Impact of Endocrine Therapy for Cancer on Periodontal Health: A Systematic Review
Simple Summary
Abstract
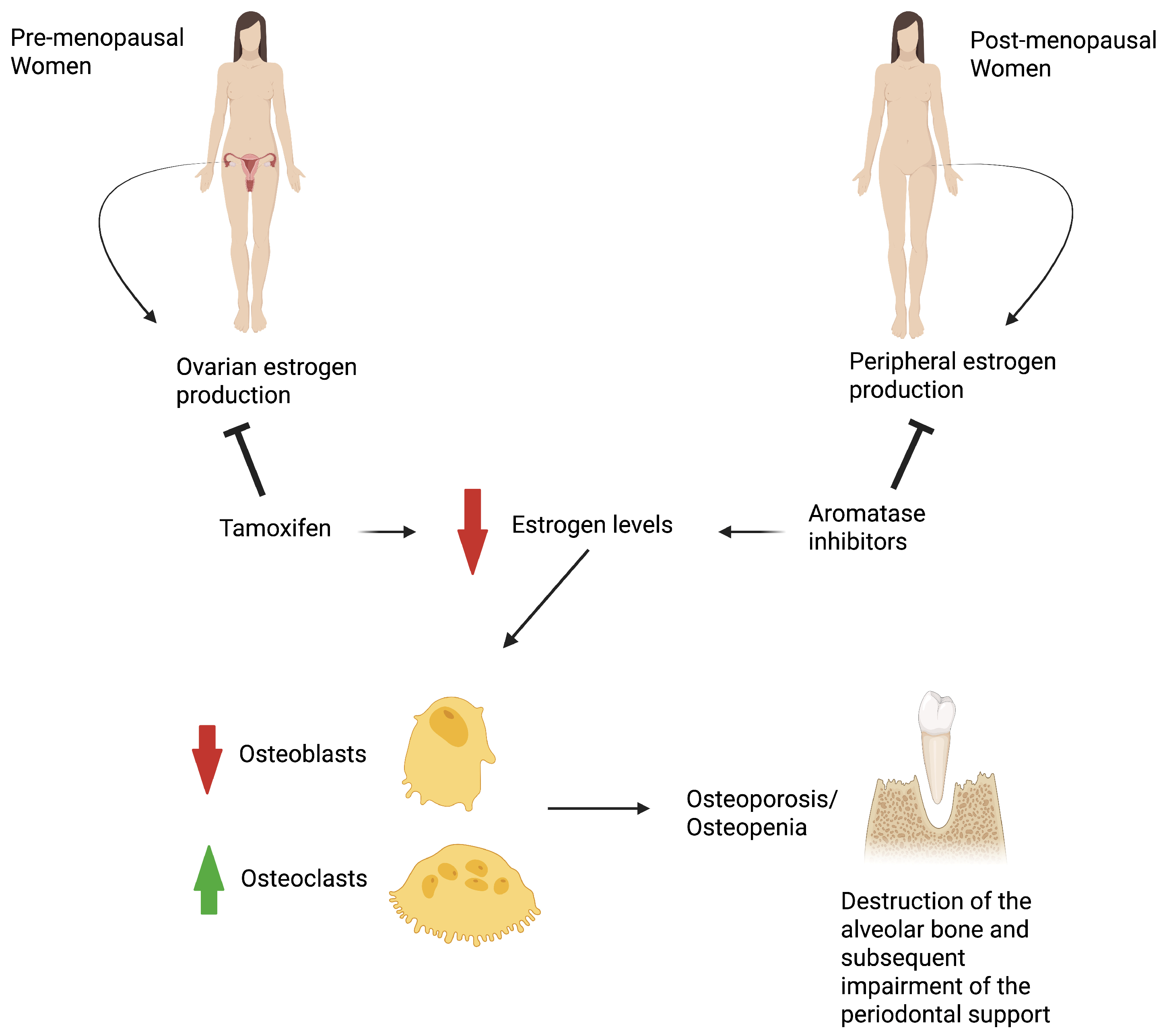
1. Introduction
2. Materials and Methods
2.1. Focused Question
- (FQ1) In patients with cancer, what is the effect of endocrine therapy on periodontal status and tooth loss compared to nonusers or healthy individuals?
- (FQ2) Do different sex hormone treatments produce varying effects on periodontal tissues?
2.2. Eligibility Criteria
2.3. Literature Search Strategy
2.4. Article Selection and Reviewer Agreement
2.5. Data Extraction and Risk of Bias Assessment
2.6. Summary Measures and Synthesis of Results
3. Results
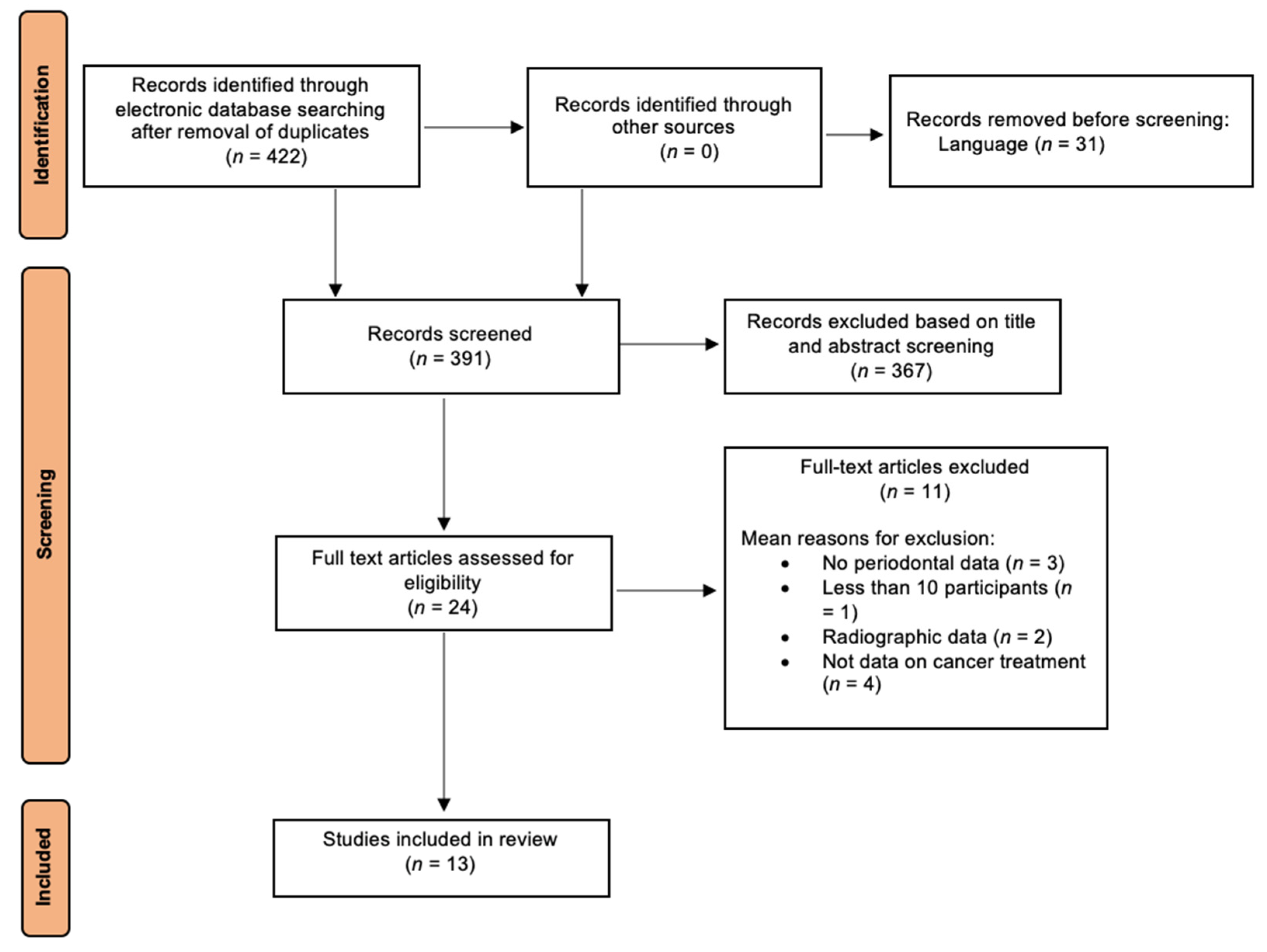
3.1. Study Selection
3.2. Study Characteristics
3.3. Synthesis of the Results
3.3.1. Impact of Hormone Therapy on Periodontal Health in Breast Cancer
- Aromatase inhibitors therapy
- Tamoxifen therapy
- Endocrine therapy (Aromatase inhibitors or Tamoxifen)
3.3.2. Impact of Hormone Therapy on Periodontal Health in Prostate Cancer
3.3.3. Comparison Between Different Hormone Therapies (FQ2)
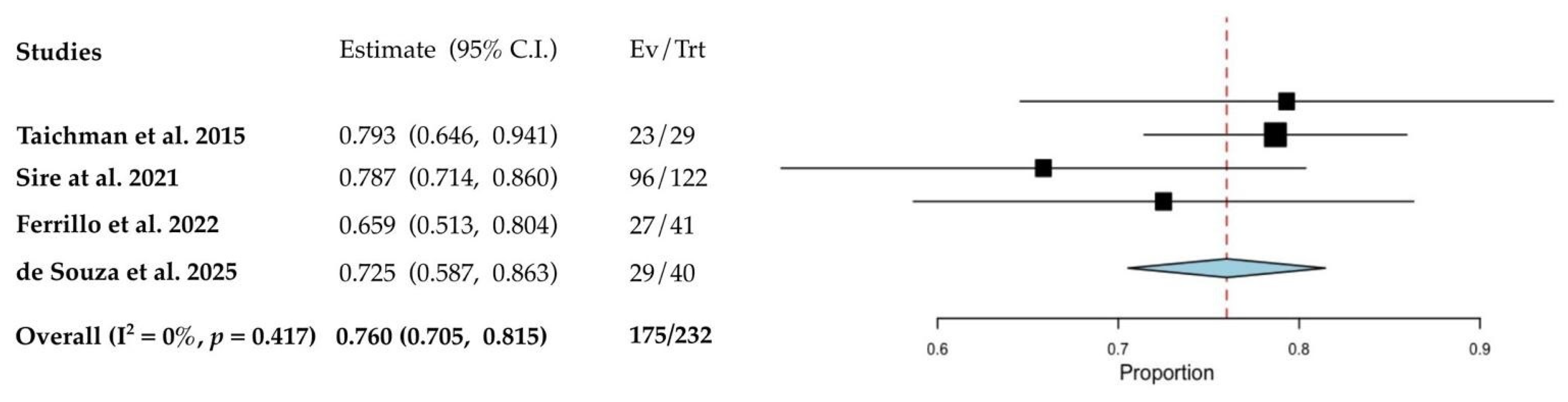
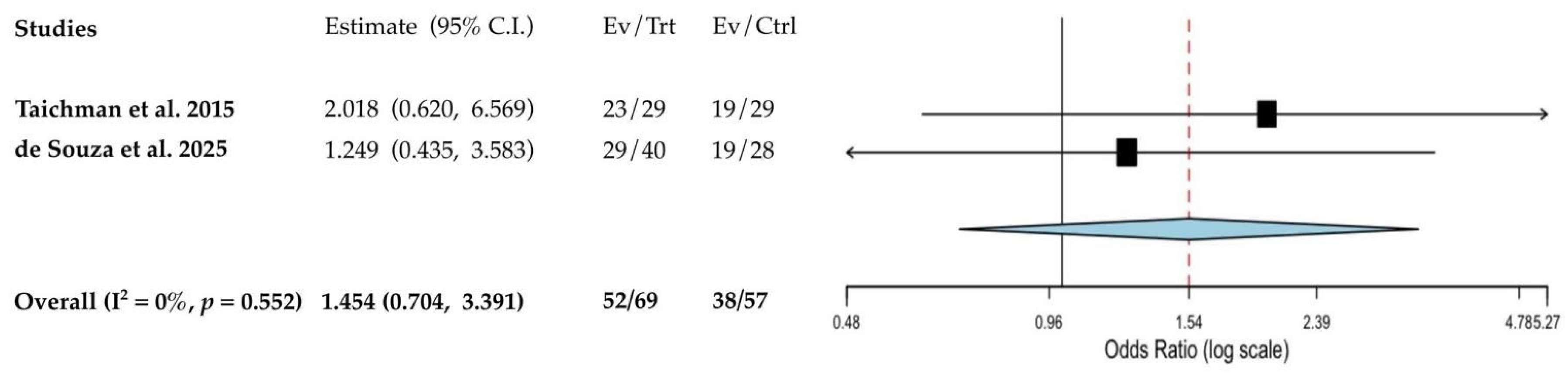
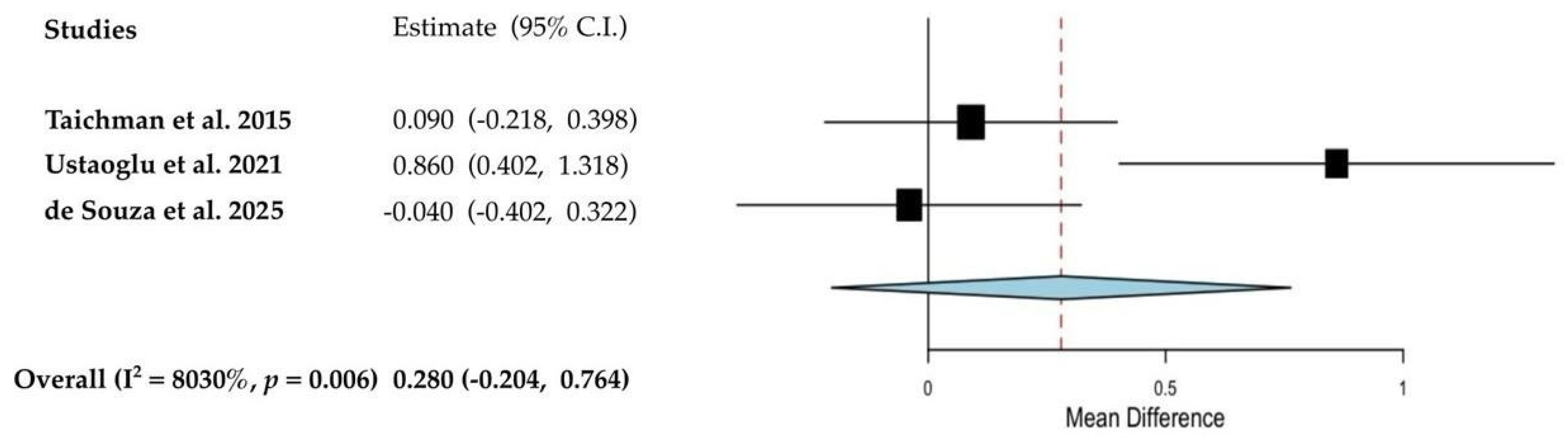
3.4. Quantitative Analyses of the Results
3.5. Risk of Bias
4. Discussion
4.1. Strenght and Limitations
4.2. Implications for Research and Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABH | Alveolar bone height |
| ADT | Androgen deprivation therapy |
| AI | Aromatase Inhibitor |
| AL | Attachment loss |
| BC | Breast cancer |
| BoP | Bleeding on probing |
| CAL | Clinical attachment level |
| CI | Confidence interval |
| DMFT | Number of decayed, missing and filled permanent teeth |
| FQ | Focused question |
| GI | Gingival index |
| HR | Hazard ratio |
| JBI | Joanna Briggs Institute Critical Appraisal Checklist |
| Moose | Meta-analysis of Observational Studies in Epidemiology |
| NHANES | National Health and Nutrition Examination Survey |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds ratio |
| PC | Prostate cancer |
| PPD | Probing pocket depth |
| PI | Plaque index |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| PCR | Plaque control record |
| PSR | Periodontal Screening and Recording Index |
| REC | Gingival recession |
| SD | Standard deviation |
| SE | Standard error |
| SMD | Standardized mean difference |
| TL | Tooth loss |
| WTCI | Winkel tongue coating index |
Appendix A
| Study | Year | Representativeness Exposed Cohort | Selection Non-Exposed Cohort | Ascertainment Exposure | Outcome of Interest not Present at Start of Study | Comparability Cohorts: Design or Analysis Controlled for Confounders | Assessment Outcome | Follow-up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Sun et al. [45] | 2025 | * | * | * | * | * | * | * | 7 |
| Study | Year | Criteria for Inclusion Clearly Defined | Study Subjects and Setting Described in Detail | Exposure Measured in a Valid and Reliable Way | Objective Criteria Used to Measure the Condition | Confounding Factors Identified | Strategies to Deal with Confounding Factors Stated | Outcomes Measured in a Valid and Reliable Way | Appropriate Statistical Analysis | Final Score |
|---|---|---|---|---|---|---|---|---|---|---|
| de Souza et al. [38] | 2024 | no | yes | n/a | n/a | no | no | yes | yes | high |
| Ferrillo et al. [39] | 2022 | yes | yes | yes | yes | yes | yes | yes | yes | low |
| Taichman et al. [42] | 2015 | yes | yes | yes | yes | yes | yes | yes | yes | low |
| Julca-Baltazar et al. [43] | 2024 | yes | yes | n/a | n/a | yes | no | no | yes | high |
| de Araujo Sensever et al. [44] | 2022 | no | yes | no | no | yes | yes | yes | yes | high |
| Park et al. [46] | 2023 | no | yes | no | n/a | no | no | yes | no | high |
| Ustaoğlu et al. [47] | 2021 | yes | yes | yes | yes | no | no | yes | no | moderate |
| de Sire et al. [48] | 2021 | yes | yes | yes | yes | no | no | yes | yes | moderate |
| Taichman et al. [49] | 2018 | yes | yes | yes | yes | n/a | n/a | no | yes | moderate |
| Famili et al. [50] | 2007 | yes | yes | n/a | n/a | yes | yes | yes | yes | high |
References
- GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2024. Available online: https://gco.iarc.who.int/today (accessed on 25 August 2025).
- AIOM; AIRTUM & Osservatorio Nazionale Screening. I Numeri del Cancro in Italia. Intermedia Editore. 2024. Available online: https://www.aiom.it/wp-content/uploads/2024/12/2024_NDC-def.pdf (accessed on 25 August 2025).
- Sleightholm, R.; Neilsen, B.K.; Elkhatib, S.; Flores, L.; Dukkipati, S.; Zhao, R.; Choudhury, S.; Gardner, B.; Carmichael, J.; Smith, L.; et al. Percentage of hormone receptor positivity in breast cancer provides prognostic value: A single-institute study. J. Clin. Med. Res. 2021, 13, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Li, M.; Bray, F.; Kvale, R.; Serraino, D.; Lorenzoni, V.; Auvinen, A.; Dal Maso, L. Prostate cancer incidence and mortality in Europe and implications for screening activities: Population-based study. BMJ. 2024, 4, e077738. [Google Scholar] [CrossRef]
- Yu, S.; Cai, X.; Wang, X.; Lin, X.; Cai, S. Disease burden of breast cancer and risk factors in Europe 44 countries, 1990–2019: Findings of the global burden of disease study 2019. Front. Endocrinol. 2024, 23, 1405204. [Google Scholar] [CrossRef]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Guzzinati, S.; Toffolutti, F.; Francisci, S.; De Paoli, A.; Giudici, F.; De Angelis, R.; Demuru, E.; Botta, L.; Tavilla, A.; Gatta, G.; et al. Patients with cancer who will be cured and projections of complete prevalence in Italy from 2018 to 2030. ESMO Open 2024, 9, 103635. [Google Scholar] [CrossRef]
- Taichman, L.S.; Havens, A.M.; Van Poznak, C.H. Potential implications of adjuvant endocrine therapy for the oral health of postmenopausal women with breast cancer. Breast Cancer Res. Treat. 2013, 137, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Thariat, J.; Bensadoun, R.J.; Barasch, A.; Murphy, B.A.; Kolnick, L.; Popplewell, L.; Maghami, E. Oral complications of cancer and cancer therapy: From cancer treatment to survivorship. CA Cancer J. Clin. 2012, 62, 400–422. [Google Scholar] [CrossRef]
- Qamar, S.; Rozi, S.; Sawani, S.; Awan, M.S.; Akhtar, S.; Siddiqui, M.I.; Abbas, S.A.; Taimoor, S.; Raza Khan, F. Oral health related quality of life in head and neck cancer survivors within the first year following treatment: A cross-sectional study in Karachi, Pakistan. Sci. Rep. 2024, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Iovoli, A.J.; Turecki, L.; Qiu, M.L.; Khan, M.; Smith, K.; Yu, H.; Ma, S.J.; Farrugia, M.K.; Singh, A.K. Severe oral mucositis after intensity-modulated radiation therapy for head and neck cancer. JAMA Netw. Open 2023, 6, e2337265. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A. Sex steroid hormones and cell dynamics in the periodontium. Crit. Rev. Oral Biol. Med. 1994, 5, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, P.; Gapski, R.; Al-Shammari, K.; Wang, H.L. Influence of sex hormones on the periodontium. J. Clin. Periodontol. 2003, 30, 671–681. [Google Scholar] [CrossRef]
- Su, X.; Jin, K.; Zhou, X.; Zhang, Z.; Zhang, C.; Li, Y.; Yang, M.; Huang, X.; Xu, S.; Wei, Q.; et al. The association between sex hormones and periodontitis among American adults: A cross-sectional study. Front. Endocrinol. 2023, 14, 1125819. [Google Scholar] [CrossRef]
- Gilliver, S.C. Sex steroids as inflammatory regulators. J. Steroid. Biochem. Mol. Biol. 2010, 120, 105–115. [Google Scholar] [CrossRef]
- Michael, H.; Härkönen, P.L.; Väänänen, H.K.; Hentunen, T.A. Estrogen and testosterone use different cellular pathways to inhibit osteoclastogenesis and bone resorption. J. Bone Miner. Res. 2005, 20, 2224–3222. [Google Scholar] [CrossRef]
- Clarke, B.L.; Khosla, S. Androgens and bone. Steroids 2009, 74, 296–305. [Google Scholar] [CrossRef]
- Khunthananithi, P.; Lertpimonchai, A.; Sritara, C.; Srithanyarat, S.S.; Thienpramuk, L.; Mongkornkarn, S. Decreased bone mineral density is associated with an increased number of teeth with periodontitis progression: A 5-year retrospective cohort study. Clin. Oral Investig. 2023, 28, 51. [Google Scholar] [CrossRef]
- National Cancer Institute. Hormone Therapy to Treat Cancer; National Cancer Institute: Bethesda, MD, USA, 2025. Available online: https://www.cancer.gov/about-cancer/treatment/types/hormone-therapy (accessed on 25 June 2025).
- Schwartz, N.; Verma, A.; Muktipaty, C.; Bivens, C.; Schwartz, Z.; Boyan, B.D. Estradiol receptor profile and estrogen responsiveness in laryngeal cancer and clinical outcomes. Steroids 2019, 142, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Beatson, G.T. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Trans. Med. Chir. Soc. Edinb. 1896, 15, 153–179. [Google Scholar]
- Harper, M.; Walpole, A. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature 1966, 212, 87. [Google Scholar] [CrossRef]
- Johnson, M.D.; Zuo, H.; Lee, K.H.; Trebley, J.P.; Rae, J.M.; Weatherman, R.V.; Desta, Z.; Flockhart, D.A.; Skaar, T.C. Pharmacological characterization of 4-hydroxy-N-desmethyl Tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res. Treat. 2004, 85, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kilker, R.L.; Hartl, M.W.; Rutherford, T.M.; Planas-Silva, M.D. Cyclin D1 expression is dependent on estrogen receptor function in tamoxifen-resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2004, 92, 63–71. [Google Scholar] [CrossRef]
- Riggins, R.B.; Schrecengost, R.S.; Guerrero, M.S.; Bouton, A.H. Pathways to tamoxifen resistance. Cancer Lett. 2007, 256, 1–24. [Google Scholar] [CrossRef]
- Generali, D.; Berardi, R.; Caruso, M.; Cazzaniga, M.; Garrone, O.; Minchella, I.; Paris, I.; Pinto, C.; De Placido, S. Aromatase inhibitors: The journey from the state of the art to clinical open questions. Front. Oncol. 2023, 13, 1249160. [Google Scholar] [CrossRef] [PubMed]
- Nabholtz, J.M. Long-term safety of aromatase inhibitors in the treatment of breast cancer. Ther. Clin. Risk Manag. 2008, 4, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Ocen, J.; Staffurth, J. Hormonal therapy for cancer. Medicine 2023, 51, 28–31. [Google Scholar] [CrossRef]
- Ascione, L.; Castellano, G.; Curigliano, G.; Zagami, P. Endocrine therapy for early breast cancer in the era of oral selective estrogen receptor degraders: Challenges and future perspectives. Curr. Opin. Oncol. 2024, 36, 465–473. [Google Scholar] [CrossRef]
- de Bataille, C.; Castellan, M.; Massabeau, C.; Jouve, E.; Lacaze, J.L.; Sibaud, V.; Vigarios, E. Oral mucosal changes induced by adjuvant endocrine therapies in breast cancer patients: Clinical aspects and proposal for management. Support. Care Cancer 2021, 29, 1719–1722. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Forrest, J.L.; Miller, S.A. Evidence-based decision making in action: Part 1—Finding the best clinical evidence. J. Contemp. Dent. Pract. 2002, 3, 10–26. [Google Scholar] [CrossRef]
- Newcastle-Ottawa Scale. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 July 2025).
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- de Souza, A.F.; Barra, S.G.; Rocha, A.L.; Bemquerer, L.M.; Oliveira, S.R.; Carvalho, L.N.; Amaral, T.M.P.; Brasileiro, C.B.; Costa, F.O.; Souza, L.N.; et al. Bone mineral density in patients using aromatase inhibitors: A clinical, nutritional, and quality of life assessment. Braz. Oral Res. 2025, 39, e023. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Lippi, L.; Antonelli, A.; Calafiore, D.; Ammendolia, V.; Fortunato, L.; Renò, F.; Giudice, A.; et al. Oral health in breast cancer women with vitamin D deficiency: A machine learning study. J. Clin. Med. 2022, 11, 4662. [Google Scholar] [CrossRef] [PubMed]
- Taichman, L.S.; Van Poznak, C.H.; Inglehart, M.R. Self-reported oral health and quality of life of postmenopausal breast cancer survivors on aromatase inhibitors and women without cancer diagnoses: A longitudinal analysis. Support. Care Cancer 2016, 24, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Eagle, I.; Benavides, E.; Eber, R.; Kolenic, G.; Jung, Y.; Van Poznak, C.; Taichman, L.S. Periodontal health in breast cancer patients on aromatase inhibitors versus postmenopausal controls: A longitudinal analysis. J. Clin. Periodontol. 2016, 43, 659–667. [Google Scholar] [CrossRef]
- Taichman, L.S.; Inglehart, M.R.; Giannobile, W.V.; Braun, T.; Kolenic, G.; Van Poznak, C. Periodontal health in women with early-stage postmenopausal breast cancer newly on aromatase inhibitors: A pilot study. J. Periodontol. 2015, 86, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Julca-Baltazar, J.J.; Asmat-Abanto, A.S.; Pantoja-Lázaro, A.R.; Gorritti-Rubio, A.P.; Minchón-Medina, C.A. Tooth loss in breast cancer patients: A comparison between tamoxifen-treated and non-treated patients. Med. Oral Patol. Oral Cir. Bucal. 2024, 29, e552–e558. [Google Scholar] [CrossRef]
- de Araujo Sensever, F.; Jardim, L.C.; Ferrazzo, K.L.; Skupien, J.A.; Antoniazzi, R.P. Association between tamoxifen and tooth loss in women with breast cancer. Support. Care Cancer 2022, 30, 8193–8199. [Google Scholar] [CrossRef]
- Sun, L.M.; Tsai, F.J.; Lin, C.L.; Wu, Y.H. Women with breast cancer exhibit a higher risk for periodontitis: A nationwide cohort study. J. Dent. Sci. 2025, 20, 962–970. [Google Scholar] [CrossRef]
- Park, S.H.; Strauss, S.M. Original research: Oral health concerns of female breast cancer survivors on adjuvant endocrine therapy. Am. J. Nurs. 2023, 123, 24–29. [Google Scholar] [CrossRef]
- Ustaoğlu, G.; Göller Bulut, D.; Üyetürk, Ü.; Uysal, Ö. Evaluation of periodontal health in breast cancer patients undergoing tamoxifen or aromatase inhibitors drugs therapy: A cross-sectional study. Spec. Care Dent. 2021, 41, 41–48. [Google Scholar] [CrossRef]
- de Sire, A.; Ferrillo, M.; Gennari, A.; Cisari, C.; Pasqua, S.; Foglio Bonda, P.L.; Invernizzi, M.; Migliario, M. Bone health, vitamin D status and oral hygiene screening in breast cancer women before starting osteoporosis treatment: A cross-sectional study. J. Biol. Regul. Homeost. Agents 2021, 35, 397–402. [Google Scholar] [CrossRef]
- Taichman, L.S.; Van Poznak, C.H.; Inglehart, M.R. Oral health-related concerns, behavior, and communication with health care providers of patients with breast cancer: Impact of different treatments. Spec. Care Dent. 2018, 38, 36–45. [Google Scholar] [CrossRef]
- Famili, P.; Cauley, J.A.; Greenspan, S.L. The effect of androgen deprivation therapy on periodontal disease in men with prostate cancer. J. Urol. 2007, 177, 921–924. [Google Scholar] [CrossRef]
- Nasi, J.H. Background to, and implementation of, the Periodontal Screening and Recording (PSR) procedure in the USA. Internat. Dent. J. 1994, 44 (Suppl. S1), 585–588. [Google Scholar]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, S.; Davidson, N.E. Extending adjuvant endocrine therapy in breast cancer: Who, what, why? Oncology 2019, 33, 243–246. [Google Scholar] [PubMed]
- Kaklamani, V.G.; Gradishar, W.J. Endocrine therapy in the current management of postmenopausal estrogen receptor-positive metastatic breast cancer. Oncologist 2017, 22, 507–517. [Google Scholar] [CrossRef]
- Lin, J.; Pei, T.; Yang, H. Association between modifiable lifestyle pattern and periodontitis: A cross-sectional study based on NHANES. BMC Oral Health 2024, 24, 591. [Google Scholar] [CrossRef]
- Almohamad, M.; Krall Kaye, E.; Mofleh, D.; Spartano, N.L. The association of sedentary behavior and physical activity with periodontal disease in NHANES 2011–2012. J. Clin. Periodontol. 2022, 49, 758–767. [Google Scholar] [CrossRef]
- Jardim, L.C.; Flores, P.T.; do Carmo Dos Santos Araújo, M.; Chiesa, J.; de Moraes, C.M.B.; Antoniazzi, R.P. Oral health-related quality of life in breast cancer survivors. Support. Care Cancer 2020, 28, 65–71. [Google Scholar] [CrossRef]
- Vaklavas, C.; Stringer-Reasor, E.M.; Elkhanany, A.M.; Ryan, K.J.; Li, Y.; Theuer, C.P.; Acosta, E.P.; Wei, S.; Yang, E.S.; Grizzle, W.E.; et al. A phase I/II study of preoperative letrozole, everolimus, and carotuximab in stage 2 and 3 hormone receptor-positive and HER2-negative breast cancer. Breast Cancer Res. Treat. 2023, 198, 217–229. [Google Scholar] [CrossRef]
- Marotta, N.; Ferrillo, M.; Giudice, A.; Lippi, L.; Calafiore, D.; Curci, C.; Migliario, M.; Invernizzi, M.; Ammendolia, A.; de Sire, A. Correlation between bone mineral density, vitamin D deficiency, and oral health in women with breast cancer. Int. J. Bone Frag. 2023, 3, 90–94. [Google Scholar] [CrossRef]
- Hasegawa, S.; Yanagita, M.; Tatsumi, M.; Yamashita, M.; Kitamura, M.; Murakami, S. Aromatase inhibitor anastrozole modifies cellular functions in gingival fibroblasts and endothelial cells: Possible periodontal complications of aromatase inhibitor treatment. J. Periodont. Res. 2021, 56, 828–836. [Google Scholar] [CrossRef]
- Valimaa, H.; Savolainen, S.; Soukka, T. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epi-thelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lucisano, M.P.; da Silva, R.A.B.; de Sousa Pereira, A.P. Alteration of the oral microbiota may be a responsible factor, along with estrogen deficiency, by the development of larger periapical lesions. Clin. Oral Investig. 2021, 25, 3651–3662. [Google Scholar] [CrossRef]
- Ojanotko, A.; Nienstedt, W.; Harri, M.P. Metabolism of testosterone by human healthy and inflamed gingiva in vitro. Arch. Oral Biol. 1980, 25, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, Á.F.; de Paiva Gonçalves, V.; Carvalho, J.S.; Spolidorio, D.M.P.; Spolidorio, L.C. Testosterone replacement relieves ligature-induced periodontitis by mitigating inflammation, increasing pro-resolving markers and promoting angiogenesis in rats: A preclinical study. Arch. Oral Biol. 2023, 146, 105605. [Google Scholar] [CrossRef]
- Memon, M.A.; Aleem, B.; Memon, H.A.; Lee, K.Y. Assessing salivary matrix metalloproteinase-8 in prostate cancer patients undergoing androgen deprivation therapy. Clin. Exp. Dent. Res. 2022, 8, 1277–1283. [Google Scholar] [CrossRef]
| (“neoplasms”[MeSH Terms] OR “adenocarcinoma”[Title/Abstract] OR “carcinoma”[Title/Abstract] OR “cancer”[Title/Abstract]) AND (“antineoplastic agents, hormonal”[MeSH Terms] OR “androgen receptor antagonist*”[Title/Abstract] OR “anti androgen*”[Title/Abstract] OR “LHRH”[Title/Abstract] OR “luteinizing hormone-releasing hormone”[Title/Abstract] OR “luteinizing hormone releasing hormone analog*”[Title/Abstract] OR “luteinizing hormone releasing hormone agonist*”[Title/Abstract] OR “androgen”[Title/Abstract] OR “androgen deprivation therap*”[Title/Abstract] OR “selective estrogen receptor modulator*”[Title/Abstract] OR “endocrine therap*”[Title/Abstract] OR (“aromatase inhibitor*”[Title/Abstract] OR “hormone therap*”[Title/Abstract]) OR “tamoxifen”[Title/Abstract]) AND ((“periodontitis”[MeSH Terms] OR “mouth diseases”[MeSH Terms] OR “oral health”[MeSH Terms] OR “gingivitis”[MeSH Terms] OR “tooth loss”[MeSH Terms] OR “periodont*”[Title/Abstract] OR “gingiv*”[Title/Abstract] OR “oral health”[Title/Abstract] OR “oral condit*”[Title/Abstract]) OR “oral stat*”[Title/Abstract]) |
| First Author Year, Country | Focused Question | Study Design, Setting, Time Period, Source of Funding | Sample Size (n) | Test Group (n) | Control Group (n) | Definition of Periodontal Disease |
|---|---|---|---|---|---|---|
| Studies on breast cancer | ||||||
| Aromatase inhibitors | ||||||
| de Souza et al., 2025, Brazil [38] | FQ1 | Cross-sectional, Cancer Unit Mater Dei Hospital, October 2019 to August 2021, no external funding | 72 | Adult women with BC assuming AIs (n = 40), of whom 24 taking letrozole, 14 anastrozole, and 2 exemestane, having at least 6 teeth, 40% of them assuming biphosphonates, mean age 61.65 yrs, 67.5% White, 20% Black, 15% mixed, 27.5% smokers | Adult women without BC not using AIs (n = 28), having at least 6 teeth, 18.7% of them using bisphosphonates, mean age 60.64 yrs, 75% White, 10.7% Black, 14.3% mixed, 42.9% smokers | 2017 World classification of periodontal health and Stage I–IV periodontitis |
| Ferrillo et al., 2022, Italy [39] | FQ1 | Cross-sectional, University Hospital “Maggiore della Carità” Novara, April 2021 to March 2022, no external funding | 41 | Post-menopausal BC women with vitamin D deficiency undergoing AI therapy, 29.3% smokers, mean age 66.1 ± 8.47 yrs, 29.27% smokers | Periodontal Screening and Recording Index (PSR) | |
| Taichman et al., 2016 and Eagle et al., 2016 USA [40,41] | FQ1 | Longitudinal study, University of Michigan Hospital, April 2009 to September 2013, no external funding. 18-month follow-up examination of the study by Taichman et al. 2015 [42] | 58 | Post-menopausal women (n = 29) with BC (Stage I-IIIA with no evidence of metastatic disease) treated with any AI (anastrozole, exemestane, or letrozole), mean age 61.7 ± 7.6 yrs, 89.7% White and 10.3% non-White, 3.4% smokers | Post-menopausal women without BC (n = 29), mean age 61.6 ± 5.4 yrs, 89.6% White and 10.4% non-White, 3.4% smokers | Subjective periodontal and dental health based on 5-point scale questions from the NHANES and 11 binary questions on periodontal disease-related symptoms |
| Taichman et al., 2015, USA [42] | FQ1 | Cross-sectional, University of Michigan Hospital, April 2009 to September 2010, no external funding | 58 | Post-menopausal women (n = 29) with early-stage BC (Stage I-IIIA) on AI adjuvant treatment (anastrozole n = 20, exemestane n = 2, and letrozole n = 7, within 2 to 11 months of start), having > 15 teeth, mean age 61.7 ± 7.6 yrs, 89.7% White and 10.3% non-White, 3.4% smokers | Post-menopausal women without BC (n = 29), not on AI therapy, having > 15 teeth, mean age 61.6 ± 5.4 yrs, 89.6% White and 10.4% non-White, 3.4% smokers | Case of periodontitis defined as at least one site with AL of ≥3 mm, and classified as mild (AL = 3 mm), moderate (AL ≥ 4 mm but <6 mm) and severe (AL ≥ 6 mm) |
| Tamoxifen | ||||||
| Julca-Baltazar et al., 2024, Peru [43] | FQ1 | Cross-sectional, High Complexity Hospital and Regional Institute of Neoplastic Diseases (Trujillo, Peru), July to September 2023, no external funding | 200 | Adult women with BC assuming tamoxifen (n = 100) | Adult women with BC not assuming tamoxifen (n = 100) | NR |
| de Araujo Sensever et al., 2022, Brazil [44] | FQ1 | Cross-sectional, Hospital Southern Brazil, January to August 2017, no external funding | 140 | Adult women with BC taking tamoxifen for up to 12 months (n = 41) | Adult women with BC taking tamoxifen for more than 12 months (n = 97) | NR |
| Outcome Measures | Impact on Periodontal Health | Additional Findings | ||||
| Aromatase Inhibitors | ||||||
| PI; BoP; PPD; CAL; DMFT | Cases Periodontitis stage II–IV: 72.5%; mean PPD: 1.89 mm; mean CAL: 2.24 mm; mean BOP: 7.26%; mean PI: 10.95%; mean DMFT: 21.23 Controls Periodontitis stage II–IV: 67.9%; mean PPD: 2.02 mm; mean CAL: 2.28 mm; mean BOP: 8.13%; mean PI: 20.13%, mean DMFT: 20.00 | PI of controls was significantly higher than that of women who used AIs. The groups were similar for DMFT index, PPD, CAL, and BoP. However, the number of patients with stage II–IV periodontitis tended to be higher in the case group. | ||||
| OHI; GBI; PCR; DMFT; WTCI | Moderate periodontitis: 63.1% Severe periodontitis: 15.6%; OHI > 3: 43.9%; PCR index > 50%: 46.3%; DMFT (mean ± SD): 16.07 ± 7.05; WTCI grade 2: 36.6% | High prevalence of osteoporosis (56.10%) and smokers (29.3%). Prevalence of periodontitis higher than in the general population. | ||||
| PI; BoP; PPD; CAL; ABH | Cases Number of subjective periodontal disease indicators (mean): Baseline: 2.0 18-month PH: 2.4 Change baseline-18 months for clinical parameters (mean ± SE) PI: 0.24 ± 0.37; BoP: 0.02 ± 0.36; PPD: 0.35 ± 0.28 mm; CAL: 0.45 ± 0.38 mm; ABH: 0.32 ± 0.36 mm Controls Number of subjective periodontal disease indicators (mean): Baseline: 1.6 18-month PH: 1.1 Change baseline-18 months for clinical parameters (mean ± SE) PI: 0.36 ± 0.14; BoP: 0.14 ± 0.13; PPD: 0.01 ± 0.22 mm; CAL: 0.03 ± 0.22 mm; ABH: 0.19 ± 0.22 mm | Women taking AI had a significantly worse mean subjective periodontal health score than controls, which tended to worsen during the first 18 months of AI use. Statistically significant greater PPD increase, CAL loss and ABH loss were observed in the case than in the control group, while BoP increased more in the control group. The use of bisphosphonate, vitamin D, and calcium usage reduced ABH loss only in the case group. | ||||
| PI; BoP; PPD; AL; REC; ABH; perceived oral health (Likert scale) | Cases Moderate periodontitis: 48.3%; Severe periodontitis: 31% Periodontal parameters (mean ± SD) N° sites with PI: 55.4 ± 3.4 N° sites with BoP: 27.8 ± 23.4 PPD: 2.0 ± 0.27 mm AL: 1.5 ± 0.75 mm Worst site AL: 1.5 ± 0.75 mm REC: 0.36 ± 0.67 mm ABH: 2.65 ± 0.63 mm Perceived dental health: 3.14 ± 0.18 Perceived gum health: 2.97 ± 1.29 Importance of dental health: 4.72 ± 0.75 Controls Moderate periodontitis: 58.3%; Severe periodontitis: 6.9% Periodontal parameters (mean ± SD) N° sites with PI: 16.3 ± 6.6 N° sites with BoP: 16.7 ± 12.3 PPD: 2.0 ± 0.29 mm AL: 1.4 ± 0.39 mm Worst site AL: 1.5 ± 0.75 mm REC: 0.28 ± 0.44 mm ABH: 2.69 ± 0.46 mm Perceived dental health: 3.69 ± 0.96 Perceived gum health: 3.34 ± 1.04 Importance of dental health: 4.97 ± 0.18 | Compared with controls, cases had a higher prevalence of severe periodontitis, more sites with BoP, and greater dental plaque, and they tended to rate their oral health lower. In adjusted linear regression (accounting for income, tobacco use, and prior radiation or chemotherapy), AI use was associated with attachment loss exceeding 2 mm (95% CI: 0.46–3.92). | ||||
| Tamoxifen | ||||||
| TL | Cases Overall TL (mean ± SD): 2.04 ± 1.58 ≤1 year of drug use (mean ± SD): 1.63 ± 1.77 >1 year of drug use (mean ± SD): 2.32 ± 1.37 Controls TL (mean ± SD): 1.80 ± 1.51 | Women who used tamoxifen for more than one year presented greater TL compared to controls as well as those who consumed tamoxifen and did not receive previous chemotherapy or radiotherapy. | ||||
| TL based on the M component of DMFT | Cases TL (mean ± SD): 10.45 ± 8.77 Controls TL (mean ± SD): 13.99 ± 8.78 | In the adjusted model, the odds of having more than 12 missing teeth were 2.75 times higher among women who used tamoxifen for over one year compared with those treated for less than one year. | ||||
| First Author Year, Country | Focused Question | Study Design, Study Setting, Time Period, Source of Funding | Sample Size (n) | Test Group (n) | Control Group (n) | Definition of Periodontal Disease |
| Studies on breast cancer | ||||||
| Tamoxifen and Aromatase Inhibitors | ||||||
| Sun et al., 2025, China [45] | FQ2 | Retrospective cohort, Taiwan National Health Registry, January 2010 to December 2019, no external funding | 16.492 | Women with BC (n = 8246), treated with tamoxifen (n = 42,746), anastrozole (n = 5524), exemestane (n = 5705) and letrozole (n = 35,654), mean age 55.1 ± 12.3 yrs | Women without BC (n = 8246), matched 1:1 in terms of age, income, comorbidities, and urbanization level, mean age 55.6 ± 14.7 yrs | Periodontitis diagnosis based on the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM and ICD-10-CM) |
| Park et al., 2023, USA [46] | FQ1 | Cross-sectional, NHANES dataset, January 2009 to March 2020, no external funding | 423 | Adult women with BC undergoing adjuvant endocrine therapy (n = 94), of whom 30 assuming tamoxifen, 33 anastrozole, 18 letrozole and 13 exemestane, 54.8% under 65 yrs, 16.6% smokers, 75.8% White, 10.7% Black, 13.5% Other | Adult women with BC not undergoing adjuvant endocrine therapy (n = 329), 38% under 65 yrs of age (10.7% smokers), 81.8% White, 6.7% Black, 11.5% Other | NR |
| Ustaoğlu et al., 2021, Turkey [47] | FQ1 FQ2 | Cross-sectional, Department of Medical Oncology Bolu Abant Izzet Baysal University, April 2009 to September 2013, no external funding | 155 | Women with early-stage BC (Stage I to IIIA) treated with at least one course of endocrine therapy (n = 103), of whom: | Systemically healthy women (n = 52), mean age 48.33 ± 10.08 yrs, 38 of them in the post-menopausal period. | NR |
| ||||||
| All patients of Al group and 44 of the tamoxifen group in the post-menopausal period. | ||||||
| de Sire et al., 2021, Italy [48] | FQ1 | Cross-sectional, University Hospital “Maggiore della Carità” Novara, January to June 2020, no external funding | 122 | Post-menopausal women with invasive BC treated with surgery at least 12 months earlier, taking tamoxifen (n = 48) or AI (n = 74) therapy, mean age 55.6 ± 10.4 yrs, 18% smokers | Periodontal Screening and Recording Index (PSR) | |
| Taichman et al., 2018, USA [49] | FQ1 FFQ2 | Cross-sectional, University of Michigan Hospital Michigan, June 2014 to June 2015, no external funding | 181 | Post-menopausal women with early-stage BC (n = 140) on adjuvant treatment for at least 3 months and having > 20 natural teeth, 28 women assuming chemotherapy (mean age 58.0 ± 9.9 yrs, smokers 4%), 52 tamoxifen (mean age 56.3 ± 8.3 yrs, smokers 2%) and 60 AI (mean age 62.5 ± 7.7 yrs, smokers 2%) | Post-menopausal women without BC (n = 41), having > 20 natural teeth, mean age 66.0 ± 9.4 yrs, smokers 12% | Subjective perception of teeth and gum health |
| Studies on prostate cancer | ||||||
| Famili et al., 2007, USA [50] | FQ1 | Cross-sectional, University Pittsburgh, no external funding | 68 | Men with nonmetastatic PC treated with ADT (n = 41), mean age 70.5 yrs, 7.3% smokers, 7.3% Black and 92.7% White | Men with nonmetastatic PC not on ADT (n = 27), mean age 68.5 yrs, 3.7% smokers, 11.1% Black and 88.9% White | Case of periodontitis defined as at least one site with AL of ≥3 mm |
| Tamoxifen and Aromatase Inhibitors | ||||||
| Risk of developing periodontitis over a mean follow-up time of 6.15 ± 3.01 years | 2679 BC women developed periodontitis of whom: | The risk of periodontitis was significantly lower in women who received hormone therapy compared with those who did not. | ||||
| ||||||
| Prevalence of gum disease, TL, Number of decayed teeth, Need for immediate dental care | Cases TL (mean ± SE): 12.4 ± 0.51 Coronal cavities (mean ± SE): 1.97± 0.17 Decayed teeth (%): 27.5 Gum disease (%): 27.2 Recommended for imminent dental care (%): 43.4 Controls TL (mean ± SE): 11.7 ± 0.91 Coronal cavities (mean ± SE): 4.44 ± 0.57 Decayed teeth (%): 13.4 Gum disease (%): 13.2 Recommended for imminent dental care (%): 26.1 | Endocrine therapy use was associated with increased prevalence of tooth decay and periodontal pathology, and these patients were more often identified as needing prompt dental intervention than those not receiving such therapy. | ||||
| PI; BoP; GI; PPD; CAL; N° of decayed teeth; N° of teeth requiring extraction for advanced periodontal involvement, fracture, or extensive carious lesion | Cases (mean ± SE) Tamoxifen users AI users | AI users exhibited the fewest teeth and highest CAL, while PI, GI, and PPD did not differ significantly across groups. PI was lower in patients using AIs for <2 years. | ||||
| PI: | 1.74 ± 0.68 | 1.87 ± 0.54 | ||||
| BoP: | 58.71 ± 40.20 | 61.50 ± 39.21 | ||||
| GI: | 1.60 ± 0.60 | 1.76 ± 0.33 | ||||
| PPD (mm): | 2.15 ± 0.70 | 2.55 ± 1.11 | ||||
| CAL (mm): | 2.65 ± 0.70 | 3.34 ± 1.34 | ||||
| N° of teeth: | 17.00 ± 8.56 | 15.23 ± 8.23 | ||||
| N° of teeth to | ||||||
| be extracted: | 0.22 ± 0.67 | 0.23 ± 0.68 | ||||
| N° decayed teeth: | 0.127 ± 0.105 | 0.08 ± 0.16 | ||||
| Controls (mean ± SE) PI: 1.50 ± 0.53 BoP: 48.40 ± 42.04 GI: 1.52 ± 0.46 PPD (mm): 2.21 ± 0.92 CAL (mm): 2.48 ± 1.39 N° of teeth: 19.17 ± 5.13 N° of teeth to be extracted: 0.63 ± 1.20 N° decayed teeth: 0.22 ± 0.22 | ||||||
| OHI; GBI; PCR; DMFT | Moderate periodontitis: 55.7% Severe periodontitis: 12.2%; insufficient OHI: 53.2%; GBI < 25%: 93.4%; PCR index > 50%: 51.6%; DMFT (mean ± SD): 17.44 ± 6.76 | BC women on hormonal therapy showed a high prevalence of mild-to-moderate periodontitis and poor oral care. | ||||
| Questionnaire on subjective oral health perception (scale from 1 to 5) and frequency of oral symptoms | Cases (mean ± SD) Chemotherapy Health of teeth: 3.21 ± 0.74 Health of gums: 3.36 ± 0.91 Frequency of teeth sensitive: 1.55 ± 1.01 Frequency of bleeding gums: 1.50 ± 1.00 Frequency of bad breath: 1.67 ± 1.09 Tamoxifen Health of teeth: 3.65 ± 1.08 Health of gums: 3.48 ± 0.98 Frequency of teeth sensitive: 2.25 ± 1.39 Frequency of bleeding gums: 1.78 ± 0.99 Frequency of bad breath: 1.88 ± 0.96 AI Health of teeth: 3.43 ± 0.89 Health of gums: 3.40 ± 0.78 Frequency of teeth sensitive: 1.93 ± 1.20 Frequency of bleeding gums: 1.60 ± 0.96 Frequency of bad breath: 1.95 ± 1.11 Controls (mean ± SD) Health of teeth: 2.73 ± 0.89 Health of gums: 2.93 ± 0.98 Frequency of teeth sensitive: 2.12 ± 1.14 Frequency of bleeding gums: 1.84 ± 0.94 Frequency of bad breath: 2.02 ± 0.97 | Controls had worse perception of teeth and gum health compared to BC women irrespective of the drug regimen, but tamoxifen and AI users reported higher frequency of sensitive teeth than chemotherapy users. | ||||
| Studies on prostate cancer | ||||||
| PI; BoP; PPD; AL; REC | Cases Prevalence of periodontitis: 80.95% Frequency of tooth mobility: 14.63% Frequency of BoP: 68.3% Frequency of PPD 3–4 mm: 100% Frequency of REC: 90.24% Frequency of AL: 100% Controls Prevalence of periodontitis: 3.70% Frequency of tooth mobility: 0% Frequency of BoP: 25.9% Frequency of PPD 3–4 mm: 3.70% Frequency of REC: 7.41% Frequency of AL: 7.41% | Men with prostate cancer undergoing ADT were more likely to have periodontal disease than men not on ADT despite having similar oral hygiene habits. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, F.; Franco, F.; Mognetti, B.; Berta, G.N. Impact of Endocrine Therapy for Cancer on Periodontal Health: A Systematic Review. Cancers 2025, 17, 3066. https://doi.org/10.3390/cancers17183066
Romano F, Franco F, Mognetti B, Berta GN. Impact of Endocrine Therapy for Cancer on Periodontal Health: A Systematic Review. Cancers. 2025; 17(18):3066. https://doi.org/10.3390/cancers17183066
Chicago/Turabian StyleRomano, Federica, Francesco Franco, Barbara Mognetti, and Giovanni Nicolao Berta. 2025. "Impact of Endocrine Therapy for Cancer on Periodontal Health: A Systematic Review" Cancers 17, no. 18: 3066. https://doi.org/10.3390/cancers17183066
APA StyleRomano, F., Franco, F., Mognetti, B., & Berta, G. N. (2025). Impact of Endocrine Therapy for Cancer on Periodontal Health: A Systematic Review. Cancers, 17(18), 3066. https://doi.org/10.3390/cancers17183066








