Decision-Making Biomarkers Guiding Therapeutic Strategies in Hepatocellular Carcinoma: From Prediction to Personalized Care
Simple Summary
Abstract
1. Introduction
1.1. The Growing Need for Individualized Treatment Strategies
1.2. Role of Biomarkers in Treatment Decision-Making
2. Biomarkers in Systemic Therapy Decision-Making
2.1. Landscape of Systemic Therapies for Advanced HCC Recommendations as First-Line Therapy in Advanced HCC
2.2. Potential Biomarkers in First-Line Treatment of Advanced HCC
2.3. Predictive Biomarkers of Immunotherapy Response
2.4. PD-L1 Expression, Tumor Mutational Burden, Microsatellite Instability, and Gene Signatures
2.5. Immune Cell Infiltration and Inflammatory Biomarkers
2.6. Biomarkers for Immune-Related Adverse Events (irAEs)
Risk Prediction and Early Detection
2.7. Managing irAEs Through Biomarker-Guided Approaches
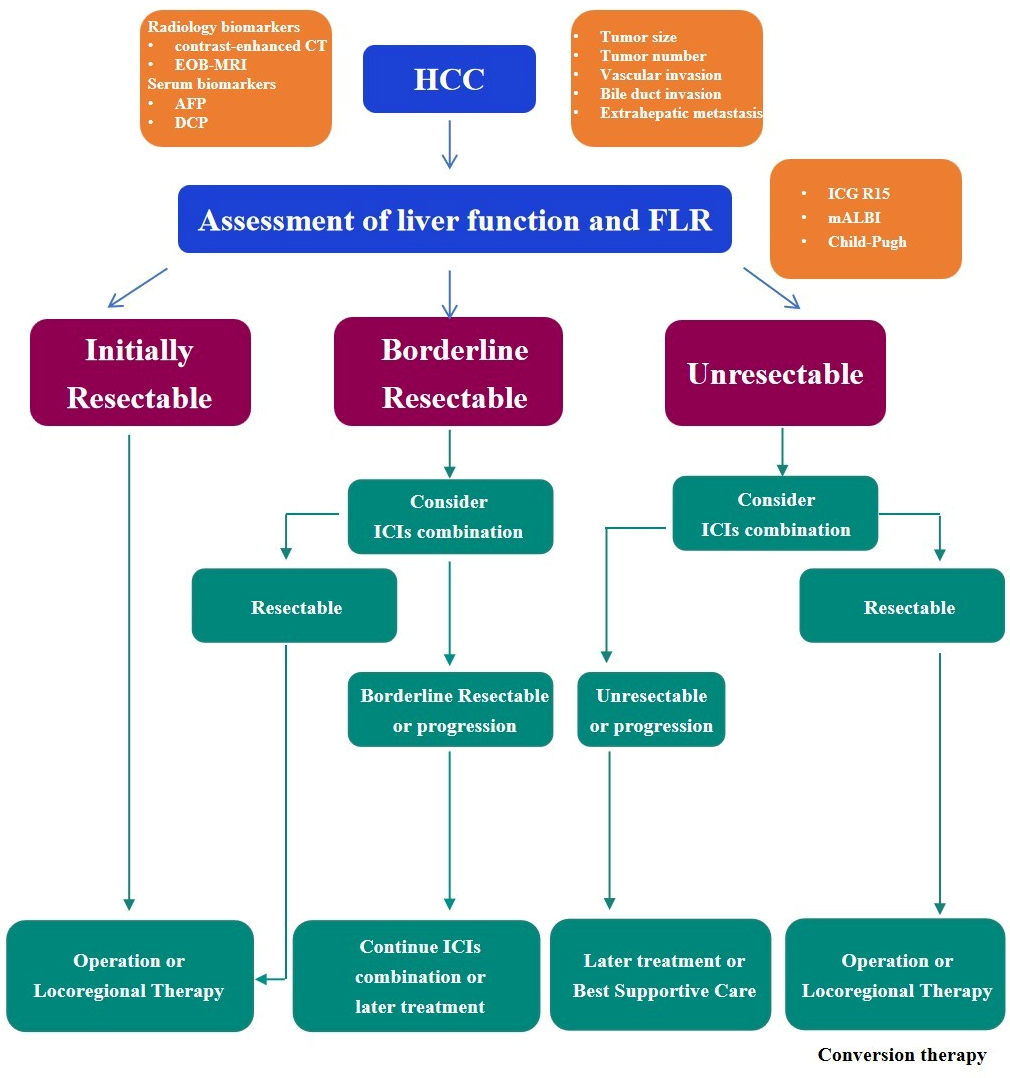
3. Surgical Decision-Making and Biomarker Guidance
3.1. Conversion Therapy: Expanding Resectability
Definition of Conversion Therapy and Its Clinical Relevance
3.2. Outcomes in Patients Undergoing Surgery After Systemic Therapy
3.3. The Concept of Borderline Resectable HCC
3.3.1. Imaging Criteria and Multidisciplinary Evaluation
3.3.2. Lack of Consensus and the Need for Biomarker-Based Stratification
3.4. Prognostic Biomarkers for Surgery Candidates
Histopathological Features and Molecular Profiles
3.5. Predicting Recurrence and Long-Term Survival
4. Biomarkers Across the Disease Continuum
4.1. Predicting Disease Progression and Relapse
ctDNA, AFP Dynamics, and Methylation Markers
4.2. Early Detection of Recurrence Through Liquid Biopsy
4.3. Integration of Imaging Biomarkers
Radiomics and Functional Imaging
4.4. Linking Imaging Phenotypes to Molecular Profiles
4.5. Blood-Based Biochemical Markers
ALBI Grade, DCP, NLR, and Composite Scoring Systems
5. Conclusions
5.1. Summary of Current Evidence
5.2. Challenges and Future Opportunities
5.3. Toward a Biomarker-Driven Clinical Decision-Making Model
- Multimodal data integration: This combines genomic variants, immune microenvironment features, and radiomics to construct adaptive risk stratification models.
- Closed-loop decision mechanism: This establishes a “treatment-response assessment-therapy adjustment” feedback cycle.
- Intelligent analytics engine: This leverages AI algorithms to analyze multi-omics data streams, generating personalized therapeutic roadmaps that transcend population-based guidelines. This model transforms static biomarkers into dynamic decision variables, achieving an organic integration of predictive monitoring and pre-adaptive therapy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Lim, R.Y.; Koh, B.; Ng, C.H.; Kulkarni, A.V.; Liu, K.; Wijarnpreecha, K.; Kim, B.K.; Muthiah, M.D.; Lee, S.W.; Zheng, M.H.; et al. Hepatocellular Carcinoma Surveillance and Survival in a Contemporary Asia-Pacific Cohort. JAMA Netw. Open 2025, 8, e2520294. [Google Scholar] [CrossRef]
- Le, M.H.; Liu, J.K.; Lee, K.; Cheung, R.; Nguyen, M.H. Late Diagnosis of Chronic Hepatitis B in the United States: A Population-Based, Retrospective Cohort Study From 2007 to 2021. Aliment. Pharmacol. Ther. 2025, 62, 340–348. [Google Scholar] [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Wong, G.; Anstee, Q.M.; Henry, L. The Global Burden of Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 1978–1991. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.S.; Lee, M.; Lee, H.A.; Park, E.S.; Choi, J.Y.; Baek, H.S.; Kim, T.H.; Lin, H.; Yip, T.C.-F.; Lee, H.W.; et al. A novel risk prediction model for hepatocellular carcinoma in MASLD: A multinational, multicenter cohort study. Clin. Gastroenterol. Hepatol. 2025, S1542-3565(25)00569-00565. [Google Scholar] [CrossRef]
- Cusi, K.; Abdelmalek, M.F.; Apovian, C.M.; Balapattabi, K.; Bannuru, R.R.; Barb, D.; Bardsley, J.K.; Beverly, E.A.; Corbin, K.D.; ElSayed, N.A.; et al. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in People with Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association. Diabetes Care 2025, 48, 1057–1082. [Google Scholar]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. [Google Scholar] [PubMed]
- Fu, Y.; Maccioni, L.; Wang, X.W.; Greten, T.F.; Gao, B. Alcohol-associated liver cancer. Hepatology 2024, 80, 1462–1479. [Google Scholar] [CrossRef]
- Shah, R.; Ahovegbe, L.; Niebel, M.; Shepherd, J.; Thomson, E.C. Non-epidemic HCV genotypes in low- and middle-income countries and the risk of resistance to current direct-acting antiviral regimens. J. Hepatol. 2021, 75, 462–473. [Google Scholar]
- Zhang, C.H.; Cheng, Y.; Zhang, S.; Fan, J.; Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2029–2041. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, R.; Biswas, S.; Bu, Q.; Xu, Z.; Qiao, L.; Zhou, Y.; Tang, J.; Zhou, J.; Zhou, H.; et al. Integrated single-cell transcriptomics reveals the hypoxia-induced inflammation-cancer transformation in NASH-derived hepatocellular carcinoma. Cell Prolif. 2024, 57, e13576. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A. The influence of diabetes in the pathogenesis and the clinical course of hepatocellular carcinoma: Recent findings and new perspectives. Curr. Diabetes Rev. 2013, 9, 382–386. [Google Scholar] [CrossRef]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Lin, H.Y.; Jeon, A.J.; Chen, K.; Lee, C.; Wu, L.; Chong, S.L.; Anene-Nzelu, C.G.; Foo, R.S.; Chow, P.K. The epigenetic basis of hepatocellular carcinoma—Mechanisms and potential directions for biomarkers and therapeutics. Br. J. Cancer 2025, 132, 869–887. [Google Scholar] [CrossRef]
- Wu, G.; Bajestani, N.; Pracha, N.; Chen, C.; Makary, M.S. Hepatocellular Carcinoma Surveillance Strategies: Major Guidelines and Screening Advances. Cancers 2024, 16, 3933. [Google Scholar] [CrossRef]
- Vutien, P.; Kim, N.J.; Nguyen, M.H. The Diagnosis and Staging of Hepatocellular Carcinoma: A Review of Current Practices. Clin. Liver Dis. 2025, 29, 33–48. [Google Scholar] [CrossRef]
- Hatzidakis, A.; Müller, L.; Krokidis, M.; Kloeckner, R. Local and Regional Therapies for Hepatocellular Carcinoma and Future Combinations. Cancers 2022, 14, 2469. [Google Scholar] [CrossRef]
- Ma, Z.; Xiao, Z.; Yin, P.; Wen, K.; Wang, W.; Yan, Y.; Lin, Z.; Li, Z.; Wang, H.; Zhang, J.; et al. Comparison of survival benefit and safety between surgery following conversion therapy versus surgery alone in patients with surgically resectable hepatocellular carcinoma at CNLC IIb/IIIa stage: A propensity score matching study. Int. J. Surg. 2024, 110, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.Y.; Jiang, J.Q.; Sun, J.H.; Huang, J.T.; Wang, W.D.; Wang, Q.; Ding, W.B.; Zhu, X.L.; Ni, C.F. Prognostic Performance of the China Liver Cancer Staging System in Hepatocellular Carcinoma Following Transarterial Chemoembolization. J. Clin. Transl. Hepatol. 2023, 11, 1321–1328. [Google Scholar] [CrossRef]
- Ito, K.; Takemura, N.; Inagaki, F.; Mihara, F.; Kokudo, N. Difference in treatment algorithms for hepatocellular carcinoma between world’s principal guidelines. Glob. Health Med. 2020, 2, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef]
- Donadon, M.; Solbiati, L.; Dawson, L.; Barry, A.; Sapisochin, G.; Greig, P.D.; Shiina, S.; Fontana, A.; Torzilli, G. Hepatocellular Carcinoma: The Role of Interventional Oncology. Liver Cancer 2016, 6, 34–43. [Google Scholar] [CrossRef]
- Gupta, P.; Maralakunte, M.; Kumar-M, P.; Chandel, K.; Chaluvashetty, S.B.; Bhujade, H.; Kalra, N.; Sandhu, M.S. Overall survival and local recurrence following RFA, MWA, and cryoablation of very early and early HCC: A systematic review and Bayesian network meta-analysis. Eur. Radiol. 2021, 31, 5400–5408. [Google Scholar] [CrossRef]
- Adwan, H.; Vogl, T.J.; Balaban, Ü.; Nour-Eldin, N.A. Percutaneous Thermal Ablation Therapy of Hepatocellular Carcinoma (HCC): Microwave Ablation (MWA) versus Laser-Induced Thermotherapy (LITT). Diagnostics 2022, 12, 564. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Zhang, B.; Yue, J.; Shi, X.; Cui, K.; Liu, Z.; Chang, Z.; Sun, Z.; Li, M.; et al. Neoadjuvant tislelizumab plus stereotactic body radiotherapy and adjuvant tislelizumab in early-stage resectable hepatocellular carcinoma: The Notable-HCC phase 1b trial. Nat. Commun. 2024, 15, 3260. [Google Scholar] [CrossRef]
- Podesta, C.; Kayani, M.; Goody, R.; Samson, A. Combination treatment of HCC with SBRT and immune checkpoint inhibition. Crit. Rev. Oncol./Hematol. 2023, 192, 104191. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Dhondt, E.; Lambert, B.; Hermie, L.; Huyck, L.; Vanlangenhove, P.; Geerts, A.; Verhelst, X.; Aerts, M.; Vanlander, A.; Berrevoet, F.; et al. (90)Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology 2022, 303, 699–710. [Google Scholar]
- Zhu, P.; Liao, W.; Zhang, W.G.; Chen, L.; Shu, C.; Zhang, Z.W.; Huang, Z.Y.; Chen, Y.F.; Lau, W.Y.; Zhang, B.X.; et al. A Prospective Study Using Propensity Score Matching to Compare Long-term Survival Outcomes After Robotic-assisted, Laparoscopic, or Open Liver Resection for Patients with BCLC Stage 0-A Hepatocellular Carcinoma. Ann. Surg. 2023, 277, e103–e111. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar]
- Parikh, N.D.; Waljee, A.K.; Singal, A.G. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015, 21, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Yarchoan, M.; Yopp, A.; Sapisochin, G.; Pinato, D.J.; Pillai, A. Neoadjuvant and adjuvant systemic therapy in HCC: Current status and the future. Hepatol. Commun. 2024, 8, e0430. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Yarchoan, M.; Singal, A.G.; Marron, T.U.; Schwartz, M.; Pikarsky, E.; Kudo, M.; Finn, R.S. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2024, 21, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar]
- Sangro, B.; Galle, P.R.; Kelley, R.K.; Charoentum, C.; De Toni, E.N.; Ostapenko, Y.; Heo, J.; Cheng, A.L.; Wilson Woods, A.; Gupta, C.; et al. Patient-Reported Outcomes From the Phase III HIMALAYA Study of Tremelimumab Plus Durvalumab in Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2024, 42, 2790–2799. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beal, E.; Finn, R.S.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Hoang, H.T.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 1830–1850. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Ladd, A.D.; Duarte, S.; Sahin, I.; Zarrinpar, A. Mechanisms of drug resistance in HCC. Hepatology 2024, 79, 926–940. [Google Scholar] [PubMed]
- Yau, T.; Galle, P.R.; Decaens, T.; Sangro, B.; Qin, S.; da Fonseca, L.G.; Karachiwala, H.; Blanc, J.F.; Park, J.W.; Gane, E.; et al. Nivolumab plus ipilimumab versus lenvatinib or sorafenib as first-line treatment for unresectable hepatocellular carcinoma (CheckMate 9DW): An open-label, randomised, phase 3 trial. Lancet 2025, 405, 1851–1864. [Google Scholar]
- Yang, X.; Yang, C.; Zhang, S.; Geng, H.; Zhu, A.X.; Bernards, R.; Qin, W.; Fan, J.; Wang, C.; Gao, Q. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell 2024, 42, 180–197. [Google Scholar] [CrossRef]
- Fernandes, E.; Rodrigues, P.D.; Álvares-da-Silva, M.R.; Scaffaro, L.A.; Farenzena, M.; Teixeira, U.F.; Waechter, F.L. Treatment strategies for locally advanced hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2019, 4, 12. [Google Scholar] [CrossRef]
- Hoshida, Y.; Toffanin, S.; Lachenmayer, A.; Villanueva, A.; Minguez, B.; Llovet, J.M. Molecular classification and novel targets in hepatocellular carcinoma: Recent advancements. Semin. Liver Dis. 2010, 30, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Sun, X.; Hoshida, Y. Molecular classification of hepatocellular carcinoma: Potential therapeutic implications. Hepatic Oncol. 2015, 2, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, Y.; Nijman, S.M.; Kobayashi, M.; Chan, J.A.; Brunet, J.P.; Chiang, D.Y.; Villanueva, A.; Newell, P.; Ikeda, K.; Hashimoto, M.; et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009, 69, 7385–7392. [Google Scholar] [CrossRef]
- Suzuki, H.; Mishra, S.; Paul, S.; Hoshida, Y. Molecular and immune landscape of hepatocellular carcinoma for therapeutic development. J. Liver Cancer 2025, 25, 9–18. [Google Scholar] [CrossRef]
- Xing, X.; Cai, L.; Ouyang, J.; Wang, F.; Li, Z.; Liu, M.; Wang, Y.; Zhou, Y.; Hu, E.; Huang, C.; et al. Proteomics-driven noninvasive screening of circulating serum protein panels for the early diagnosis of hepatocellular carcinoma. Nat. Commun. 2023, 14, 8392. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, D.W.; Cho, E.J.; Lee, J.Y.; Kim, J.; Kwon, C.; Kim-Ha, J.; Hong, S.K.; Choi, Y.; Yi, N.J.; et al. A circulating cell-free DNA methylation signature for the detection of hepatocellular carcinoma. Mol. Cancer 2023, 22, 164. [Google Scholar] [CrossRef]
- Gupta, S.; Bent, S.; Kohlwes, J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann. Intern. Med. 2003, 139, 46–50. [Google Scholar] [PubMed]
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009, 137, 110–118. [Google Scholar]
- Zhu, R.; Yang, J.; Xu, L.; Dai, W.; Wang, F.; Shen, M.; Zhang, Y.; Zhang, H.; Chen, K.; Cheng, P.; et al. Diagnostic Performance of Des-γ-carboxy Prothrombin for Hepatocellular Carcinoma: A Meta-Analysis. Gastroenterol. Res. Pract. 2014, 2014, 529314. [Google Scholar] [CrossRef]
- Fang, Y.S.; Wu, Q.; Zhao, H.C.; Zhou, Y.; Ye, L.; Liu, S.S.; Li, X.X.; Du, W.D. Do combined assays of serum AFP, AFP-L3, DCP, GP73, and DKK-1 efficiently improve the clinical values of biomarkers in decision-making for hepatocellular carcinoma? A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1065–1076. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Kudo, M. ALBI Score as a Novel Tool in Staging and Treatment Planning for Hepatocellular Carcinoma: Advantage of ALBI Grade for Universal Assessment of Hepatic Function. Liver Cancer 2017, 6, 377–379. [Google Scholar] [CrossRef]
- Ho, S.Y.; Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Lei, H.J.; Lee, R.C.; Hou, M.C.; Huo, T.I. A New Prognostic Model Based on Albumin-Bilirubin Grade for Hepatocellular Carcinoma Beyond the Milan Criteria. Dig. Dis. Sci. 2020, 65, 658–667. [Google Scholar]
- Liang, B.Y.; Zhang, E.L.; Li, J.; Long, X.; Wang, W.Q.; Zhang, B.X.; Zhang, Z.W.; Chen, Y.F.; Zhang, W.G.; Mei, B.; et al. A combined pre- and intra-operative nomogram in evaluation of degrees of liver cirrhosis predicts post-hepatectomy liver failure: A multicenter prospective study. Hepatobiliary Surg. Nutr. 2024, 13, 198–213. [Google Scholar]
- Fernández-Palanca, P.; Payo-Serafín, T.; Méndez-Blanco, C.; San-Miguel, B.; Tuñón, M.J.; González-Gallego, J.; Mauriz, J.L. Neuropilins as potential biomarkers in hepatocellular carcinoma: A systematic review of basic and clinical implications. Clin. Mol. Hepatol. 2023, 29, 293–319. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Bicer, F.; Kure, C.; Ozluk, A.A.; El-Rayes, B.F.; Akce, M. Advances in Immunotherapy for Hepatocellular Carcinoma (HCC). Curr. Oncol. 2023, 30, 9789–9812. [Google Scholar] [CrossRef]
- Donne, R.; Lujambio, A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology 2023, 77, 1773–1796. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Lei, H.J.; Wang, L.C.; Yeh, Y.C.; Chau, G.Y.; Hsia, C.Y.; Chou, S.C.; Luo, J.C.; Hou, M.C.; Huang, Y.H. M2BPGi Correlated with Immunological Biomarkers and Further Stratified Recurrence Risk in Patients with Hepatocellular Carcinoma. Liver Cancer 2025, 14, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Huang, P.; Wu, Q.; Yang, Y.; Zhou, K.; Zhang, M.; Li, Q. Promising first-line immuno-combination therapies for unresectable hepatocellular carcinoma: A cost-effectiveness analysis. Cancer Med. 2024, 13, e70094. [Google Scholar] [CrossRef]
- Rimassa, L.; Finn, R.S.; Sangro, B. Combination immunotherapy for hepatocellular carcinoma. J. Hepatol. 2023, 79, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, S.; Kawaoka, T.; Murakami, S.; Miura, R.; Shirane, Y.; Johira, Y.; Kosaka, M.; Fujii, Y.; Fujino, H.; Ono, A.; et al. Significance of changes in tumor markers in patients treated with durvalumab plus tremelimumab combination therapy as a surrogate marker for tumor response to unresectable hepatocellular carcinoma. Hepatol. Res. 2024. [Google Scholar] [CrossRef]
- Kuwano, A.; Tanaka, K.; Takahira, J.; Suzuki, H.; Ohishi, Y.; Motomura, K. WNT/β-catenin Signaling and CD8+ Tumor-infiltrating Lymphocytes in Tremelimumab Plus Durvalumab for Advanced Hepatocellular Carcinoma. Vivo 2024, 38, 2774–2781. [Google Scholar]
- Zhu, A.X.; Abbas, A.R.; de Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.H.; He, A.R.; Ryoo, B.Y.; et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022, 28, 1599–1611. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Morita, M.; Nishida, N.; Sakai, K.; Aoki, T.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; et al. Immunological Microenvironment Predicts the Survival of the Patients with Hepatocellular Carcinoma Treated with Anti-PD-1 Antibody. Liver Cancer 2021, 10, 380–393. [Google Scholar] [CrossRef]
- Kuwano, A.; Yada, M.; Narutomi, F.; Nagasawa, S.; Tanaka, K.; Kurosaka, K.; Ohishi, Y.; Masumoto, A.; Motomura, K. Therapeutic efficacy of atezolizumab plus bevacizumab for hepatocellular carcinoma with WNT/β-catenin signal activation. Oncol. Lett. 2022, 24, 216. [Google Scholar] [CrossRef]
- Hatanaka, T.; Hiraoka, A.; Tada, T.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Association of early bevacizumab interruption with efficacy of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma: A landmark analysis. Hepatol. Res. 2022, 52, 462–470. [Google Scholar] [CrossRef]
- Limousin, W.; Laurent-Puig, P.; Ziol, M.; Ganne-Carrié, N.; Nahon, P.; Ait-Omar, A.; Seror, O.; Sidali, S.; Campani, C.; Blanc, P.; et al. Molecular-based targeted therapies in patients with hepatocellular carcinoma and hepato-cholangiocarcinoma refractory to atezolizumab/bevacizumab. J. Hepatol. 2023, 79, 1450–1458. [Google Scholar]
- Yang, W.; Nguyen, R.; Safri, F.; Shiddiky, M.; Warkiani, M.E.; George, J.; Qiao, L. Liquid Biopsy in Hepatocellular Carcinoma: ctDNA as a Potential Biomarker for Diagnosis and Prognosis. Curr. Oncol. Rep. 2025, 27, 791–802. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The biology, function, and applications of exosomes in cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef]
- Ye, Q.; Ling, S.; Zheng, S.; Xu, X. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. Mol. Cancer 2019, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Abou-Alfa, G.K.; Zheng, B.; Liu, J.F.; Bai, J.; Du, L.T.; Qian, Y.S.; Fan, R.; Liu, X.L.; Wu, L.; et al. Genome-scale profiling of circulating cell-free DNA signatures for early detection of hepatocellular carcinoma in cirrhotic patients. Cell Res. 2021, 31, 589–592. [Google Scholar] [CrossRef]
- Chan, Y.T.; Zhang, C.; Wu, J.; Lu, P.; Xu, L.; Yuan, H.; Feng, Y.; Chen, Z.S.; Wang, N. Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol. Cancer 2024, 23, 189. [Google Scholar] [CrossRef]
- Ugonabo, O.; Udoh, U.S.; Rajan, P.K.; Reeves, H.; Arcand, C.; Nakafuku, Y.; Joshi, T.; Finley, R.; Pierre, S.V.; Sanabria, J.R. The Current Status of the Liver Liquid Biopsy in MASH Related HCC: Overview and Future Directions. Biomolecules 2023, 13, 1369. [Google Scholar] [CrossRef] [PubMed]
- Franzè, M.S.; Saffioti, F.; Mavroeidis, V.K. Interactions between tumor microenvironment and resistance to transarterial and systemic treatments for HCC. Cancer Drug Resist. 2025, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yang, B.; Liao, Z. Biomarkers in Immunotherapy-Based Precision Treatments of Digestive System Tumors. Front. Oncol. 2021, 11, 650481. [Google Scholar] [CrossRef]
- Lin, Z.F.; Qin, L.X.; Chen, J.H. Biomarkers for response to immunotherapy in hepatobiliary malignancies. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 413–419. [Google Scholar] [CrossRef]
- Pavelescu, L.A.; Enache, R.M.; Roşu, O.A.; Profir, M.; Creţoiu, S.M.; Gaspar, B.S. Predictive Biomarkers and Resistance Mechanisms of Checkpoint Inhibitors in Malignant Solid Tumors. Int. J. Mol. Sci. 2024, 25, 9659. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [PubMed]
- Ang, C.; Klempner, S.J.; Ali, S.M.; Madison, R.; Ross, J.S.; Severson, E.A.; Fabrizio, D.; Goodman, A.; Kurzrock, R.; Suh, J.; et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget 2019, 10, 4018–4025. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Hause, R.J.; Pritchard, C.C.; Shendure, J.; Salipante, S.J. Classification and characterization of microsatellite instability across 18 cancer types. Nat. Med. 2016, 22, 1342–1350. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, P.; Zhang, Z.; Wang, Z.; Zhou, K.; Song, M.; Ji, Y.; Zang, F.; Lou, L.; Rao, K.; et al. Integrated multi-omics profiling to dissect the spatiotemporal evolution of metastatic hepatocellular carcinoma. Cancer Cell 2024, 42, 135–156.e17. [Google Scholar] [CrossRef]
- Xing, X.; Hu, E.; Ouyang, J.; Zhong, X.; Wang, F.; Liu, K.; Cai, L.; Zhou, Y.; Wang, Y.; Chen, G.; et al. Integrated omics landscape of hepatocellular carcinoma suggests proteomic subtypes for precision therapy. Cell Rep. Med. 2023, 4, 101315. [Google Scholar] [CrossRef]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, Y.; Wang, X.; Dong, Z.; Chen, Y.; Zhang, R.; Huang, J.; Jin, X.; Yao, J.; Ge, A.; et al. Response Stratification in the First-Line Combined Immunotherapy of Hepatocellular Carcinoma at Genomic, Transcriptional and Immune Repertoire Levels. J. Hepatocell. Carcinoma 2021, 8, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, J.; Tu, X.; Li, B.; Tong, Z.; Wang, T.; Zheng, Y.; Shi, H.; Zeng, X.; Chen, W.; et al. Single-cell immune signature for detecting early-stage HCC and early assessing anti-PD-1 immunotherapy efficacy. J. Immunother. Cancer 2022, 10, e003133. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, B.; Pomej, K.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Waidmann, O.; Himmelsbach, V.; Schulze, K.; von Felden, J.; Fründt, T.W.; et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—Development and validation of the CRAFITY score. J. Hepatol. 2022, 76, 353–363. [Google Scholar]
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.L.; Yau, T.; Furuse, J.; Park, J.W.; Boyd, Z.; et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2020, 73, 1460–1469. [Google Scholar] [CrossRef]
- Kim, C.G.; Kim, C.; Yoon, S.E.; Kim, K.H.; Choi, S.J.; Kang, B.; Kim, H.R.; Park, S.H.; Shin, E.C.; Kim, Y.Y.; et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J. Hepatol. 2021, 74, 350–359. [Google Scholar] [CrossRef]
- He, M.; Liu, Y.; Chen, S.; Deng, H.; Feng, C.; Qiao, S.; Chen, Q.; Hu, Y.; Chen, H.; Wang, X.; et al. Serum amyloid A promotes glycolysis of neutrophils during PD-1 blockade resistance in hepatocellular carcinoma. Nat. Commun. 2024, 15, 1754. [Google Scholar] [CrossRef]
- Fukushima, T.; Morimoto, M.; Kobayashi, S.; Ueno, M.; Uojima, H.; Hidaka, H.; Kusano, C.; Chuma, M.; Numata, K.; Tsuruya, K.; et al. Association Between Immune-Related Adverse Events and Survival in Patients with Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab. Oncologist 2023, 28, e526–e533. [Google Scholar] [CrossRef]
- Nam, H.; Lee, J.; Han, J.W.; Lee, S.K.; Yang, H.; Lee, H.L.; Sung, P.S.; Kim, H.Y.; Kim, S.H.; Song, M.J.; et al. Analysis of Immune-Related Adverse Events of Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma: A Multicentre Cohort Study. Liver Cancer 2024, 13, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, K.; Wei, W.; Ling, Y.; Li, P.; Li, S.; Wang, Y.; Xie, D.; Guo, R.; Cai, M. Immune-related adverse events predict responses to PD-1 blockade immunotherapy in hepatocellular carcinoma. Int. J. Cancer 2021, 149, 959–966. [Google Scholar]
- De Martin, E.; Michot, J.M.; Rosmorduc, O.; Guettier, C.; Samuel, D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep. 2020, 2, 100170. [Google Scholar] [CrossRef]
- Dharmapuri, S.; Özbek, U.; Jethra, H.; Jun, T.; Marron, T.U.; Saeed, A.; Huang, Y.H.; Muzaffar, M.; Pinter, M.; Balcar, L.; et al. Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio appear predictive of immune treatment related toxicity in hepatocellular carcinoma. World J. Gastrointest. Oncol. 2023, 15, 1900–1912. [Google Scholar]
- Hwang, S.Y.; Rezaee-Zavareh, M.S.; Attia, A.M.; Kaymen, E.A.; Tran, N.; Abou-Alfa, G.K.; Parikh, N.D.; Singal, A.G.; Yang, J.D. Immune-Related Adverse Events Are Associated with Improved Outcomes After Immune Checkpoint Inhibitor Treatment in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2025. ahead of print. [Google Scholar]
- Jing, Y.; Liu, J.; Ye, Y.; Pan, L.; Deng, H.; Wang, Y.; Yang, Y.; Diao, L.; Lin, S.H.; Mills, G.B.; et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat. Commun. 2020, 11, 4946. [Google Scholar] [CrossRef]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Manson, G.; Norwood, J.; Marabelle, A.; Kohrt, H.; Houot, R. Biomarkers associated with checkpoint inhibitors. Ann. Oncol. 2016, 27, 1199–1206. [Google Scholar] [CrossRef]
- Ponvilawan, B.; Khan, A.W.; Subramanian, J.; Bansal, D. Non-Invasive Predictive Biomarkers for Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors. Cancers 2024, 16, 1225. [Google Scholar] [CrossRef]
- Tay, S.H.; Toh, M.; Thian, Y.L.; Vellayappan, B.A.; Fairhurst, A.M.; Chan, Y.H.; Aminkeng, F.; Bharwani, L.D.; Huang, Y.; Mak, A.; et al. Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature. Front. Immunol. 2022, 13, 807050. [Google Scholar] [CrossRef]
- Ruf, T.; Kramer, R.; Forschner, A.; Leiter, U.; Meier, F.; Reinhardt, L.; Dücker, P.; Ertl, C.; Tomsitz, D.; Tietze, J.K.; et al. Second-line therapies for steroid-refractory immune-related adverse events in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2024, 203, 114028. [Google Scholar]
- Liu, X.; Tang, H.; Zhou, Q.; Zeng, Y.; Lu, B.; Chen, D.; Li, Y.; Qian, J.; Chen, M.; Zhao, J.; et al. Gut microbiota composition in patients with advanced malignancies experiencing immune-related adverse events. Front. Immunol. 2023, 14, 1109281. [Google Scholar] [CrossRef] [PubMed]
- Iesaka, H.; Kameda, H.; Miya, A.; Nomoto, H.; Cho, K.Y.; Nakamura, A.; Abe, T.; Shinohara, N.; Atsumi, T. Fulminant ACTH decrease following diabetic ketoacidosis induced by immune checkpoint inhibitor combination therapy with nivolumab and ipilimumab: A case report. Medicine 2023, 102, e36664. [Google Scholar] [CrossRef] [PubMed]
- Burri, E.; Mangana, J.; Cheng, P.F.; Schneider, A.; Weber, A.; Dummer, R.; Ramelyte, E. Infliximab in steroid-refractory immune-related hepatitis does not demonstrate hepatotoxicity and may shorten time on steroids. J. Immunother. Cancer 2024, 12, e008074. [Google Scholar] [CrossRef]
- Ye, R.; Zheng, H.; Yang, D.; Lin, J.; Li, L.; Li, Y.; Pan, H.; Dai, H.; Zhao, L.; Zhou, Y.; et al. irAE-colitis induced by CTLA-4 and PD-1 blocking were ameliorated by TNF blocking and modulation of gut microbial. Biomed. Pharmacother. 2024, 177, 116999. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Lin, H.; Wang, J.; Cao, B. Predictive biomarkers for immune-related adverse events in cancer patients treated with immune-checkpoint inhibitors. BMC Immunol. 2024, 25, 8. [Google Scholar]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of hepatocellular cancer after resection: Patterns, treatments, and prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar]
- Wang, M.D.; Xu, X.J.; Wang, K.C.; Diao, Y.K.; Xu, J.H.; Gu, L.H.; Yao, L.Q.; Li, C.; Lv, G.Y.; Yang, T. Conversion therapy for advanced hepatocellular carcinoma in the era of precision medicine: Current status, challenges and opportunities. Cancer Sci. 2024, 115, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiu, J.; Zheng, Y.; Shi, Y.; Zou, R.; He, W.; Yuan, Y.; Zhang, Y.; Wang, C.; Qiu, Z.; et al. Conversion to Resectability Using Transarterial Chemoembolization Combined with Hepatic Arterial Infusion Chemotherapy for Initially Unresectable Hepatocellular Carcinoma. Ann. Surg. Open 2021, 2, e057. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Zhu, X.D.; Huang, C.; Shen, Y.H.; Ji, Y.; Ge, N.L.; Qu, X.D.; Chen, L.; Shi, W.K.; Li, M.L.; Zhu, J.J.; et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer 2021, 10, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Aoki, T.; Ueshima, K.; Tsuchiya, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Achievement of Complete Response and Drug-Free Status by Atezolizumab plus Bevacizumab Combined with or without Curative Conversion in Patients with Transarterial Chemoembolization-Unsuitable, Intermediate-Stage Hepatocellular Carcinoma: A Multicenter Proof-Of-Concept Study. Liver Cancer 2023, 12, 321–338. [Google Scholar]
- Takeda, H.; Nishijima, N.; Nasu, A.; Komekado, H.; Kita, R.; Kimura, T.; Kudo, M.; Osaki, Y. Long-term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol. Res. 2019, 49, 594–599. [Google Scholar] [CrossRef]
- Hidaka, M.; Hara, T.; Soyama, A.; Sasaki, R.; Matsushima, H.; Tanaka, T.; Hamada, T.; Imamura, H.; Adachi, T.; Kanetaka, K.; et al. The Outcome of Conversion Liver Resection Surgery by Lenvatinib Treatment: A Single Center Experience. Anticancer. Res. 2022, 42, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Huang, C.; Shen, Y.H.; Xu, B.; Ge, N.L.; Ji, Y.; Qu, X.D.; Chen, L.; Chen, Y.; Li, M.L.; et al. Hepatectomy After Conversion Therapy Using Tyrosine Kinase Inhibitors Plus Anti-PD-1 Antibody Therapy for Patients with Unresectable Hepatocellular Carcinoma. Ann. Surg. Oncol. 2023, 30, 2782–2790. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, S.; Hu, B.; Wan, T.; Tang, H.; Zhao, F.; Jiao, T.; Li, J.; Zhang, Z.; Cai, J.; et al. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: A single-arm, phase II trial. J. Immunother. Cancer 2023, 11, e007366. [Google Scholar] [CrossRef]
- Chiang, C.L.; Chiu, K.; Chan, K.; Lee, F.; Li, J.; Wan, C.; Dai, W.C.; Lam, T.C.; Chen, W.; Wong, N.; et al. Sequential transarterial chemoembolisation and stereotactic body radiotherapy followed by immunotherapy as conversion therapy for patients with locally advanced, unresectable hepatocellular carcinoma (START-FIT): A single-arm, phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 169–178. [Google Scholar] [CrossRef]
- Tai, D.; Loke, K.; Gogna, A.; Kaya, N.A.; Tan, S.H.; Hennedige, T.; Ng, D.; Irani, F.; Lee, J.; Lim, J.Q.; et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): A single arm, single centre, phase 2 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 1025–1035. [Google Scholar] [CrossRef]
- Shindoh, J.; Kawamura, Y.; Kobayashi, Y.; Kobayashi, M.; Akuta, N.; Okubo, S.; Suzuki, Y.; Hashimoto, M. Prognostic Impact of Surgical Intervention After Lenvatinib Treatment for Advanced Hepatocellular Carcinoma. Ann. Surg. Oncol. 2021, 28, 7663–7672. [Google Scholar] [CrossRef]
- Itoh, S.; Toshida, K.; Morita, K.; Kurihara, T.; Nagao, Y.; Tomino, T.; Toshima, T.; Harada, N.; Mori, M.; Yoshizumi, T. Clinical effectiveness of surgical treatment after lenvatinib administration for hepatocellular carcinoma. Int. J. Clin. Oncol. 2022, 27, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Akuta, N.; Shindoh, J.; Matsumura, M.; Okubo, S.; Tominaga, L.; Fujiyama, S.; Hosaka, T.; Saitoh, S.; Sezaki, H.; et al. Efficacy of the Combination of Systemic Sequential Therapy and Locoregional Therapy in the Long-Term Survival of Patients with BCLC Stage C Hepatocellular Carcinoma. Cancers 2023, 15, 3789. [Google Scholar] [CrossRef]
- Akahoshi, K.; Shindoh, J.; Tanabe, M.; Ariizumi, S.; Eguchi, S.; Okamura, Y.; Kaibori, M.; Kubo, S.; Shimada, M.; Taketomi, A.; et al. Oncological Resectability Criteria for Hepatocellular Carcinoma in the Era of Novel Systemic Therapies: The Japan Liver Cancer Association and Japanese Society of Hepato-Biliary-Pancreatic Surgery Expert Consensus Statement 2023. Liver Cancer 2024, 13, 579–589. [Google Scholar]
- Nakamura, I.; Yoh, T.; Nishimura, T.; Okuno, M.; Okamoto, T.; Sueoka, H.; Iida, K.; Tada, M.; Ishii, T.; Seo, S.; et al. A classification model for resectability in hepatocellular carcinoma patients. Hepatol. Res. 2024. ahead of print. [Google Scholar]
- Akabane, M.; Kawashima, J.; Altaf, A.; Woldesenbet, S.; Cauchy, F.; Aucejo, F.; Popescu, I.; Kitago, M.; Martel, G.; Ratti, F.; et al. International Validation and Refinement of Oncological Borderline Resectability Criteria for Hepatocellular Carcinoma Using Tumor Burden Score to Predict Survival. Ann. Surg. Open 2025, 6, e557. [Google Scholar] [CrossRef]
- Shindoh, J.; Matsumura, M.; Komatsu, S.; Fukumoto, T.; Ichida, A.; Hasegawa, K.; Ishii, T.; Hatano, E.; Nakamura, M.; Ohtsuka, M. Prognostic factors and clinical significance of preoperative systemic therapy in patients with borderline resectable hepatocellular carcinoma: A JSHBPS project study 2023, Part 2. J. Hepato-Biliary-Pancreat. Sci. 2025, 32, 374–384. [Google Scholar]
- Shindoh, J.; Kawamura, Y.; Akahoshi, K.; Matsumura, M.; Okubo, S.; Akuta, N.; Tanabe, M.; Kokudo, N.; Suzuki, Y.; Hashimoto, M. Clinical Utility of the Novel Oncological Criteria of Resectability for Advanced Hepatocellular Carcinoma. Liver Cancer 2024, 13, 601–609. [Google Scholar] [CrossRef]
- Karasuyama, T.; Ishii, T.; Yoh, T.; Ogiso, S.; Takeda, H.; Takai, A.; Kishi, N.; Shimizu, H.; Ito, T.; Haga, H.; et al. Aggressive Multidisciplinary Treatment for Unresectable Hepatocellular Carcinoma: The Achievement of a Pathologic Complete Response and Long-Term Survival. Ann. Surg. Oncol. 2025, 32, 1819–1820. [Google Scholar] [CrossRef]
- Matsumoto, N.; Ogawa, M.; Kaneko, M.; Kumagawa, M.; Watanabe, Y.; Hirayama, M.; Nakagawara, H.; Masuzaki, R.; Kanda, T.; Moriyama, M.; et al. Quantitative Ultrasound Image Analysis Helps in the Differentiation of Hepatocellular Carcinoma (HCC) From Borderline Lesions and Predicting the Histologic Grade of HCC and Microvascular Invasion. J. Ultrasound Med. 2021, 40, 689–698. [Google Scholar] [PubMed]
- Poté, N.; Caruso, S.; Caderaro, J.; Cauchy, F.; Lagadec, F.; Couchy, G.; Raffenne, J.; Augustin, J.; Vernuccio, F.; Vilgrain, V.; et al. Borderline Hepatocellular Adenomas: A Practical Diagnostic Approach Based on Pathologic and Molecular Features. Mod. Pathol. 2023, 36, 100211. [Google Scholar] [CrossRef] [PubMed]
- Haruki, K.; Harimoto, N.; Furukawa, K.; Taniai, T.; Yanagaki, M.; Igarashi, Y.; Tsunematsu, M.; Shirai, Y.; Shirabe, K.; Ikegami, T. Proposal for Prognosis-Oriented Definition of Borderline Resectable Hepatocellular Carcinoma. J. Am. Coll. Surg. 2024, 238, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Kokudo, N.; Kokudo, T.; Song, P.; Tang, W. Role of liver resection in the era of advanced systemic therapy for hepatocellular carcinoma. Glob. Health Med. 2024, 6, 170–173. [Google Scholar]
- Yang, Z.; Fu, Y.; Wang, Q.; Pan, Y.; Wang, J.; Chen, J.; Hu, D.; Zhou, Z.; Chen, M.; Zhang, Y. Dynamic changes of serum α-fetoprotein predict the prognosis of bevacizumab plus immunotherapy in hepatocellular carcinoma. Int. J. Surg. 2025, 111, 751–760. [Google Scholar] [CrossRef]
- Lin, N.; Lin, Y.; Xu, J.; Liu, D.; Li, D.; Meng, H.; Gallant, M.A.; Kubota, N.; Roy, D.; Li, J.S.; et al. A multi-analyte cell-free DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol. Commun. 2022, 6, 1753–1763. [Google Scholar] [CrossRef]
- Guo, D.Z.; Zhang, S.Y.; Dong, S.Y.; Yan, J.Y.; Wang, Y.P.; Cao, Y.; Rao, S.X.; Fan, J.; Yang, X.R.; Huang, A.; et al. Prognostic model for predicting outcome and guiding treatment decision for unresectable hepatocellular carcinoma treated with lenvatinib monotherapy or lenvatinib plus immunotherapy. Front. Immunol. 2023, 14, 1141199. [Google Scholar] [CrossRef]
- Guo, D.Z.; Zhang, S.Y.; Dong, S.Y.; Yan, J.Y.; Wang, Y.P.; Cao, Y.; Rao, S.X.; Fan, J.; Yang, X.R.; Huang, A.; et al. Circulating immune index predicting the prognosis of patients with hepatocellular carcinoma treated with lenvatinib and immunotherapy. Front. Oncol. 2023, 13, 1109742. [Google Scholar] [CrossRef]
- Bo, Z.; Chen, B.; Zhao, Z.; He, Q.; Mao, Y.; Yang, Y.; Yao, F.; Yang, Y.; Chen, Z.; Yang, J.; et al. Prediction of Response to Lenvatinib Monotherapy for Unresectable Hepatocellular Carcinoma by Machine Learning Radiomics: A Multicenter Cohort Study. Clin. Cancer Res. 2023, 29, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Dong, S.Y.; Bai, X.L.; Song, T.Q.; Zhang, B.H.; Zhou, L.D.; Chen, Y.J.; Zeng, Z.M.; Wang, K.; Zhao, H.T.; et al. Tumor Radiomic Features on Pretreatment MRI to Predict Response to Lenvatinib plus an Anti-PD-1 Antibody in Advanced Hepatocellular Carcinoma: A Multicenter Study. Liver Cancer 2023, 12, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Hiraoka, A.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. Neutrophil-lymphocyte ratio predicts early outcomes in patients with unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab: A multicenter analysis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 698–706. [Google Scholar] [CrossRef]
- Eso, Y.; Takeda, H.; Taura, K.; Takai, A.; Takahashi, K.; Seno, H. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Predictive Marker of Response to Atezolizumab Plus Bevacizumab for Hepatocellular Carcinoma. Curr. Oncol. 2021, 28, 4157–4166. [Google Scholar] [CrossRef]
- Ochi, H.; Kurosaki, M.; Joko, K.; Mashiba, T.; Tamaki, N.; Tsuchiya, K.; Marusawa, H.; Tada, T.; Nakamura, S.; Narita, R.; et al. Usefulness of neutrophil-to-lymphocyte ratio in predicting progression and survival outcomes after atezolizumab-bevacizumab treatment for hepatocellular carcinoma. Hepatol. Res. 2023, 53, 61–71. [Google Scholar] [CrossRef]
- Matoya, S.; Suzuki, T.; Matsuura, K.; Suzuki, Y.; Okumura, F.; Nagura, Y.; Sobue, S.; Kuroyanagi, K.; Kusakabe, A.; Koguchi, H.; et al. The neutrophil-to-lymphocyte ratio at the start of the second course during atezolizumab plus bevacizumab therapy predicts therapeutic efficacy in patients with advanced hepatocellular carcinoma: A multicenter analysis. Hepatol. Res. 2023, 53, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.; Chan, K.; Chiu, K.; Lee, F.; Chen, W.; Wong, N.; Ho, R.; Lee, V.; Man, K.; Kong, F.; et al. Complete Response to Locoregional Therapy Plus Immunotherapy for Hepatocellular Carcinoma. JAMA Oncol. 2024, 10, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tang, H.; Hu, B.; Zhang, W.; Wan, T.; Han, J.; Jiao, T.; Li, J.; Li, X.; Yang, Z.; et al. Comparison of survival benefit between salvage surgery after conversion therapy versus surgery alone for hepatocellular carcinoma with portal vein tumor thrombosis: A propensity score analysis. HPB 2023, 25, 775–787. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, C.; Peng, X.; Bi, X.; Sun, Y.; Zhou, J.; Wu, F.; Zeng, H.; Wang, Y.; Zhou, H.; et al. Serum Concentration of CD137 and Tumor Infiltration by M1 Macrophages Predict the Response to Sintilimab plus Bevacizumab Biosimilar in Advanced Hepatocellular Carcinoma Patients. Clin. Cancer Res. 2022, 28, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, R.; Ito, T.; Yoshio, S.; Yamamoto, T.; Mizuno, K.; Ishigami, M.; Kawashima, H.; Yasuda, S.; Shimose, S.; Iwamoto, H.; et al. Serum osteopontin predicts the response to atezolizumab plus bevacizumab in patients with hepatocellular carcinoma. J. Gastroenterol. 2023, 58, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, C.J.; Hong, H.; Kamath, S.; Schlegel, A.; Fujiki, M.; Hashimoto, K.; Kwon, D.; Miller, C.; Walsh, R.M.; Aucejo, F. Tumor Mutational Burden From Circulating Tumor DNA Predicts Recurrence of Hepatocellular Carcinoma After Resection: An Emerging Biomarker for Surveillance. Ann. Surg. 2024, 280, 504–513. [Google Scholar] [CrossRef]
- Campani, C.; Imbeaud, S.; Couchy, G.; Ziol, M.; Hirsch, T.Z.; Rebouissou, S.; Noblet, B.; Nahon, P.; Hormigos, K.; Sidali, S.; et al. Circulating tumour DNA in patients with hepatocellular carcinoma across tumour stages and treatments. Gut 2024, 73, 1870–1882. [Google Scholar] [CrossRef]
- Zhu, Q.; Xie, J.; Mei, W.; Zeng, C. Methylated circulating tumor DNA in hepatocellular carcinoma: A comprehensive analysis of biomarker potential and clinical implications. Cancer Treat. Rev. 2024, 128, 102763. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Gassa, A.; Buchner, D.; Alakus, H.; Dong, Q.; Ren, N.; Liu, M.; Odenthal, M.; Stippel, D.; et al. Circulating tumor DNA as an emerging liquid biopsy biomarker for early diagnosis and therapeutic monitoring in hepatocellular carcinoma. Int. J. Biol. Sci. 2020, 16, 1551–1562. [Google Scholar] [CrossRef]
- Qu, C.; Wang, Y.; Wang, P.; Chen, K.; Wang, M.; Zeng, H.; Lu, J.; Song, Q.; Diplas, B.H.; Tan, D.; et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc. Natl. Acad. Sci. USA 2019, 116, 6308–6312. [Google Scholar] [CrossRef]
- Best, J.; Bilgi, H.; Heider, D.; Schotten, C.; Manka, P.; Bedreli, S.; Gorray, M.; Ertle, J.; van Grunsven, L.A.; Dechêne, A. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z. Für Gastroenterol. 2016, 54, 1296–1305. [Google Scholar] [CrossRef]
- Yang, T.; Xing, H.; Wang, G.; Wang, N.; Liu, M.; Yan, C.; Li, H.; Wei, L.; Li, S.; Fan, Z.; et al. A Novel Online Calculator Based on Serum Biomarkers to Detect Hepatocellular Carcinoma among Patients with Hepatitis B. Clin. Chem. 2019, 65, 1543–1553. [Google Scholar] [CrossRef]
- Xu, R.H.; Wei, W.; Krawczyk, M.; Wang, W.; Luo, H.; Flagg, K.; Yi, S.; Shi, W.; Quan, Q.; Li, K.; et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017, 16, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, N.; Yang, T.; Li, Y.; Li, J.; Huang, Q.; Wu, C.; Sun, L.; Zhou, X.; Cheng, X.; et al. DNMT1-mediated methylation of BEX1 regulates stemness and tumorigenicity in liver cancer. J. Hepatol. 2021, 75, 1142–1153. [Google Scholar] [CrossRef]
- Reddy, D.; Bhattacharya, S.; Shah, S.; Rashid, M.; Gupta, S. DNA methylation mediated downregulation of histone H3 variant H3.3 affects cell proliferation contributing to the development of HCC. Biochim. Biophys. Acta-Mol. Basis Dis. 2022, 1868, 166284. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Porter, K.; Bhattacharya, A.; Book, A.J.; Neis, B.M.; Xiong, K.M.; Ramasubramanian, T.S.; Edwards, D.K.V.; Chen, I.; Johnson, S.; et al. Validation of a Novel Multitarget Blood Test Shows High Sensitivity to Detect Early Stage Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2022, 20, 173–182.e7. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Ramasubramanian, T.S.; Bhattacharya, A.; Olson, M.C.; Edwards, D.K.V.; Roberts, L.R.; Kisiel, J.B.; Reddy, K.R.; Lidgard, G.P.; Johnson, S.C.; et al. A Novel Blood-Based Panel of Methylated DNA and Protein Markers for Detection of Early-Stage Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2021, 19, 2597–2605.e4. [Google Scholar] [CrossRef]
- El-Garem, H.; Ammer, A.; Shehab, H.; Shaker, O.; Anwer, M.; El-Akel, W.; Omar, H. Circulating microRNA, miR-122 and miR-221 signature in Egyptian patients with chronic hepatitis C related hepatocellular carcinoma. World J. Hepatol. 2014, 6, 818–824. [Google Scholar] [CrossRef]
- Ding, L.H.; Fallgren, C.M.; Yu, Y.; McCarthy, M.; Edmondson, E.F.; Ullrich, R.L.; Weil, M.M.; Story, M.D. Orthologs of human circulating miRNAs associated with hepatocellular carcinoma are elevated in mouse plasma months before tumour detection. Sci. Rep. 2022, 12, 10927. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ye, Y.; Wang, Z.; Guan, L.; Bao, S.; Li, B.; Li, W. Circulating microRNAs for the diagnosis of hepatocellular carcinoma. Dig. Liver Dis. 2019, 51, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kondo, S.; Matsuzaki, J.; Esaki, M.; Okusaka, T.; Shimada, K.; Murakami, Y.; Enomoto, M.; Tamori, A.; Kato, K.; et al. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients With Liver Disease. Hepatol. Commun. 2020, 4, 284–297. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, W.; Wang, Y.; Zhao, M.; Li, Y.; Ren, L. Serum long non-coding RNA SCARNA10 serves as a potential diagnostic biomarker for hepatocellular carcinoma. BMC Cancer 2022, 22, 431. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G.; Zhao, Y.; Gao, H.; Li, L.; Yin, Y.; Jiang, J.; Wang, L.; Mang, Y.; Gao, Y.; et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol. Cancer 2023, 22, 55. [Google Scholar] [CrossRef]
- Huang, X.Y.; Huang, Z.L.; Huang, J.; Xu, B.; Huang, X.Y.; Xu, Y.H.; Zhou, J.; Tang, Z.Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 20. [Google Scholar] [CrossRef]
- Sun, N.; Lee, Y.T.; Zhang, R.Y.; Kao, R.; Teng, P.C.; Yang, Y.; Yang, P.; Wang, J.J.; Smalley, M.; Chen, P.J.; et al. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat. Commun. 2020, 11, 4489. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, C.; Lee, Y.T.; Tran, B.V.; Wang, J.; Kim, H.; Lee, J.; Zhang, R.Y.; Wang, J.J.; Hu, J.; et al. HCC EV ECG score: An extracellular vesicle-based protein assay for detection of early-stage hepatocellular carcinoma. Hepatology 2023, 77, 774–788. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Z.H.; Wang, J.X.; Zhao, Z.; Peng, T.Y. Tumor Cell-Derived Exosomal Circ-0072088 Suppresses Migration and Invasion of Hepatic Carcinoma Cells Through Regulating MMP-16. Front. Cell Dev. Biol. 2021, 9, 726323. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, S.; Li, X.; Miao, Y.; Han, Y.; Ju, R.; Cui, X.; Li, Y. A deep learning model based on self-supervised learning for identifying subtypes of proliferative hepatocellular carcinoma from dynamic contrast-enhanced MRI. Insights Imaging 2025, 16, 89. [Google Scholar] [PubMed]
- Sato, A.; Okada, M.; Tago, K.; Nakazawa, Y.; Mizuno, M.; Miyauchi, T.; Kobashi, Y. Multiparametric gadoxetic acid-enhanced MR versus dual-layer spectral detector CT for differentiating hepatocellular carcinoma from hypervascular pseudolesions. Acta Radiol. 2025, 66, 712–721. [Google Scholar] [CrossRef]
- Lee, S.W.; Jeong, S.Y.; Kim, S.J. Diagnostic performance of FDG PET/CT radiomics in predicting microvascular invasion in hepatocellular carcinoma compared to conventional metabolic parameters: A systematic review and meta-analysis. Ann. Nucl. Med. 2025. ahead of print. [Google Scholar]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.; Mahmoud, H.; Abd El-Hafeez, T.; ElAraby, M.E. The power of deep learning in simplifying feature selection for hepatocellular carcinoma: A review. BMC Med. Inform. Decis. Mak. 2024, 24, 287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sheng, Y.; Jiang, Z.; Liu, H.; Lu, H.; Xing, W. What Imaging Modality Is More Effective in Predicting Early Recurrence of Hepatocellular Carcinoma after Hepatectomy Using Radiomics Analysis: CT or MRI or Both. Diagnostics 2023, 13, 2012. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, S.K.; Fetzer, D.T.; Seow, J.H.; McGillen, K.; Burrowes, D.P.; Fung, C.; Udare, A.S.; Wilson, S.R.; Kamaya, A. Optimizing US for HCC surveillance. Abdom. Radiol. 2025, 50, 2453–2463. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, J.H.; Choi, M.H.; Lee, C.H.; Kang, T.W.; Kim, H.A.; Ku, Y.M.; Lee, J.M.; Kim, S.H.; Kim, K.A.; et al. Comparison of non-contrast abbreviated MRI and ultrasound as surveillance modalities for HCC. J. Hepatol. 2024, 81, 461–470. [Google Scholar] [CrossRef]
- Yang, N.; Ma, Z.; Zhang, L.; Ji, W.; Xi, Q.; Li, M.; Jin, L. Radiomics-based automated machine learning for differentiating focal liver lesions on unenhanced computed tomography. Abdom. Radiol. 2025, 50, 2126–2139. [Google Scholar] [CrossRef]
- Bharti, P.; Mittal, D.; Ananthasivan, R. Preliminary Study of Chronic Liver Classification on Ultrasound Images Using an Ensemble Model. Ultrason. Imaging 2018, 40, 357–379. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, A.; Wu, X.; Hu, X.; Bai, G.; Fan, Y.; Stål, P.; Brismar, T.B. Radiomics models for preoperative prediction of the histopathological grade of hepatocellular carcinoma: A systematic review and radiomics quality score assessment. Eur. J. Radiol. 2023, 166, 111015. [Google Scholar] [CrossRef]
- Vithayathil, M.; Koku, D.; Campani, C.; Nault, J.C.; Sutter, O.; Ganne-Carrié, N.; Aboagye, E.O.; Sharma, R. Machine learning based radiomic models outperform clinical biomarkers in predicting outcomes after immunotherapy for hepatocellular carcinoma. J. Hepatol. 2025, 83, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ji, Q.; Gao, Y.; Yang, X.; Guo, D.; Gu, D.; Yuan, C.; Tian, J.; Ding, D. A multi-scale, multi-region and attention mechanism-based deep learning framework for prediction of grading in hepatocellular carcinoma. Med. Phys. 2023, 50, 2290–2302. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Tong, Y.; Chen, H.; Su, R.; Tao, Y.; Zhang, G.; Sun, Z. Tumor Growth Pattern and Intra- and Peritumoral Radiomics Combined for Prediction of Initial TACE Outcome in Patients with Primary Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 1927–1944. [Google Scholar] [CrossRef]
- Xie, T.; Wei, Y.; Xu, L.; Li, Q.; Che, F.; Xu, Q.; Cheng, X.; Liu, M.; Yang, M.; Wang, X.; et al. Self-supervised contrastive learning using CT images for PD-1/PD-L1 expression prediction in hepatocellular carcinoma. Front. Oncol. 2023, 13, 1103521. [Google Scholar] [CrossRef] [PubMed]
- Petukhova-Greenstein, A.; Zeevi, T.; Yang, J.; Chai, N.; DiDomenico, P.; Deng, Y.; Ciarleglio, M.; Haider, S.P.; Onyiuke, I.; Malpani, R.; et al. MR Imaging Biomarkers for the Prediction of Outcome after Radiofrequency Ablation of Hepatocellular Carcinoma: Qualitative and Quantitative Assessments of the Liver Imaging Reporting and Data System and Radiomic Features. J. Vasc. Interv. Radiol. 2022, 33, 814–824.e3. [Google Scholar] [CrossRef]
- Kuang, F.; Gao, Y.; Zhou, Q.; Lu, C.; Lin, Q.; Al Mamun, A.; Pan, J.; Shi, S.; Tu, C.; Shao, C. MRI Radiomics Combined with Clinicopathological Factors for Predicting 3-Year Overall Survival of Hepatocellular Carcinoma After Hepatectomy. J. Hepatocell. Carcinoma 2024, 11, 1445–1457. [Google Scholar] [CrossRef]
- Qu, W.F.; Tian, M.X.; Lu, H.W.; Zhou, Y.F.; Liu, W.R.; Tang, Z.; Yao, Z.; Huang, R.; Zhu, G.Q.; Jiang, X.F.; et al. Development of a deep pathomics score for predicting hepatocellular carcinoma recurrence after liver transplantation. Hepatol. Int. 2023, 17, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Huang, W.; Pan, X.; Ruan, F.; Li, X.; Tan, S.; Long, L. Predicting overall survival in hepatocellular carcinoma patients via a combined MRI radiomics and pathomics signature. Transl. Oncol. 2025, 51, 102174. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, L.; Sun, Z.; Jiang, H.; Zhang, J. A radiomics signature associated with underlying gene expression pattern for the prediction of prognosis and treatment response in hepatocellular carcinoma. Eur. J. Radiol. 2023, 167, 111086. [Google Scholar] [CrossRef]
- Wang, G.; Ding, F.; Chen, K.; Liang, Z.; Han, P.; Wang, L.; Cui, F.; Zhu, Q.; Cheng, Z.; Chen, X.; et al. CT-based radiomics nomogram to predict proliferative hepatocellular carcinoma and explore the tumor microenvironment. J. Transl. Med. 2024, 22, 683. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Lin, X.H.; Guo, H.Y.; Shi, X.; Zhang, D.Y.; Sun, J.L.; Zhang, G.C.; Xu, R.C.; Wang, F.; Yu, X.N.; et al. Multi-Omics profiling identifies aldehyde dehydrogenase 2 as a critical mediator in the crosstalk between Treg-mediated immunosuppression microenvironment and hepatocellular carcinoma. Int. J. Biol. Sci. 2024, 20, 2763–2778. [Google Scholar] [CrossRef]
- Chaudhary, K.; Poirion, O.B.; Lu, L.; Garmire, L.X. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin. Cancer Res. 2018, 24, 1248–1259. [Google Scholar]
- Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Chiou, Y.Y.; Huang, Y.H.; Lee, F.Y.; Lin, H.C.; Hou, M.C.; Huo, T.I. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J. Gastroenterol. Hepatol. 2017, 32, 879–886. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, B.; Zeng, J.; Wang, Z.; Xiao, L.; Deng, G. Preoperative neutrophil-lymphocyte ratio predicts the risk of microvascular invasion in hepatocellular carcinoma: A meta-analysis. Int. J. Biol. Markers 2019, 34, 213–220. [Google Scholar] [CrossRef]
- Lim, K.C.; Chow, P.K.; Allen, J.C.; Chia, G.S.; Lim, M.; Cheow, P.C.; Chung, A.Y.; Ooi, L.L.; Tan, S.B. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann. Surg. 2011, 254, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Rungsakulkij, N.; Mingphruedhi, S.; Suragul, W.; Tangtawee, P.; Muangkaew, P.; Aeesoa, S. Platelet-to-Lymphocyte Ratio and Large Tumor Size Predict Microvascular Invasion after Resection for Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 3435–3441. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cai, J.; Li, H.; Zeng, K.; He, L.; Fu, H.; Zhang, J.; Chen, L.; Yao, J.; Zhang, Y.; et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: A Meta-Analysis and Systematic Review. Cell. Physiol. Biochem. 2017, 44, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Devarajan, K.; Singal, A.G.; Marrero, J.A.; Dai, J.; Feng, Z.; Rinaudo, J.A.; Srivastava, S.; Evans, A.; Hann, H.W.; et al. The Doylestown Algorithm: A Test to Improve the Performance of AFP in the Detection of Hepatocellular Carcinoma. Cancer Prev. Res. 2016, 9, 172–179. [Google Scholar] [CrossRef]
| Category/Biomarker | Key Findings |
|---|---|
| AFP | |
| Baseline AFP levels | In the IMbrave150 study, baseline AFP levels were a key stratification factor. Patients with AFP < 400 ng/mL may derive more significant benefit from Ate/Bev therapy compared with those with AFP ≥ 400 ng/mL. |
| Post-treatment AFP changes | Exploratory analyses of the IMbrave150 studies revealed that a ≥75% reduction or ≤10% elevation in AFP levels at week 6 post-treatment was significantly associated with improved OS and PFS. |
| DCP | |
| Post-treatment DCP changes | In the HIMALAYA study, the results demonstrated that patients exhibiting a DCP reduction of >40% at week 4 achieved treatment response in approximately 72% of cases. |
| Tumor-related genes and protein expression | |
| CD274 and TEFF | In the IMbrave150 study, high pre-existing expression of CD274 and TEFF was associated with greater benefit from Ate/Bev therapy. Patients with complete CR/PR had higher expression of ABRS, CD274, and TEFF than those with stable disease/progressive disease (SD/PD). |
| CD8+ T cells | In the IMbrave150 study, the concentration of CD8+ T cells in tumor tissue correlated with PFS and OS benefits from Ate/Bev therapy. In the HIMALAYA study, elevated levels of CD8+ T cells were associated with superior response rates to the STRIDE regimen. |
| TREG/TEFF ratio | A low TREG/TEFF ratio was associated with more significant improvements in PFS and OS after Ate/Bev therapy. |
| Wnt/β-catenin Signaling Pathway | Patients with inactivation of the Wnt/β-catenin signaling pathway showed enhanced response rates to the STRIDE regimen. |
| 1st-Line Therapy | Incidence ≥ G3 irAEs | Common irAEs |
|---|---|---|
| IMbrave150 | 36.0% | Hypertension AST increased ALT increased |
| HIMALAYA | 25.8% | AST increased Lipase increased Amylase increased |
| CHECKMATE-9DW | 41.0% | AST increased ALT increased Lipase increased |
| Predictive Scenarios | Biomarker Category | Biomarker |
|---|---|---|
| Prognostic Biomarkers for Surgical Candidates | Tumor biomarkers | AFP |
| DCP | ||
| ctDNA | ||
| Imaging Response Markers | RECIST or mRECIST | |
| contrast-enhanced MRI | ||
| Liver Functional Reserve Markers | ICG-R15 | |
| ALBI | ||
| Postoperative recurrence and long-term survival prognosis | Pathological risk factors | MVI |
| Satellite nodule | ||
| Differentiation | ||
| Molecular Markers | CTCs/ctDNA | |
| NLR/PLR | ||
| Specific genetic expression | ||
| Immune markers | PD-L1 expression | |
| CD8+ T cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Imai, N. Decision-Making Biomarkers Guiding Therapeutic Strategies in Hepatocellular Carcinoma: From Prediction to Personalized Care. Cancers 2025, 17, 3105. https://doi.org/10.3390/cancers17193105
Liu D, Imai N. Decision-Making Biomarkers Guiding Therapeutic Strategies in Hepatocellular Carcinoma: From Prediction to Personalized Care. Cancers. 2025; 17(19):3105. https://doi.org/10.3390/cancers17193105
Chicago/Turabian StyleLiu, Dongming, and Norihiro Imai. 2025. "Decision-Making Biomarkers Guiding Therapeutic Strategies in Hepatocellular Carcinoma: From Prediction to Personalized Care" Cancers 17, no. 19: 3105. https://doi.org/10.3390/cancers17193105
APA StyleLiu, D., & Imai, N. (2025). Decision-Making Biomarkers Guiding Therapeutic Strategies in Hepatocellular Carcinoma: From Prediction to Personalized Care. Cancers, 17(19), 3105. https://doi.org/10.3390/cancers17193105







