonti, C.I. Impact of setting of care on pain management in patients with cancer: A multicentre cross-sectional study. Ann. Oncol. 2010, 21, 2088–2093. [Google Scholar] [CrossRef]
- Mercadante, S.; Bruera, E. Good … but Bad News. J. Clin. Oncol. 2015, 33, 2119. [Google Scholar] [CrossRef]
- Greco, M.T.; Roberto, A.; Corli, O.; Deandrea, S.; Bandieri, E.; Cavuto, S.; Apolone, G. Reply to S. Mercadante et al. J. Clin. Oncol. 2015, 33, 2119–2120. [Google Scholar] [CrossRef][Green Version]
- Greco, M.T.; Roberto, A.; Corli, O.; Deandrea, S.; Bandieri, E.; Cavuto, S.; Apolone, G. Quality of cancer pain management: An update of a systematic review of undertreatment of patients with cancer. J. Clin. Oncol. 2014, 32, 4149–4154. [Google Scholar] [CrossRef]
- Mitera, G.; Zeiadin, N.; Kirou-Mauro, A.; DeAngelis, C.; Wong, J.; Sanjeevan, T.; Sinclair, E.; Danjoux, C.; Barnes, E.; Tsao, M.; et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J. Pain Symptom Manag. 2010, 39, 259–267. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation therapy–associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Scirocco, E.; Cellini, F.; Donati, C.M.; Capuccini, J.; Rossi, R.; Buwenge, M.; Montanari, L.; Maltoni, M.; Morganti, A.G. Improving the Integration between Palliative Radiotherapy and Supportive Care: A Narrative Review. Curr. Oncol. 2022, 29, 7932–7942. [Google Scholar] [CrossRef]


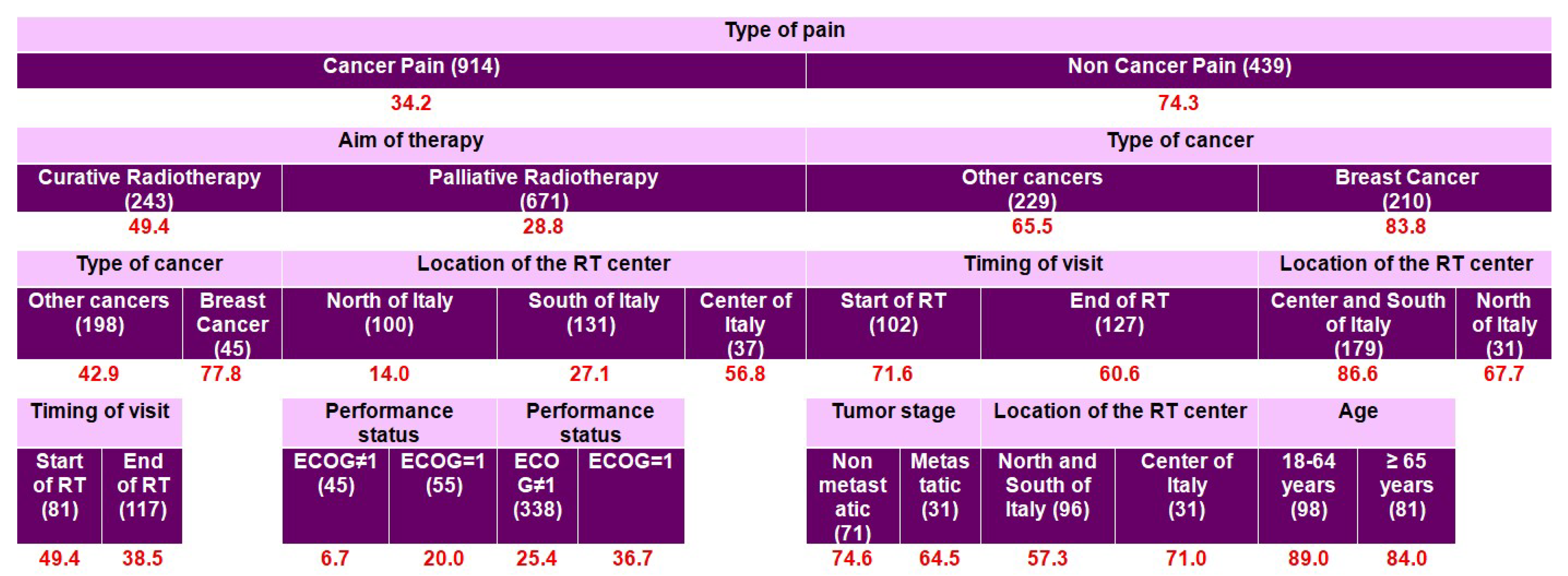
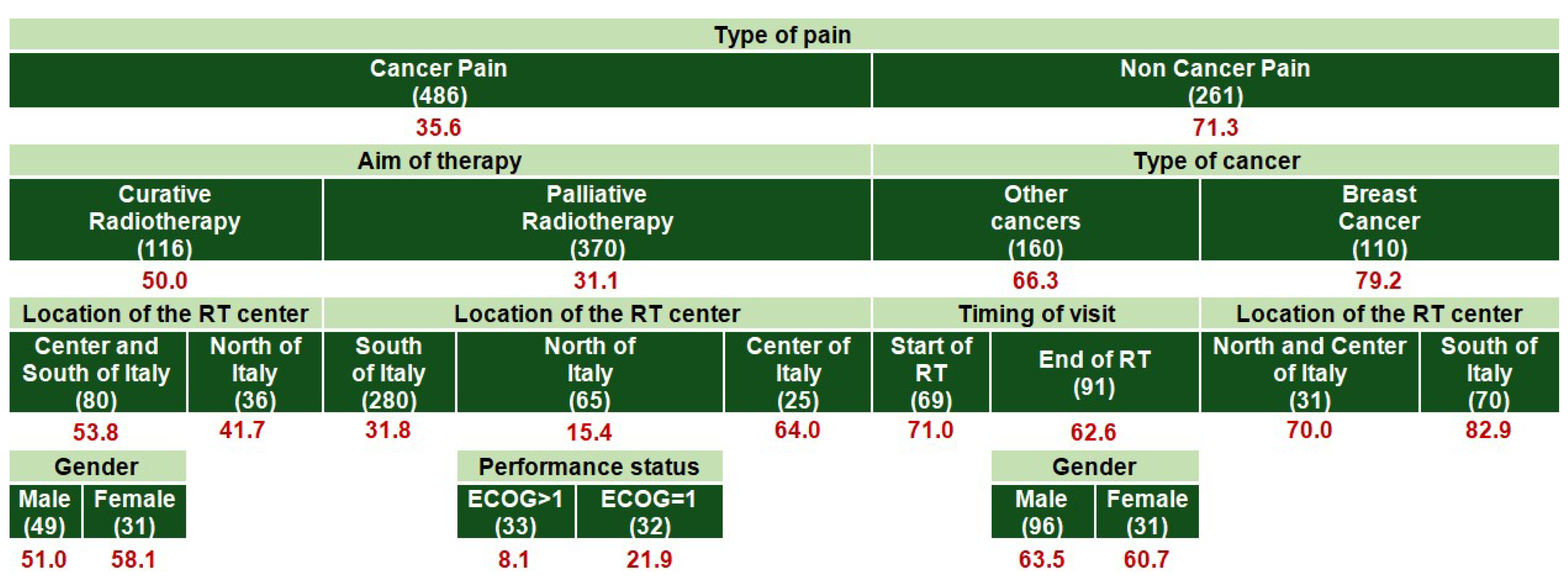

| All (1353) | PMI < 0 | PMI ≥ 0 | |||||
|---|---|---|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | p | ||||
| Gender | <0.001 | ||||||
| Male | 639 | 47.2 | 257 | 40.2 | 382 | 53.5 | |
| Female | 714 | 52.8 | 382 | 59.8 | 332 | 46.5 | |
| Age, years | 0.780 | ||||||
| 18–35 | 27 | 2.0 | 12 | 1.9 | 15 | 2.1 | |
| 36–64 | 579 | 42.8 | 268 | 41.9 | 311 | 43.6 | |
| ≥65 | 747 | 55.2 | 359 | 56.2 | 388 | 54.3 | |
| ECOG-PS | <0.001 | ||||||
| 1 | 831 | 61.4 | 468 | 73.2 | 363 | 50.8 | |
| 2 | 334 | 24.7 | 122 | 19.1 | 212 | 29.7 | |
| 3 | 160 | 11.8 | 45 | 7.0 | 115 | 16.1 | |
| 4 | 28 | 2.1 | 4 | 0.7 | 24 | 3.9 | |
| Aim of treatment | <0.001 | ||||||
| Curative | 645 | 47.7 | 421 | 65.9 | 224 | 31.4 | |
| Palliative | 708 | 52.3 | 218 | 34.1 | 490 | 68.6 | |
| Primary Tumor | <0.001 | ||||||
| Breast | 422 | 31.1 | 263 | 41.2 | 159 | 22.3 | |
| Prostate | 143 | 10.5 | 65 | 10.2 | 78 | 10.9 | |
| Gastrointestinal | 134 | 9.9 | 43 | 6.7 | 91 | 12.7 | |
| Endometrial/Cervical | 72 | 5.3 | 39 | 6.1 | 33 | 4.6 | |
| Lung | 191 | 14.1 | 58 | 9.1 | 133 | 18.6 | |
| Head and Neck | 117 | 8.6 | 62 | 9.7 | 55 | 7.7 | |
| Others | 274 | 20.2 | 109 | 17.1 | 165 | 23.1 | |
| Tumor stage | <0.001 | ||||||
| Metastatic | 737 | 54.5 | 240 | 37.6 | 497 | 69.6 | |
| Non-Metastatic | 616 | 45.5 | 399 | 62.4 | 217 | 30.4 | |
| Type of Pain | <0.001 | ||||||
| Cancer pain or mixed pain | 914 | 67.5 | 313 | 49.0 | 601 | 84.2 | |
| Non-cancer Pain | 439 | 32.5 | 326 | 51.0 | 113 | 15.8 | |
| Pain score | <0.001 | ||||||
| NRS: 1–4 | 591 | 43.7 | 231 | 36.2 | 360 | 50.4 | |
| NRS: 5–6 | 509 | 37.6 | 274 | 42.8 | 235 | 32.9 | |
| NRS: 7–10 | 253 | 18.7 | 134 | 21.0 | 119 | 16.7 | |
| Analgesic score | <0.001 | ||||||
| No therapy | 327 | 24.2 | 327 | 51.1 | 0 | 0 | |
| Analgesics | 551 | 40.7 | 275 | 43.1 | 276 | 38.7 | |
| Weak Opioids | 194 | 14.3 | 37 | 5.8 | 157 | 22.0 | |
| Strong Opioids | 281 | 20.8 | 0 | 0 | 281 | 39.3 | |
| Location of the radiotherapy center | <0.001 | ||||||
| North of Italy | 258 | 19.1 | 103 | 16.1 | 155 | 21.7 | |
| Center of Italy | 157 | 11.6 | 102 | 16.0 | 55 | 7.7 | |
| South of Italy | 938 | 69.3 | 434 | 67.9 | 504 | 70.6 | |
| Timing of visit | 0.040 | ||||||
| During Therapy | 728 | 53.8 | 325 | 50.9 | 403 | 56.4 | |
| End of Therapy | 625 | 46.2 | 314 | 49.1 | 311 | 43.6 | |
| Age Group | Primary | Secondary | Tertiary (Context-Dependent) |
|---|---|---|---|
| 18–64 years | Type of pain | Aim of therapy | Type of cancer; Performance status |
| ≥65 years | Type of pain | Aim of therapy | Type of cancer; Location of the RT center; Timing of the visit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donati, C.M.; Galietta, E.; Cellini, F.; Zamfir, A.A.; Di Rito, A.; Portaluri, M.; Santacaterina, A.; Mammini, F.; Di Franco, R.; Parisi, S.; et al. Older Age Does Not Predict Inadequate Pain Management in Cancer Patients: A Multicenter Prospective Analysis from Italian Radiotherapy Departments (ARISE-Study). Cancers 2025, 17, 3073. https://doi.org/10.3390/cancers17183073
Donati CM, Galietta E, Cellini F, Zamfir AA, Di Rito A, Portaluri M, Santacaterina A, Mammini F, Di Franco R, Parisi S, et al. Older Age Does Not Predict Inadequate Pain Management in Cancer Patients: A Multicenter Prospective Analysis from Italian Radiotherapy Departments (ARISE-Study). Cancers. 2025; 17(18):3073. https://doi.org/10.3390/cancers17183073
Chicago/Turabian StyleDonati, Costanza M., Erika Galietta, Francesco Cellini, Arina A. Zamfir, Alessia Di Rito, Maurizio Portaluri, Anna Santacaterina, Filippo Mammini, Rossella Di Franco, Salvatore Parisi, and et al. 2025. "Older Age Does Not Predict Inadequate Pain Management in Cancer Patients: A Multicenter Prospective Analysis from Italian Radiotherapy Departments (ARISE-Study)" Cancers 17, no. 18: 3073. https://doi.org/10.3390/cancers17183073
APA StyleDonati, C. M., Galietta, E., Cellini, F., Zamfir, A. A., Di Rito, A., Portaluri, M., Santacaterina, A., Mammini, F., Di Franco, R., Parisi, S., Bianculli, A., Ziccarelli, P., Ziccarelli, L., Genovesi, D., Caravatta, L., Deodato, F., Macchia, G., Fiorica, F., Cammelli, S., ... Cilla, S. (2025). Older Age Does Not Predict Inadequate Pain Management in Cancer Patients: A Multicenter Prospective Analysis from Italian Radiotherapy Departments (ARISE-Study). Cancers, 17(18), 3073. https://doi.org/10.3390/cancers17183073










