CRAFITY and AFP/PIVKA-II Kinetics Predict Prognosis in Hepatocellular Carcinoma on Immunotherapy
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Data Collection
2.3. Scoring System: CRAFITY and 100 RULE
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and Radiological Response
3.2. Distribution of CRAFITY Scores and CRAFITY-100 RULE
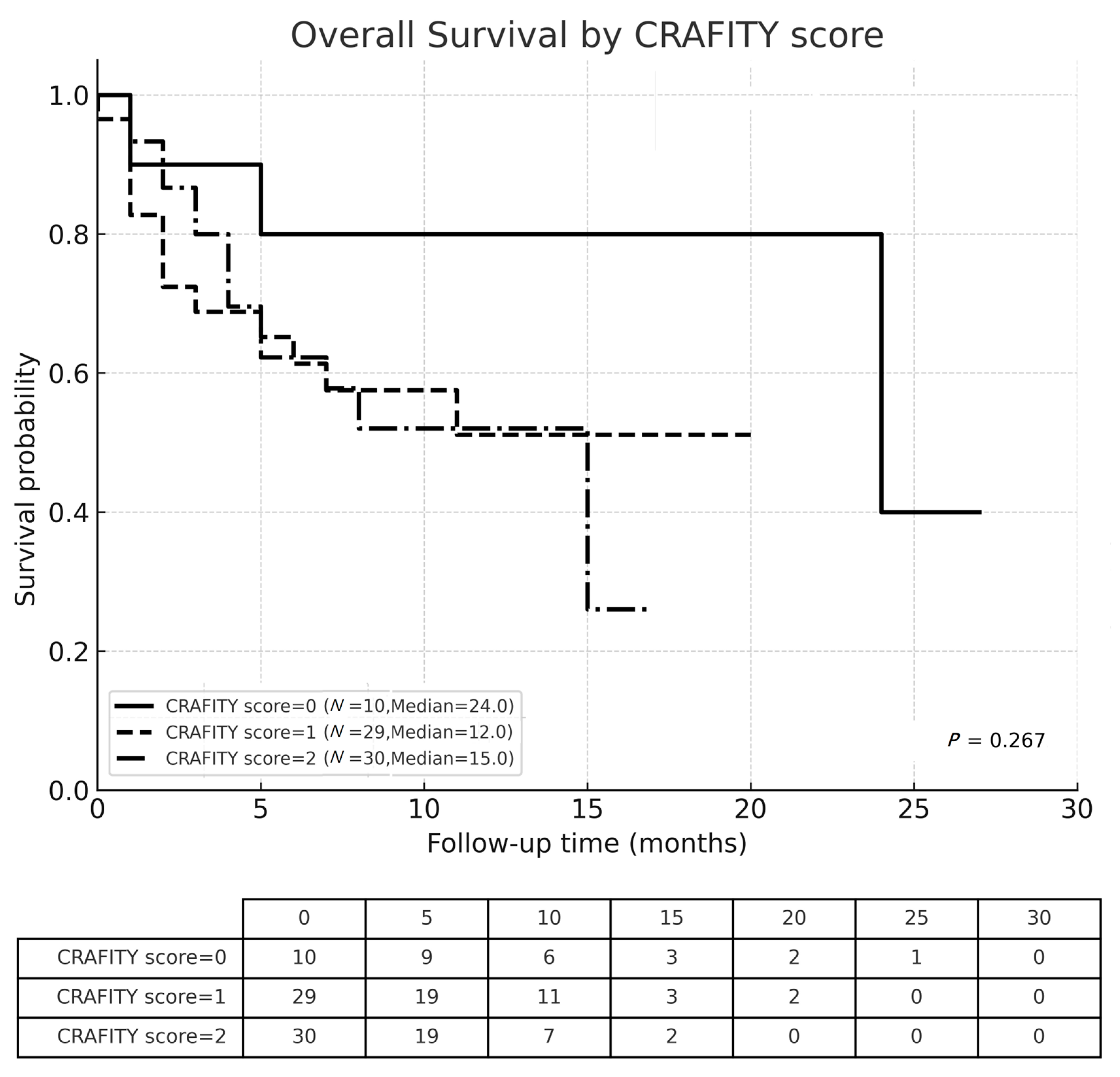
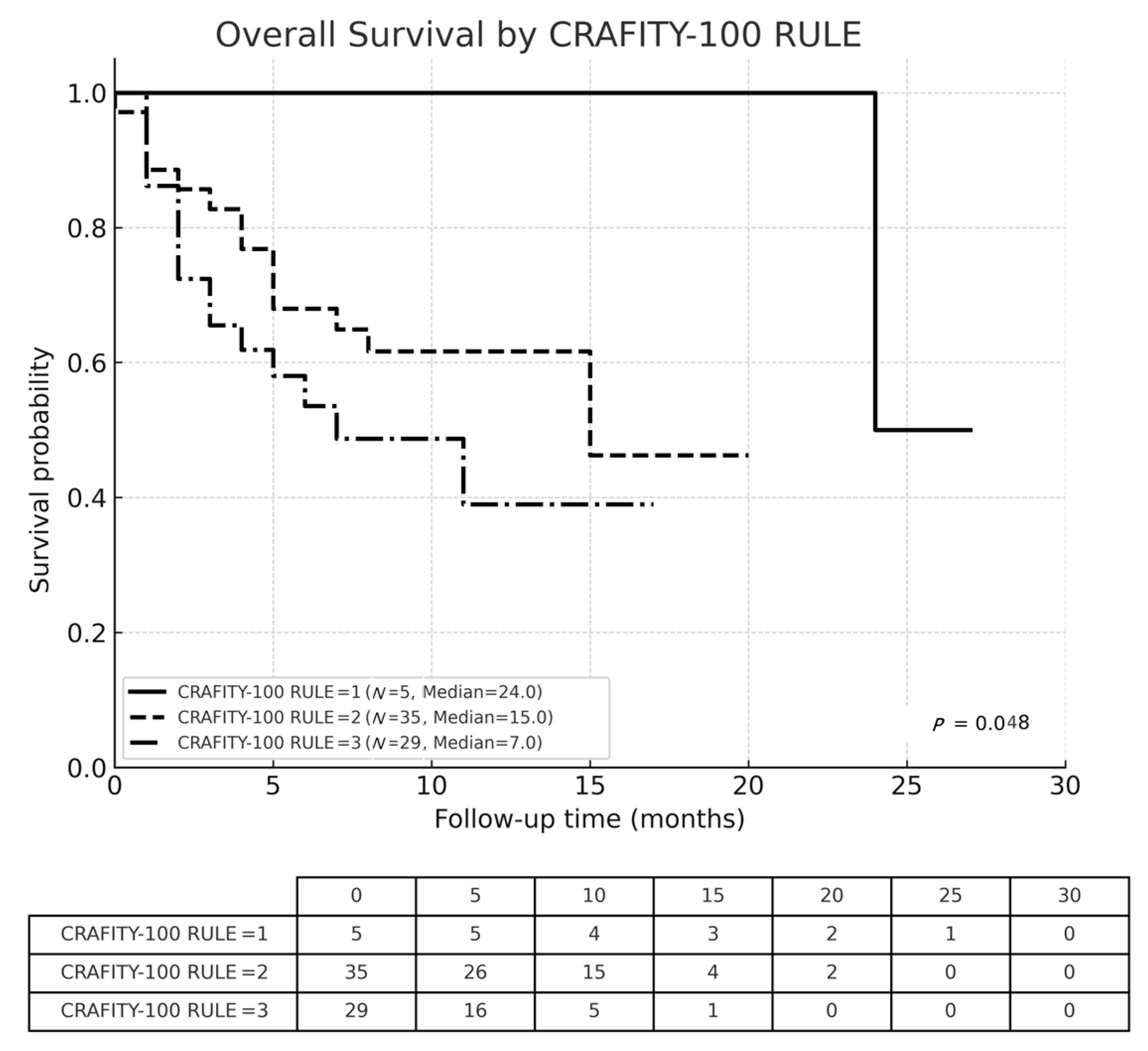
3.3. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Lachenmayer, A.; Finn, R.S. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 2015, 12, 408–424. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 randomized, open-label, multicenter study of tremelimumab and durvalumab as first-line therapy in patients with unresectable hepatocellular carcinoma: HIMALAYA. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, Y.; Chen, Y.; Huang, Y.; Lin, J.; Xiao, Z.; Cui, Z. Diagnostic and prognostic performance of serum GPC3 and PIVKA-II in AFP-negative hepatocellular carcinoma and establishment of nomogram prediction models. BMC Cancer 2025, 25, 16. [Google Scholar] [CrossRef]
- Kudo, M. Urgent global need for PIVKA-II and AFP-L3 measurements for surveillance and management of hepatocellular carcinoma. Liver Cancer 2024, 13, 113–118. [Google Scholar] [CrossRef]
- Liebman, H.A.; Furie, B.C.; Tong, M.J.; Blanchard, R.A.; Lo, K.J.; Lee, S.D.; Coleman, M.S.; Furie, B. Des-γ-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 1984, 310, 1427–1431. [Google Scholar] [CrossRef]
- Feng, H.; Li, B.; Li, Z.; Wei, Q.; Ren, L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer 2021, 21, 108. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Ciruolo, M.; Abate, M.L.; Carucci, P.; Rolle, E.; Rosso, C.; Olivero, A.; Troshina, G.; Risso, A.; Nicolosi, A.; et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist-II and glypican-3 for the detection and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers 2020, 12, 3218. [Google Scholar] [CrossRef]
- Sagar, V.M.; Herring, K.; Curbishley, S.; Hodson, J.; Fletcher, P.; Karkhanis, S.; Mehrzad, H.; Punia, P.; Shah, T.; Shetty, S.; et al. The potential of PIVKA-II as a treatment response biomarker in hepatocellular carcinoma: A prospective United Kingdom cohort study. Oncotarget 2016, 7, 2338–2350. [Google Scholar] [CrossRef]
- Norman, J.S.; Mehta, N. The role of AFP-L3 and DCP biomarkers in the diagnosis and management of hepatocellular carcinoma. Curr. Hepatol. Rep. 2025, 24, 16. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Liu, T.H.; Hsu, C.; Lu, L.C.; Shen, Y.C.; Lin, Z.Z.; Cheng, A.L.; Hsu, C.H. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019, 39, 2184–2189. [Google Scholar] [CrossRef]
- Hsu, W.F.; Wang, H.W.; Chen, C.K.; Lai, H.C.; Chuang, P.H.; Tsai, M.H.; Su, W.P.; Chen, H.Y.; Chu, C.S.; Chou, J.W.; et al. Alpha-fetoprotein response predicts treatment outcomes in patients with unresectable hepatocellular carcinoma receiving immune checkpoint inhibitors with or without tyrosine kinase inhibitors or locoregional therapies. Am. J. Cancer Res. 2021, 11, 6173–6187. [Google Scholar] [PubMed] [PubMed Central]
- Huang, C.; Zhu, X.D.; Shen, Y.H.; Xu, B.; Wu, D.; Ji, Y.; Chen, L.L.; Song, T.Q.; Zhang, W.; Zeng, Z.M.; et al. Radiographic and α-fetoprotein response predict pathologic complete response to immunotherapy plus a TKI in hepatocellular carcinoma: A multicenter study. BMC Cancer 2023, 23, 1098. [Google Scholar] [CrossRef]
- Sun, X.; Mei, J.; Lin, W.; Yang, Z.; Peng, W.; Chen, J.; Zhang, Y.; Xu, L.; Chen, M. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer 2021, 21, 775. [Google Scholar] [CrossRef]
- Lee, S.W.; Yang, S.S.; Lee, T.Y. A real-world experience on a Chinese population of patients with unresectable hepatocellular carcinoma treated with nivolumab. Gastroenterol. Res. 2024, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, B.; Pomej, K.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Waidmann, O.; Himmelsbach, V.; Schulze, K.; von Felden, J.; Fründt, T.W.; et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—Development and validation of the CRAFITY score. J. Hepatol. 2022, 76, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.A.; Agopian, V.G.; Marrero, J.A.; et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Tanabe, N.; Saeki, I.; Aibe, Y.; Matsuda, T.; Hanazono, T.; Nishi, M.; Hidaka, I.; Kuwashiro, S.; Shiratsuki, S.; Matsuura, K. Early prediction of response focused on tumor markers in atezolizumab plus bevacizumab therapy for hepatocellular carcinoma. Cancers 2023, 15, 2927. [Google Scholar] [CrossRef]
- Chan, S.L.; Mo, F.K.; Johnson, P.J.; Hui, E.P.; Ma, B.B.; Ho, W.M.; Lam, K.C.; Chan, A.T.; Mok, T.S.; Yeo, W. New utility of an old marker: Serial α-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J. Clin. Oncol. 2009, 27, 446–452. [Google Scholar] [CrossRef]
- Yang, M.; Pan, Y.; Wang, W. Prognostic significance of the CRAFITY score in hepatocellular carcinoma treated with immunotherapy: A systematic review and meta-analysis. BMC Cancer 2023, 23, 686. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Deng, N.; Ding, X.Y.; Chen, J.L.; Sun, W. CRAFITY score and nomogram predict the clinical efficacy of lenvatinib combined with immune checkpoint inhibitors in hepatocellular carcinoma. World J. Gastroenterol. 2025, 31, 101672. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, T.; Sun, B.; Zhang, K.; Zheng, Y.; Li, N.; Chen, L.; Zheng, C.; Liang, B.; Shi, H.; et al. Utility and predictive value of the CRAFITY score in advanced hepatocellular carcinoma treated with transarterial chemoembolization plus tyrosine kinase inhibitors and PD-1 inhibitor. BMC Cancer 2024, 24, 11936. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Yang, S.S.; Lien, H.C.; Peng, Y.C.; Tung, C.F.; Lee, T.Y. The combination of tyrosine kinase inhibitors and immune checkpoint inhibitors as first-line treatment for advanced-stage hepatocellular carcinoma. J. Clin. Med. 2022, 11, 4874. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, T.Y.; Yang, S.S.; Huang, Y.J.; Peng, Y.C. The impact of sequential therapies after first-line systemic therapies in unresectable hepatocellular carcinoma. J. Clin. Med. 2024, 13, 2612. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Toan, B.N.; Tan, C.K.; Hasan, I.; Setiawan, L.; Yu, M.L.; Izumi, N.; Huyen, N.N.; Chow, P.K.H.; Mohamed, R.; et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin. Mol. Hepatol. 2023, 29, 277–292. [Google Scholar] [CrossRef]
- Facciorusso, A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: Recent findings and new perspectives. Curr. Diabetes Rev. 2013, 9, 382–386. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Crucinio, N.; Muscatiello, N.; Carr, B.I.; Di Leo, A.; Barone, M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. J. Gastroenterol. Hepatol. 2015, 30, 1643–1650. [Google Scholar] [CrossRef]


| Variables Mean ± SD or N (%) | Total N = 69 (100%) | OR N = 20 (29.0%) | Non-OR N = 49 (71.0%) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 64.9 ± 11.6 | 61.2 ± 9.5 | 66.5 ± 12.1 | 0.083 | |
| >65 | 38 (55.1%) | 7 (35%) | 31 (63.3%) | 0.032 | |
| Gender | male | 55 (79.7%) | 16 (80.0%) | 39 (79.6%) | 0.624 |
| female | 14 (20.3%) | 4 (20.0%) | 10 (20.4%) | ||
| Viral hepatitis | HBV | 37 (53.6%) | 9 (45.0%) | 28 (57.1%) | 0.359 |
| HCV | 22 (31.9%) | 10 (50.0%) | 12 (24.5%) | 0.039 | |
| ALBI grade | 1 | 38 (55.1%) | 13 (65.0%) | 25 (51.0%) | 0.290 |
| 2/3 | 31 (44.9%) | 7 (35.0%) | 24 (49.0%) | ||
| BCLC stage | B | 10 (14.5%) | 5 (25.0%) | 5 (10.2%) | 0.113 |
| C | 59 (85.5%) | 15 (75.0%) | 44 (89.8%) | ||
| MVI | 43 (62.3%) | 11 (55.0%) | 32 (65.3%) | 0.423 | |
| EHS | 28 (40.6%) | 6 (30.0%) | 22 (44.9%) | 0.253 | |
| Concurrent LRT | 23 (33.3%) | 8 (40.0%) | 15 (30.6%) | 0.453 | |
| AFP (ng/mL) | 21,072.2 ± 48,053.5 | 22,042.7 ± 44,476.8 | 20,667.9 ± 49,914.8 | 0.915 | |
| PIVKA-II (mAU/mL) | 4429.2 ± 8864.9 | 3798.1 ± 9636.5 | 4692.1 ± 8616.8 | 0.708 | |
| CRP (mg/dL) | 3.2 ± 3.1 | 1.9 ± 1.8 | 3.7 ± 3.3 | 0.021 | |
| mRECIST | All (N = 69) | |
|---|---|---|
| N | % | |
| Complete response | 4 | (5.8%) |
| Partial response | 16 | (23.2%) |
| Stable disease | 21 | (30.4%) |
| Progressive disease | 28 | (40.6%) |
| Objective response (OR) | 20 | (29.0%) |
| Non-objective response (non-OR) | 49 | (71.0%) |
| Variables N (%) | Total N = 69 (100%) | OR N = 20 (29.0%) | Non-OR N = 49 (71.0%) | p-Value | |
|---|---|---|---|---|---|
| Baseline | |||||
| AFP ≥ 100 ng/ml | 41 (59.4%) | 14 (70.0%) | 27 (55.1%) | 0.253 | |
| PIVKA-II ≥ 100 mAU/mL | 51 (73.9%) | 13 (65.0%) | 38 (77.6%) | 0.281 | |
| CRP ≥ 1 mg/dL | 48 (69.6%) | 10 (50.0%) | 38 (77.6%) | 0.084 | |
| At 4 weeks after immunotherapy | |||||
| AFP decline < 10% | 40 (58.0%) | 8 (40.0%) | 32 (65.3%) | 0.049 | |
| PIVKA-II decline < 10% | 42 (60.9%) | 6 (30.0%) | 36 (73.5%) | 0.001 | |
| CRAFITY scores | 0 | 10 (14.5%) | 5 (25.0%) | 5 (10.2%) | 0.209 |
| 1 | 29 (42.0%) | 6 (30.0%) | 23 (46.9%) | ||
| 2 | 30 (43.5%) | 9 (45.0%) | 21 (42.9%) | ||
| CRAFITY-100 RULE | level I | 5 (7.3%) | 4 (20.0%) | 1 (2.0%) | 0.015 |
| level II | 35 (50.7%) | 11 (55.0%) | 24 (49.0%) | ||
| level III | 29 (42.0%) | 5 (25.0%) | 24 (49.0%) | ||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-Value | HR | (95% CI) | p-Value | |
| Age (≤65 vs. >65 y/o) | 0.77 | (0.36–1.64) | 0.502 | |||
| Gender (male vs. female) | 1.03 | (0.42–2.56) | 0.945 | |||
| HBV (HBsAg + vs. −) | 0.84 | (0.40–1.77) | 0.643 | |||
| HCV (anti-HCV + vs. −) | 1.35 | (0.60–3.08) | 0.462 | |||
| ALBI grade (1 vs. 2/3) | 0.38 | (0.18–0.83) | 0.015 | 0.54 | (0.24–1.21) | 0.138 |
| BCLC stage (B vs. C) | 0.29 | (0.07–1.22) | 0.091 | |||
| MVI (no vs. yes) | 0.31 | (0.12–0.78) | 0.013 | 0.56 | (0.21−1.52) | 0.253 |
| EHS (no vs. yes) | 0.90 | (0.43–1.93) | 0.798 | |||
| Combined LRT (yes vs. no) | 0.44 | (0.18–1.10) | 0.079 | |||
| Baseline | ||||||
| AFP (≥100 vs. <100 ng/mL) | 1.01 | (0.47–2.14) | 0.993 | |||
| PIVKAII (≥ 100 vs. <100 mAU/mL) | 4.42 | (1.33–14.69) | 0.015 | 3.22 | (0.93−11.11) | 0.064 |
| CRP (≥ 1 vs. <1 mg/dL) | 2.85 | (1.06–7.65) | 0.038 | 2.14 | (0.78−5.86) | 0.137 |
| At 4 weeks after immunotherapy | ||||||
| AFP decline < 10% (yes vs. no) | 1.87 | (0.86–4.03) | 0.112 | |||
| PIVKA-II decline < 10% (yes vs. no) | 1.21 | (0.55–2.62) | 0.638 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Huang, Y.-J.; Lin, Y.-C.; Tsai, H.-J.; Chen, C.-C.; Chang, C.-H.; Lee, T.-Y.; Peng, Y.-C. CRAFITY and AFP/PIVKA-II Kinetics Predict Prognosis in Hepatocellular Carcinoma on Immunotherapy. Cancers 2025, 17, 3058. https://doi.org/10.3390/cancers17183058
Lee S-W, Huang Y-J, Lin Y-C, Tsai H-J, Chen C-C, Chang C-H, Lee T-Y, Peng Y-C. CRAFITY and AFP/PIVKA-II Kinetics Predict Prognosis in Hepatocellular Carcinoma on Immunotherapy. Cancers. 2025; 17(18):3058. https://doi.org/10.3390/cancers17183058
Chicago/Turabian StyleLee, Shou-Wu, Yi-Jie Huang, Ying-Cheng Lin, Hsin-Ju Tsai, Chia-Chang Chen, Chung-Hsin Chang, Teng-Yu Lee, and Yen-Chun Peng. 2025. "CRAFITY and AFP/PIVKA-II Kinetics Predict Prognosis in Hepatocellular Carcinoma on Immunotherapy" Cancers 17, no. 18: 3058. https://doi.org/10.3390/cancers17183058
APA StyleLee, S.-W., Huang, Y.-J., Lin, Y.-C., Tsai, H.-J., Chen, C.-C., Chang, C.-H., Lee, T.-Y., & Peng, Y.-C. (2025). CRAFITY and AFP/PIVKA-II Kinetics Predict Prognosis in Hepatocellular Carcinoma on Immunotherapy. Cancers, 17(18), 3058. https://doi.org/10.3390/cancers17183058







