Cure of Recurrent Ovarian Cancer: A Multicenter Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Disease-Free Survival (DFS)
2.3. Survey Items in the University Hospital Group
2.4. Survey Items for the Community Hospital Group
2.5. Specimen Collection and Pathology Review
2.6. Tumor BRCA Mutation Analysis
2.7. Statistical Analysis
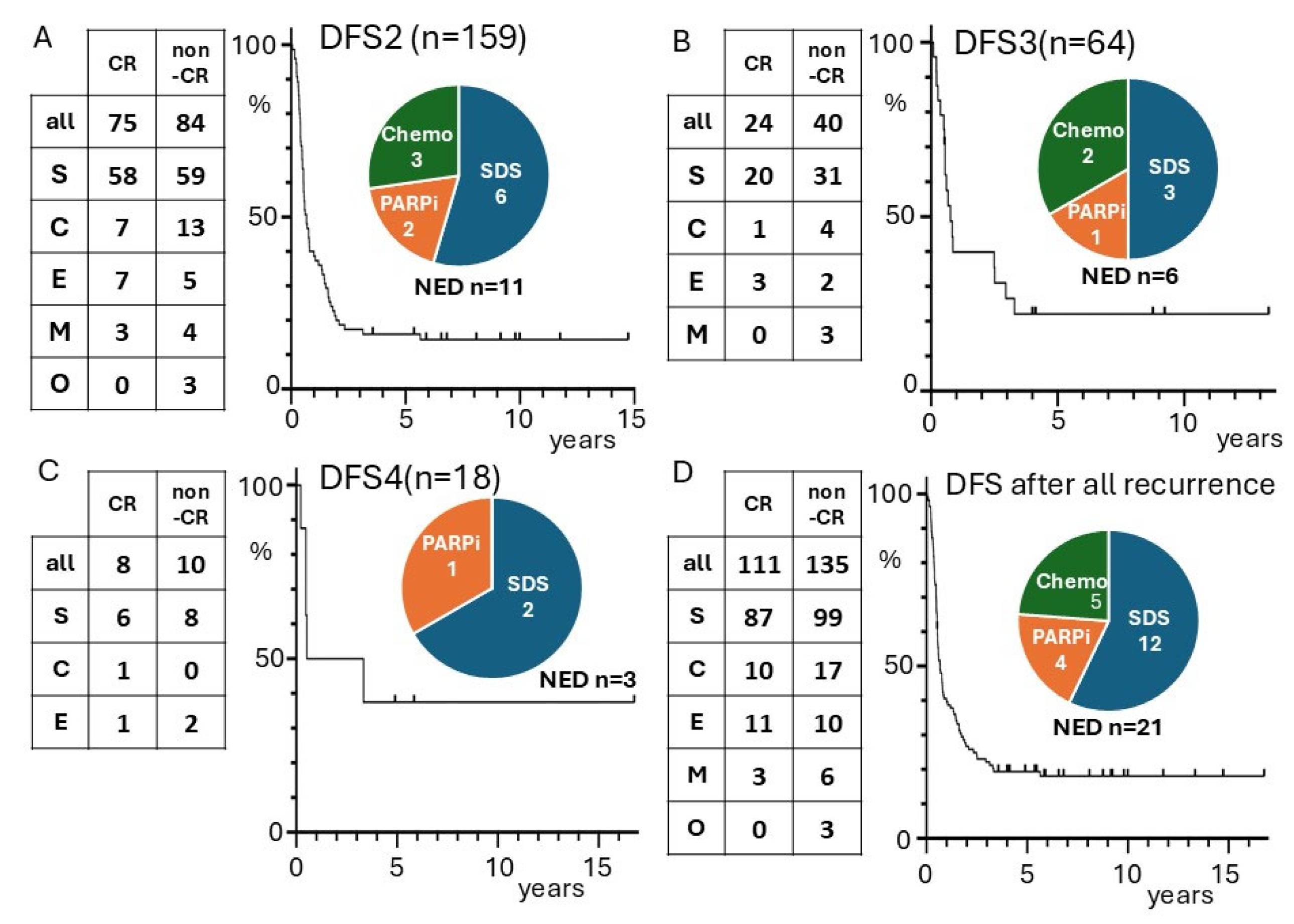
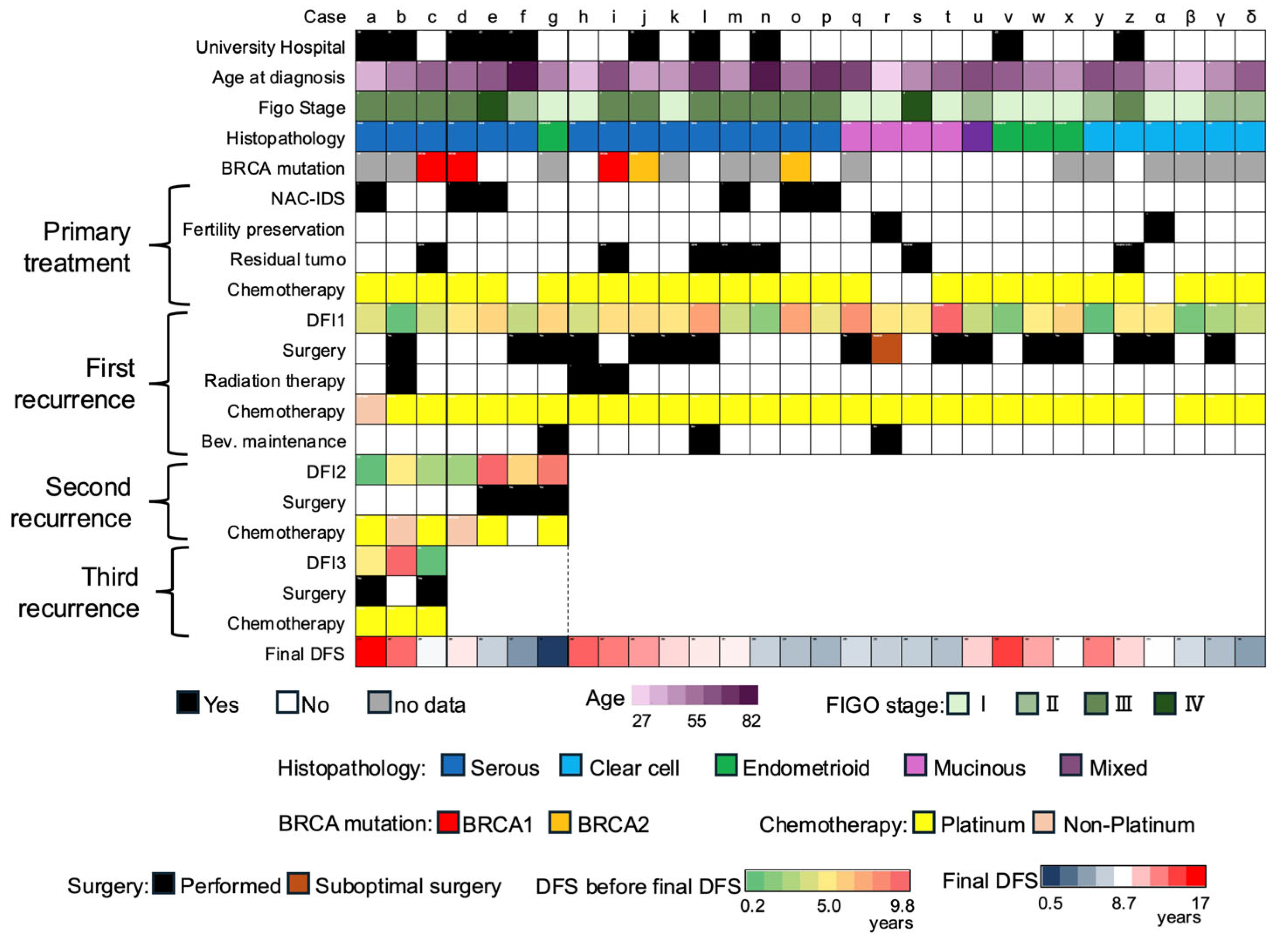
3. Results
3.1. University Hospital Groups
3.2. FFPE Specimens, CPR, and BRCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CR | complete remission |
| NED | no evidence of disease |
| DFS | disease-free survival |
| HGSC | high-grade serous carcinoma |
| PARP | poly (ADP-ribose) polymerase |
| NAC | neoadjuvant chemotherapy |
| IDS | interval debulking surgery |
| SDS | secondary debulking surgery |
| AWD | alive with disease |
| DOD | died of disease |
| FFPE | Formalin-fixed paraffin-embedded |
| CPR | central pathology review |
| RFS | recurrence-free survival |
| OS | overall survival |
References
- Nakai, H.; Higashi, T.; Kakuwa, T.; Matsumura, N. Trends in gynecologic cancer in Japan: Incidence from 1980 to 2019 and mortality from 1981 to 2021. Int. J. Clin. Oncol. 2024, 29, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Simacek, K.; Raja, P.; Chiauzzi, E.; Eek, D.; Halling, K. What Do Ovarian Cancer Patients Expect from Treatment?: Perspectives from an Online Patient Community. Cancer Nurs. 2017, 40, E17–E27. [Google Scholar] [CrossRef]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.W.; Park, S.Y.; Kim, B.G.; Nam, J.H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef]
- Friedlander, M.; Matulonis, U.; Gourley, C.; du Bois, A.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br. J. Cancer 2018, 119, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.R.; Scambia, G.; et al. Clinical and molecular characteristics of ARIEL3 patients who derived exceptional benefit from rucaparib maintenance treatment for high-grade ovarian carcinoma. Gynecol. Oncol. 2022, 167, 404–413. [Google Scholar] [CrossRef]
- Hilal, Z.; Schultheis, B.; Hartmann, F.; Dogan, A.; Cetin, C.; Krentel, H.; Schiermeier, S.; Tempfer, C.B. What Characterizes Long-term Survivors of Recurrent Ovarian Cancer? Case Report and Review of the Literature. Anticancer Res. 2016, 36, 5365–5371. [Google Scholar] [CrossRef]
- Frei, E., III. Curative cancer chemotherapy. Cancer Res. 1985, 45, 6523–6537. [Google Scholar]
- Oriuchi, N.; Nakajima, T.; Mochiki, E.; Takeyoshi, I.; Kanuma, T.; Endo, K.; Sakamoto, J. A new, accurate and conventional five-point method for quantitative evaluation of ascites using plain computed tomography in cancer patients. Jpn. J. Clin. Oncol. 2005, 35, 386–390. [Google Scholar] [CrossRef]
- Cline, M.S.; Liao, R.G.; Parsons, M.T.; Paten, B.; Alquaddoomi, F.; Antoniou, A.; Baxter, S.; Brody, L.; Cook-Deegan, R.; Coffin, A.; et al. BRCA Challenge: BRCA Exchange as a global resource for variants in BRCA1 and BRCA2. PLoS Genet. 2018, 14, e1007752. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, M.; Mogi, K.; Kitami, K.; Uno, K.; Iyoshi, S.; Tano, S.; Fujimoto, H.; Miyamoto, E.; Yoshikawa, N.; Emoto, R.; et al. Who are the long-term survivors of recurrent ovarian carcinoma?: A retrospective analysis of a multicenter study. Int. J. Clin. Oncol. 2022, 27, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Soyama, H.; Takano, M.; Miyamoto, M.; Yoshikawa, T.; Aoyama, T.; Goto, T.; Hirata, J.; Suzuki, A.; Sasa, H.; Furuya, K. Factors favouring long-term survival following recurrence in ovarian cancer. Mol. Clin. Oncol. 2017, 7, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Iwase, H.; Takada, T.; Iitsuka, C.; Nomura, H.; Abe, A.; Taniguchi, T.; Sakamoto, K.; Takizawa, K.; Takeshima, N. Clinical features of long-term survivors of recurrent epithelial ovarian cancer. Int. J. Clin. Oncol. 2015, 20, 143–149. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S.; Zola, P.; Sostegni, B.; Fuso, L.; Sartori, E. Prognostic factors and clinical outcome of patients with recurrent early-stage epithelial ovarian cancer: An Italian multicenter retrospective study. Int. J. Gynecol. Cancer 2013, 23, 461–468. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S.; Zola, P.; Sostegni, B.; Ferrero, A.M.; Teti, G.; Cristofani, R.; Sartori, E. The clinical outcome of epithelial ovarian cancer patients with apparently isolated lymph node recurrence: A multicenter retrospective Italian study. Gynecol. Oncol. 2010, 116, 358–363. [Google Scholar] [CrossRef]
- Corrigan, K.L.; Yoder, A.; De, B.; Lin, L.; Jhingran, A.; Joyner, M.M.; Eifel, P.J.; Colbert, L.E.; Lu, K.H.; Klopp, A.H. Long-term survival following definitive radiation therapy for recurrence or oligometastases in gynecological malignancies: A landmark analysis. Gynecol. Oncol. 2022, 164, 550–557. [Google Scholar] [CrossRef]
- Rome, R.; Dipnall, J.; Leung, S. Long-Term Survival After Surgery and Radiotherapy for Recurrent or Persistent Ovarian and Tubal Cancer. Int. J. Gynecol. Cancer 2018, 28, 1090–1100. [Google Scholar] [CrossRef]
- Eng, K.H.; Hanlon, B.M.; Bradley, W.H.; Szender, J.B. Prognostic factors modifying the treatment-free interval in recurrent ovarian cancer. Gynecol. Oncol. 2015, 139, 228–235. [Google Scholar] [CrossRef]
- Nakai, H.; Matsumura, N. The roles and limitations of bevacizumab in the treatment of ovarian cancer. Int. J. Clin. Oncol. 2022, 27, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Nakai, H.; Yamaguchi, K.; Hamanishi, J.; Mandai, M.; Matsumura, N. Time-Dependent Changes in Risk of Progression During Use of Bevacizumab for Ovarian Cancer. JAMA Netw. Open 2023, 6, e2326834. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef]
- Takamatsu, S.; Yoshihara, K.; Baba, T.; Shimada, M.; Yoshida, H.; Kajiyama, H.; Oda, K.; Mandai, M.; Okamoto, A.; Enomoto, T.; et al. Prognostic relevance of HRDness gene expression signature in ovarian high-grade serous carcinoma; JGOG3025-TR2 study. Br. J. Cancer 2023, 128, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Takaya, H.; Nakai, H.; Takamatsu, S.; Mandai, M.; Matsumura, N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci. Rep. 2020, 10, 2757. [Google Scholar] [CrossRef]
- Yoshihara, K.; Baba, T.; Tokunaga, H.; Nishino, K.; Sekine, M.; Takamatsu, S.; Matsumura, N.; Yoshida, H.; Kajiyama, H.; Shimada, M.; et al. Homologous recombination inquiry through ovarian malignancy investigations: JGOG3025 Study. Cancer Sci. 2023, 114, 2515–2523. [Google Scholar] [CrossRef]
- Takamatsu, S.; Brown, J.B.; Yamaguchi, K.; Hamanishi, J.; Yamanoi, K.; Takaya, H.; Kaneyasu, T.; Mori, S.; Mandai, M.; Matsumura, N. Utility of Homologous Recombination Deficiency Biomarkers Across Cancer Types. JCO Precis Oncol. 2022, 6, e2200085. [Google Scholar] [CrossRef]
- Fuh, K.C.; Shin, J.Y.; Kapp, D.S.; Brooks, R.A.; Ueda, S.; Urban, R.R.; Chen, L.M.; Chen, L.M.; Chan, J.K. Survival differences of Asian and Caucasian epithelial ovarian cancer patients in the United States. Gynecol. Oncol. 2015, 136, 491–497. [Google Scholar] [CrossRef]
- Gandara, D.R.; Kawaguchi, T.; Crowley, J.; Moon, J.; Furuse, K.; Kawahara, M.; Teramukai, S.; Ohe, Y.; Kubota, K.; Williamson, S.K.; et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: A model for assessing population-related pharmacogenomics. J. Clin. Oncol. 2009, 27, 3540–3546. [Google Scholar] [CrossRef]
- Yoshihama, T.; Kuroda, Y.; Chiyoda, T.; Takahashi, M.; Yoshimura, T.; Saotome, K.; Nanki, Y.; Sakai, K.; Kobayashi, Y.; Yamagami, W.; et al. Efficacy and safety of olaparib maintenance monotherapy for Japanese patients with platinum-sensitive relapsed ovarian, fallopian tube, and primary peritoneal cancer. Int. J. Clin. Oncol. 2022, 27, 1644–1650. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
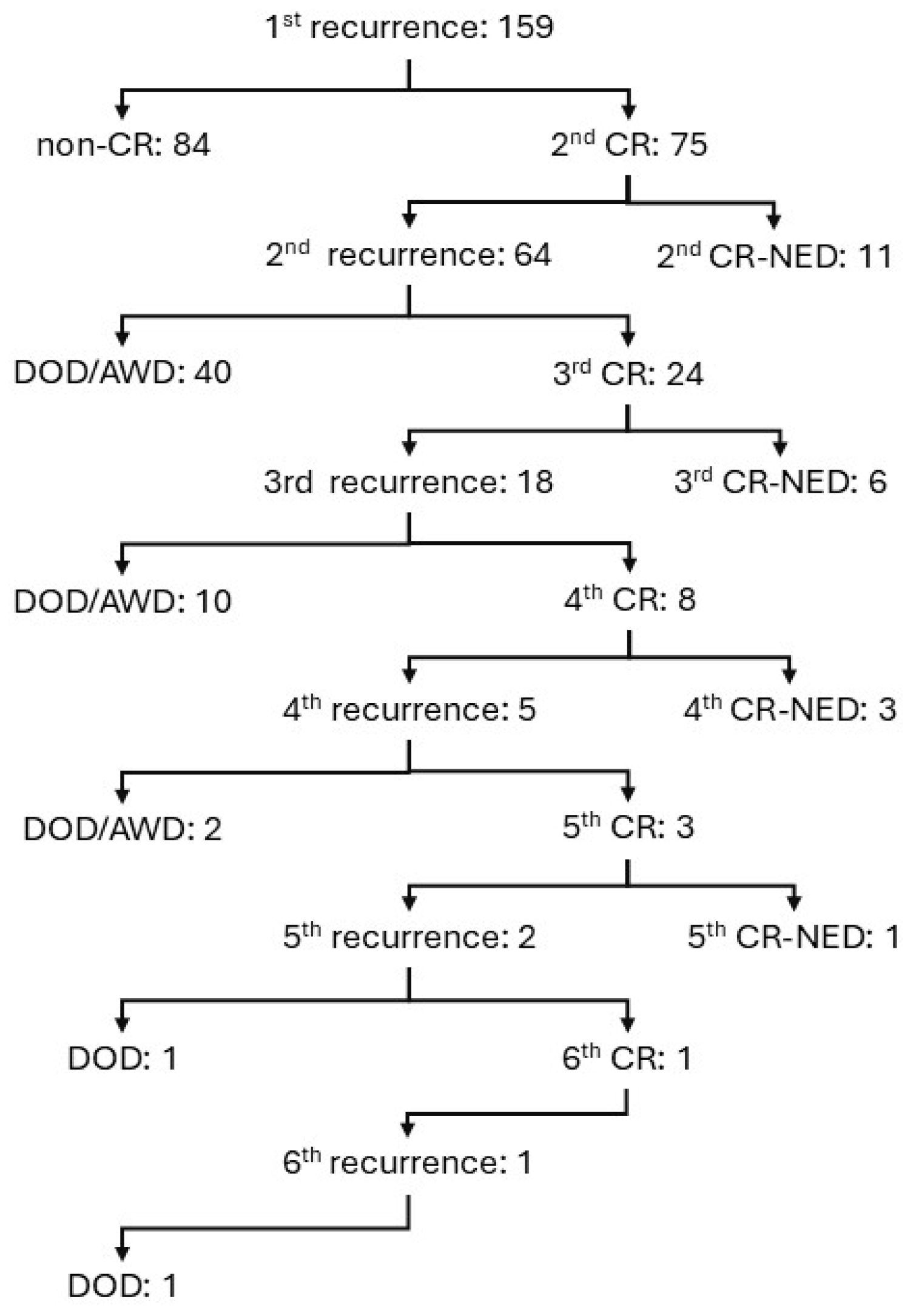
| Non-CR | DOD /AWD | CR-NED | Univariate p Value | Multivariate p Value | |
|---|---|---|---|---|---|
| number of cases | 84 | 54 | 21 | ||
| Characteristics at diagnosis | |||||
| median Age y.o. | 59.5 | 59.5 | 60.0 | 0.91 | 0.42 |
| Diabetes Mellitus (%) | 3 (4) | 3 (6) | 0 (0) | 0.72 | |
| Hypertension (%) | 19 (23) | 8 (15) | 3 (14) | 0.49 | |
| median BMI | 20.8 | 21.1 | 21.5 | 0.42 | |
| FIGO Stage III/IV (%) | 68 (81) | 49 (91) | 16 (76) | 0.21 | 0.96 |
| Metastatic site | Distant LNs.: 6 Distant Organ: 5 | Distant LNs.: 2 Distant Organ: 1 | Distant LNs.: 2 Distant Organ: 1 | ||
| Serous carcinoma (%) | 59 (70) | 41 (76) | 17 (81) | 0.76 | 0.44 |
| median CA125 IU/L | 762.5 | 308.0 | 634.7 | 0.14 | 0.87 |
| ascites > 200 mL (%) | 39 (46) | 13 (28) | 2 (12) | 0.001 | 0.02 |
| Treatment of primary tumor | |||||
| NAC-IDS (%) | 40 (48) | 28 (52) | 10 (48) | 0.99 | 0.55 |
| complete surgery (%) | 52 (62) | 37 (70) | 16 (76) | 0.65 | 0.50 |
| fertility preservation (%) | 0 (0) | 2 (4) | 1 (5) | 0.13 | 0.28 |
| no chemotherapy (%) | 0 (0) | 0 (0) | 3 (14) | 0.002 | 0.99 |
| Disease status at first recurrence | |||||
| median DFS1 mo. | 8.0 | 13.0 | 22.8 | <0.001 | <0.001 |
| median CA125 IU/L | 96.0 | 51.0 | 75.5 | 0.04 | 0.34 |
| solitary lesion (%) | 10 (12) | 15 (29) | 11 (52) | 0.004 | 0.001 |
| ascites >200 mL (%) | 6 (7.1) | 5 (10) | 0 (0) | 0.47 | 0.85 |
| Treatment of recurrent tumor | |||||
| complete surgery (%) | 19 (35) | 13 (62) | 0.04 | ||
| PARP inhibitor (%) | 11 (20) | 4 (20) | 1.00 | ||
| Bevacizumab | 11 (20) | 2 (10) | 0.33 | ||
| Case | Gene | Coding | Amino Acid Change | Variant Effect |
|---|---|---|---|---|
| c | BRCA2 | c.6952C>T | p.Arg2318Ter | nonsense |
| d | BRCA2 | c.6405_6409del | p.Asn2135Lysfs*3 | frameshift deletion |
| i | BRCA2 | c.6952C>T | p.Arg2318Ter | nonsense |
| j | BRCA1 | c.3719_3720insA | p.Ser1241ValfsTer3 | frameshift insertion |
| o | BRCA1 | c.2192_2196delAAGAA | p.Lys731ArgfsTer7 | frameshift deletion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumitomo, M.; Kotani, Y.; Murakami, K.; Abiko, K.; Sakai, K.; Otani, T.; Ueda, A.; Ukita, M.; Taga, A.; Emoto, I.; et al. Cure of Recurrent Ovarian Cancer: A Multicenter Retrospective Study. Cancers 2025, 17, 3069. https://doi.org/10.3390/cancers17183069
Sumitomo M, Kotani Y, Murakami K, Abiko K, Sakai K, Otani T, Ueda A, Ukita M, Taga A, Emoto I, et al. Cure of Recurrent Ovarian Cancer: A Multicenter Retrospective Study. Cancers. 2025; 17(18):3069. https://doi.org/10.3390/cancers17183069
Chicago/Turabian StyleSumitomo, Masahiro, Yasushi Kotani, Kosuke Murakami, Kaoru Abiko, Kazuko Sakai, Tomoyuki Otani, Akihiko Ueda, Masayo Ukita, Atsuko Taga, Ikuko Emoto, and et al. 2025. "Cure of Recurrent Ovarian Cancer: A Multicenter Retrospective Study" Cancers 17, no. 18: 3069. https://doi.org/10.3390/cancers17183069
APA StyleSumitomo, M., Kotani, Y., Murakami, K., Abiko, K., Sakai, K., Otani, T., Ueda, A., Ukita, M., Taga, A., Emoto, I., Sekiyama, K., Okudate, M., Matsubara, M., Yamanishi, Y., Nishio, K., Mandai, M., & Matsumura, N. (2025). Cure of Recurrent Ovarian Cancer: A Multicenter Retrospective Study. Cancers, 17(18), 3069. https://doi.org/10.3390/cancers17183069








