Long-Term Survival After Thyroidectomy for Thyroid Cancer: A Propensity-Matched TriNetX Study with Specialty-Stratified Analyses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Exposure and Outcomes
2.3. Classification of Surgical Specialties
2.4. Covariates
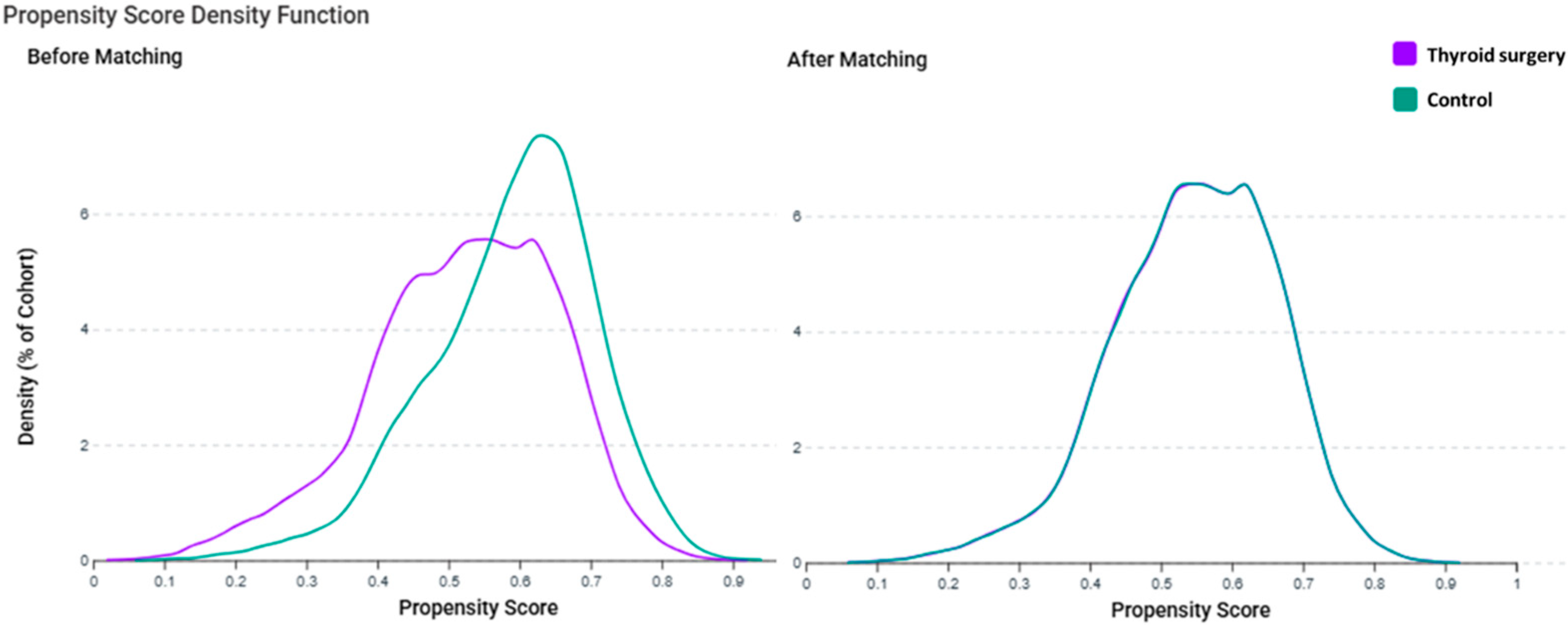
2.5. Propensity Score Matching
2.6. Subgroup
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics After Matching
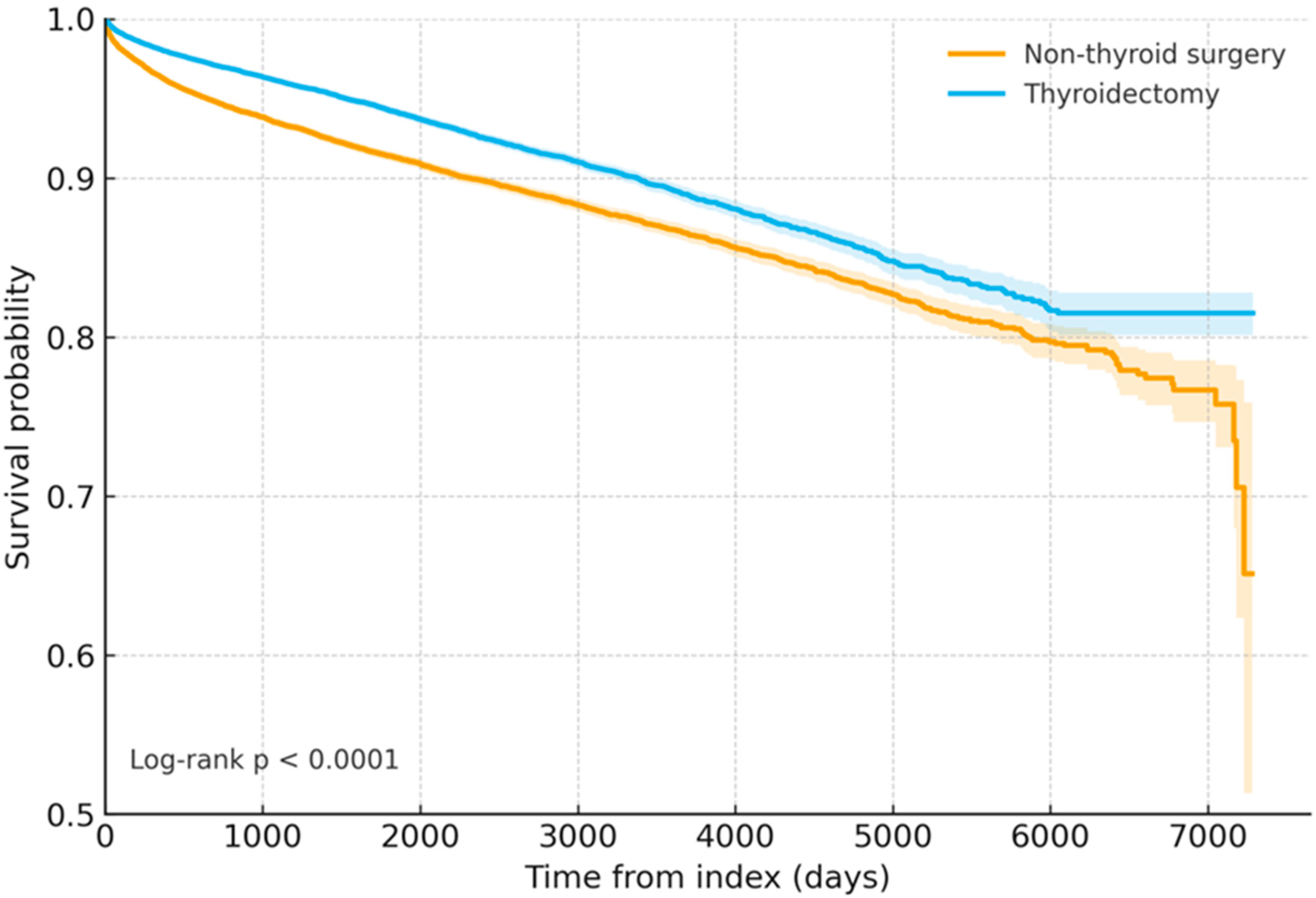
3.2. Kaplan–Meier Survival Curves
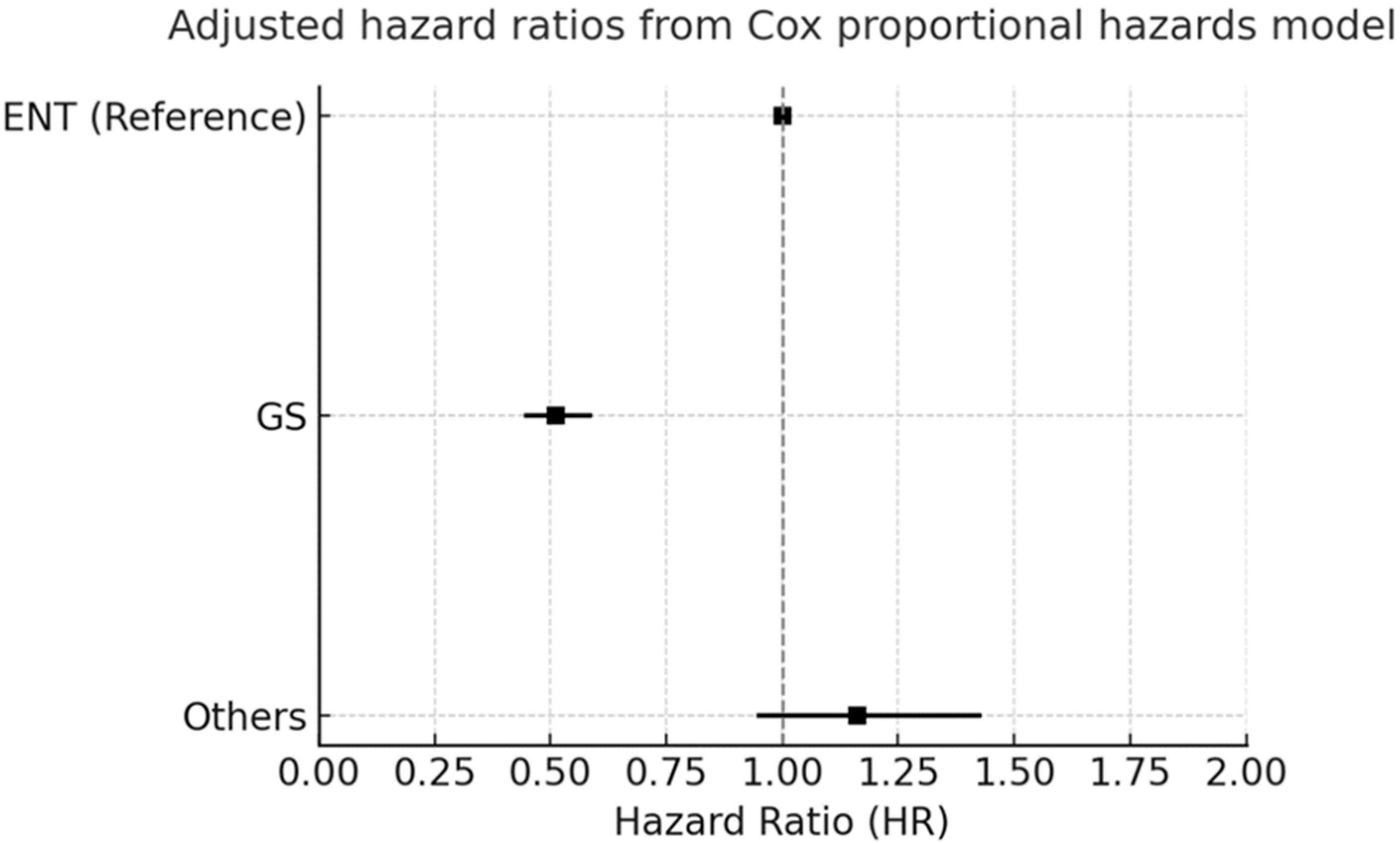
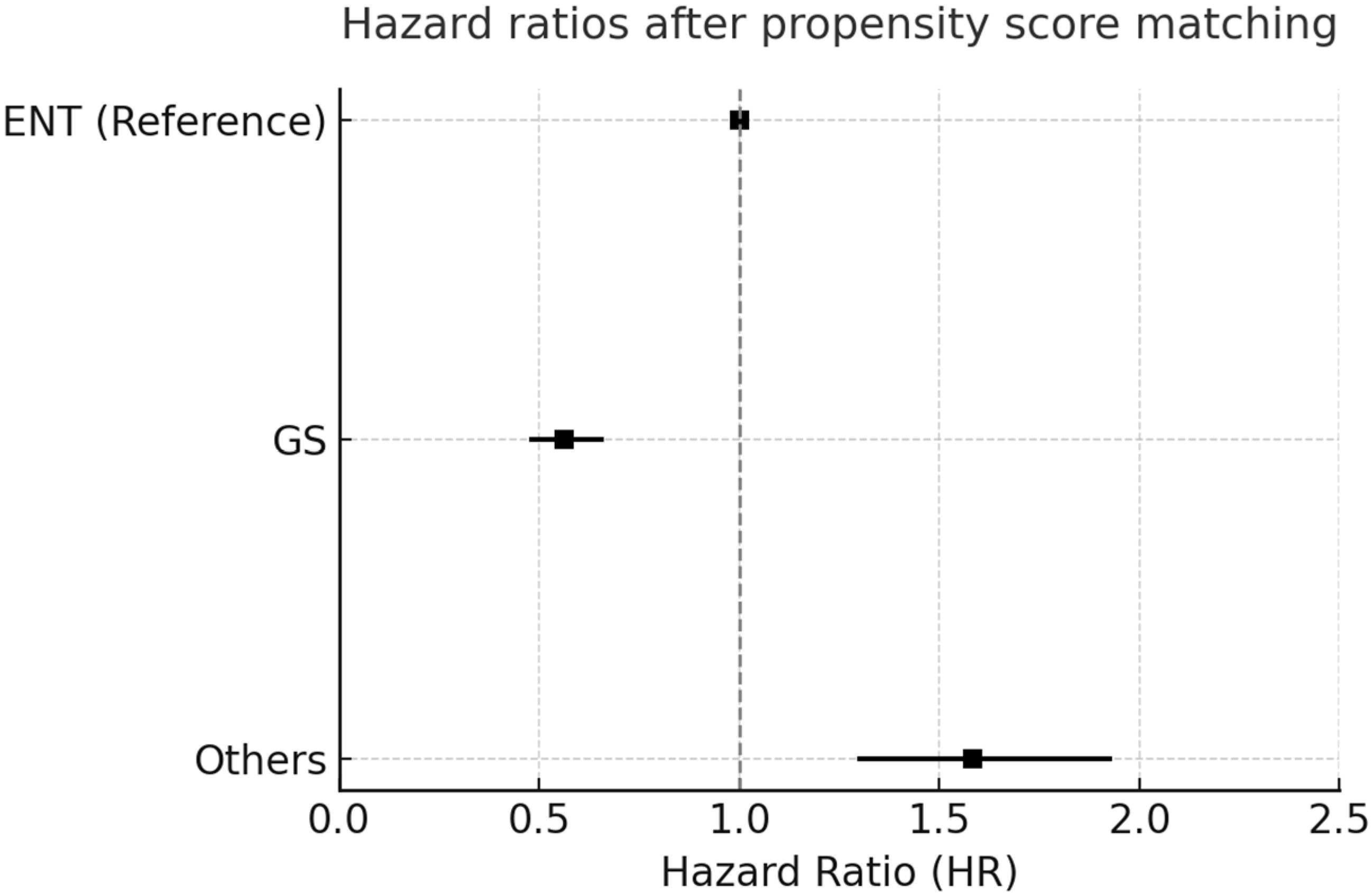
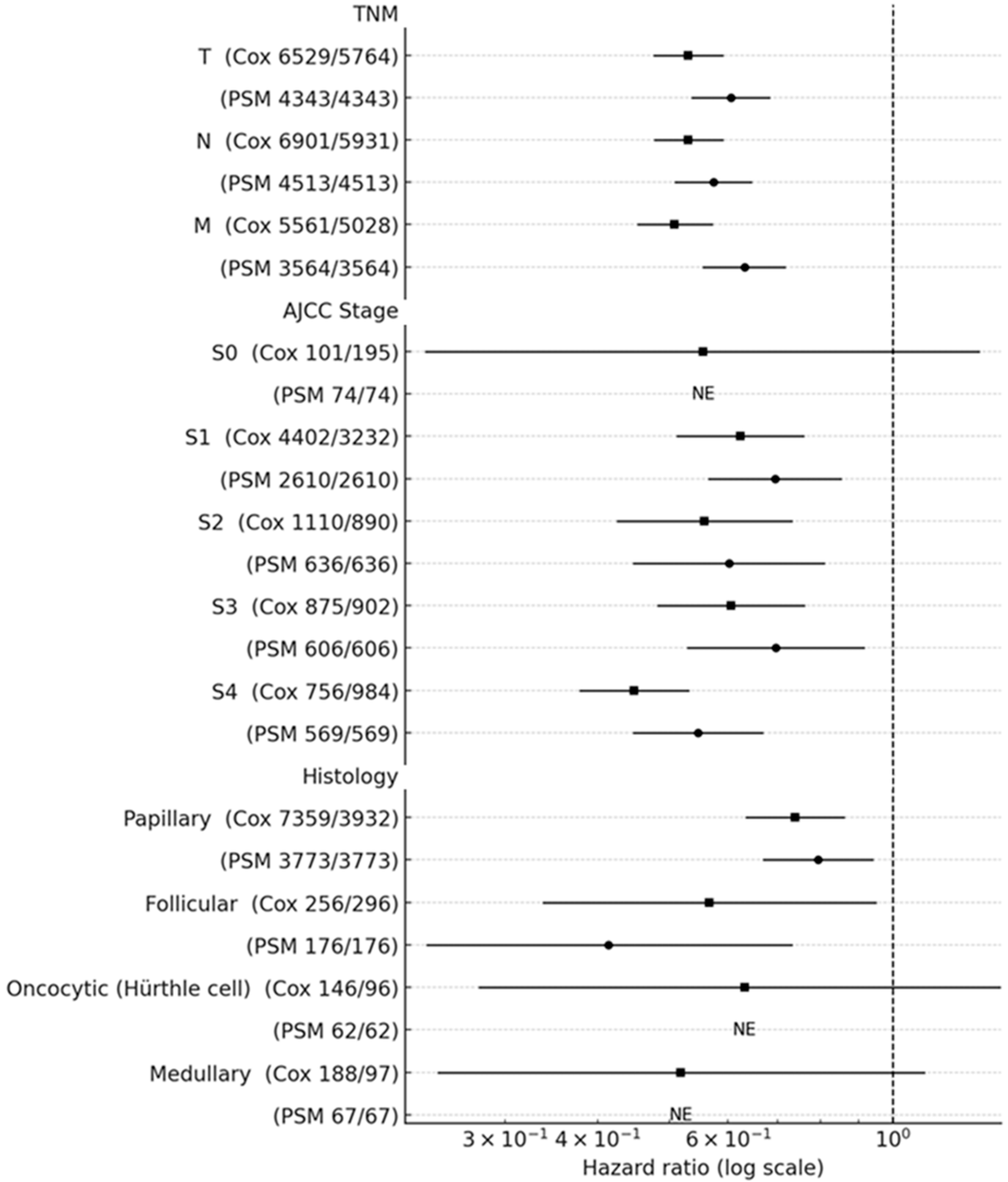
3.3. Forest Plot Analysis
3.4. Subgroup Hazard Ratios for All-Cause Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S.; Sturgeon, C. Extent of surgery affects survival for papillary thyroid cancer. Ann. Surg. 2007, 246, 375–384. [Google Scholar] [CrossRef]
- Fligor, S.C.; Lopez, B.; Uppal, N.; Lubitz, C.C.; James, B.C. Time to Surgery and Thyroid Cancer Survival in the United States. Ann. Surg. Oncol. 2021, 28, 3556–3565. [Google Scholar] [CrossRef]
- Al-Qurayshi, Z.; Khadra, H.; Chang, K.; Pagedar, N.; Randolph, G.W.; Kandil, E. Risk and survival of patients with medullary thyroid cancer: National perspective. Oral Oncol. 2018, 83, 59–63. [Google Scholar] [CrossRef]
- Stopenski, S.; Grigorian, A.; Roditi, R.; Jutric, Z.; Yamamoto, M.; Lekawa, M.; Nahmias, J. Discrepancies in Thyroidectomy Outcomes Between General Surgeons and Otolaryngologists. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 5384–5390. [Google Scholar] [CrossRef]
- Lorenz, K.; Raffaeli, M.; Barczynski, M.; Lorente-Poch, L.; Sancho, J. Volume, outcomes, and quality standards in thyroid surgery: An evidence-based analysis-European Society of Endocrine Surgeons (ESES) positional statement. Langenbeck’s Arch. Surg. 2020, 405, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Nixon, I.J.; Wang, L.Y.; Palmer, F.L.; Migliacci, J.C.; Aniss, A.; Sywak, M.; Eskander, A.E.; Freeman, J.L.; Campbell, M.J.; et al. Survival from Differentiated Thyroid Cancer: What Has Age Got to Do with It? Thyroid 2015, 25, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Papaleontiou, M.; Gauger, P.G.; Haymart, M.R. Referral of Older Thyroid Cancer Patients to a High-Volume Surgeon: Results of a Multidisciplinary Physician Survey. Endocr. Pract. 2017, 23, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Limaiem, F.; Rehman, A.; Mazzoni, T. Papillary Thyroid Carcinoma; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kaplan, E.; Angelos, P.; Applewhite, M.; Mercier, F.; Grogan, R.H. Chapter 21 Surgery of the Thyroid. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Parham, K.; Chapurin, N.; Schulz, K.; Shin, J.J.; Pynnonen, M.A.; Witsell, D.L.; Langman, A.; Nguyen-Huynh, A.; Ryan, S.E.; Vambutas, A.; et al. Thyroid Disease and Surgery in CHEER: The Nation’s Otolaryngology-Head and Neck Surgery Practice-Based Network. Otolaryngol. Head Neck Surg. 2016, 155, 22–27. [Google Scholar] [CrossRef][Green Version]
- McDow, A.D.; Saucke, M.C.; Marka, N.A.; Long, K.L.; Pitt, S.C. Thyroid Lobectomy for Low-Risk Papillary Thyroid Cancer: A National Survey of Low- and High-Volume Surgeons. Ann. Surg. Oncol. 2021, 28, 3568–3575. [Google Scholar] [CrossRef]
- Di Filippo, L.; Giugliano, G.; Tagliabue, M.; Gandini, S.; Sileo, F.; Allora, A.; Grosso, E.; Proh, M.; Basso, V.; Scaglione, D.; et al. Total thyroidectomy versus lobectomy: Surgical approach to T1-T2 papillary thyroid cancer. Acta Otorhinolaryngol. Ital. 2020, 40, 254–261. [Google Scholar] [CrossRef]
- Adam, M.A.; Pura, J.; Gu, L.; Dinan, M.A.; Tyler, D.S.; Reed, S.D.; Scheri, R.; Roman, S.A.; Sosa, J.A. Extent of surgery for papillary thyroid cancer is not associated with survival: An analysis of 61,775 patients. Ann. Surg. 2014, 260, 601–607, discussion 605–607. [Google Scholar] [CrossRef] [PubMed]
- Jishu, J.A.; Hussein, M.H.; Sadakkadulla, S.; Baah, S.; Bashumeel, Y.Y.; Toraih, E.; Kandil, E. Limited Thyroidectomy Achieves Equivalent Survival to Total Thyroidectomy for Early Localized Medullary Thyroid Cancer. Cancers 2024, 16, 4062. [Google Scholar] [CrossRef]
- Chang, C.M.; Huang, K.Y.; Hsu, T.W.; Su, Y.C.; Yang, W.Z.; Chen, T.C.; Chou, P.; Lee, C.C. Multivariate analyses to assess the effects of surgeon and hospital volume on cancer survival rates: A nationwide population-based study in Taiwan. PLoS ONE 2012, 7, e40590. [Google Scholar] [CrossRef]
- Chung, P.J.; Lee, M.; Chang, E.H.; Ferzli, G.S.; Alfonso, A.E.; Chernichenko, N.; Sugiyama, G. Does Specialty Matter? Analysis of Outcomes in Total Thyroidectomy for Goiters Between General Surgery and Otolaryngology Using American College of Surgeons NSQIP. J. Am. Coll. Surg. 2017, 225, S68. [Google Scholar] [CrossRef][Green Version]
- Stuart, E.A. Matching methods for causal inference: A review and a look forward. Stat. Sci. 2010, 25, 1–21. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Nassar, M.; Abosheaishaa, H.; Elfert, K.; Beran, A.; Ismail, A.; Mohamed, M.; Misra, A.; Essibayi, M.A.; Altschul, D.J.; Azzam, A.Y. TriNetX and Real-World Evidence: A Critical Review of Its Strengths, Limitations, and Bias Considerations in Clinical Research. ASIDE Intern. Med. 2025, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.S.; Konuthula, N.; Lam, A.; Massa, S.; Rizvi, Z.H. Risks Associated With Extent of Surgical Management for Benign, Non-Toxic Goiter. Laryngoscope Investig. Otolaryngol. 2025, 10, e70214. [Google Scholar] [CrossRef]
- Ramos-Gonzalez, G.J.; Canas, J.A.; Green, A.; Chandler, N.M.; Snyder, C.W. Predictors, Trends, and Outcomes of Parathyroid Autotransplantation in Pediatric Total Thyroidectomy. J. Surg. Res. 2025, 306, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Pace-Asciak, P.; Tufano, R.P. Editorial: Recent advances in thermal and nonthermal ablative technologies of the thyroid. Front. Endocrinol. 2025, 16, 1601452. [Google Scholar] [CrossRef]
- Al-Qahtani, K.; Al Shahrani, M.; Al Zahrani, F.; Al Ghamdi, A.; Al Alghamdi, F.; Al Alshaalan, Z.; Al-Saif, A.; Bokhari, A.; Al-Abdulkarim, A.A.; Islam, T. Comparing Thyroidectomy Techniques, Surgical Loupe and Neuromonitoring Between ENT and Endocrine Surgeons—An Observational Study. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 1618–1624. [Google Scholar] [CrossRef]
- Rosato, L.; Avenia, N.; Bernante, P.; De Palma, M.; Gulino, G.; Nasi, P.G.; Pelizzo, M.R.; Pezzullo, L. Complications of thyroid surgery: Analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J. Surg. 2004, 28, 271–276. [Google Scholar] [CrossRef]
- Bergenfelz, A.; Jansson, S.; Kristoffersson, A.; Martensson, H.; Reihner, E.; Wallin, G.; Lausen, I. Complications to thyroid surgery: Results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbeck’s Arch. Surg. 2008, 393, 667–673. [Google Scholar] [CrossRef]
- Christou, N.; Mathonnet, M. Complications after total thyroidectomy. J. Visc. Surg. 2013, 150, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Sosa, J.A.; Bowman, H.M.; Tielsch, J.M.; Powe, N.R.; Gordon, T.A.; Udelsman, R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann. Surg. 1998, 228, 320–330. [Google Scholar] [CrossRef]
- Adam, M.A.; Thomas, S.; Youngwirth, L.; Hyslop, T.; Reed, S.D.; Scheri, R.P.; Roman, S.A.; Sosa, J.A. Is There a Minimum Number of Thyroidectomies a Surgeon Should Perform to Optimize Patient Outcomes? Ann. Surg. 2017, 265, 402–407. [Google Scholar] [CrossRef]
- Haymart, M.R.; Banerjee, M.; Stewart, A.K.; Koenig, R.J.; Birkmeyer, J.D.; Griggs, J.J. Use of radioactive iodine for thyroid cancer. JAMA 2011, 306, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Gupta, S.; Agarwal, A. Focused Parathyroidectomy Using Accurate Preoperative Imaging and Intraoperative PTH: Tertiary Care Experience. Indian J. Endocrinol. Metab. 2019, 23, 347–352. [Google Scholar] [CrossRef]
- Mirallie, E.; Borel, F.; Tresallet, C.; Hamy, A.; Mathonnet, M.; Lifante, J.C.; Brunaud, L.; Menegaux, F.; Hardouin, J.B.; Blanchard, C.; et al. Impact of total thyroidectomy on quality of life at 6 months: The prospective ThyrQoL multicentre trial. Eur. J. Endocrinol. 2020, 182, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Xue, S.; Wang, P.; Hurst, Z.A.; Chang, Y.S.; Chen, G. Active Surveillance for Papillary Thyroid Microcarcinoma: Challenges and Prospects. Front. Endocrinol. 2018, 9, 736. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Kihara, M.; Higashiyama, T.; Kobayashi, K.; Miya, A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.X.; Zhang, W.; Jiang, D.Z.; Zheng, X.M.; Liu, S.; Qiu, M. Routine central neck dissection in differentiated thyroid carcinoma: A systematic review and meta-analysis. Laryngoscope 2012, 122, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Nixon, I.J.; Ganly, I.; Shah, J.P. Thyroid cancer: Surgery for the primary tumor. Oral Oncol. 2013, 49, 654–658. [Google Scholar] [CrossRef]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; The ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Dionigi, G.; Boni, L.; Rovera, F.; Bacuzzi, A.; Dionigi, R. Neuromonitoring and video-assisted thyroidectomy: A prospective, randomized case-control evaluation. Surg. Endosc. 2009, 23, 996–1003. [Google Scholar] [CrossRef]
- Peyser Cardoso, R.; Agarwal, L.; Cardoso, S.A.; Agarwal, A.; Varshney, V.; Soni, S.; Selvakumar, B.; Varshney, P. Impact of intraoperative recurrent laryngeal nerve monitoring on minimally invasive esophagectomy outcomes for esophageal cancer: A meta-analysis of case-control studies. Dis. Esophagus 2025, 38, doae116. [Google Scholar] [CrossRef]
- Bedi, H.K.; Jedrzejko, N.; Nguyen, A.; Aspinall, S.R.; Wiseman, S.M. Thyroid and parathyroid surgeon case volume influences patient outcomes: A systematic review. Surg. Oncol. 2021, 38, 101550. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.K.; Aspinall, S.; Tolley, N.; Day, J.; Lansdown, M. The volume and outcome relationship for thyroidectomy in England. Langenbeck’s Arch. Surg. 2021, 406, 1999–2010. [Google Scholar] [CrossRef]
- Hauch, A.; Al-Qurayshi, Z.; Randolph, G.; Kandil, E. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann. Surg. Oncol. 2014, 21, 3844–3852. [Google Scholar] [CrossRef] [PubMed]
- Gorbea, E.; Goldrich, D.Y.; Agarwal, J.; Nayak, R.; Iloreta, A.M. The impact of surgeon volume on total thyroidectomy outcomes among otolaryngologists. Am. J. Otolaryngol. 2020, 41, 102726. [Google Scholar] [CrossRef]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J., 3rd; et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann. Surg. 2020, 271, e21–e93. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.J.; Galofre, J.C. Thyroid Cancer Patients’ View of Clinician Professionalism and Multidisciplinary Approach to Their Management. J. Multidiscip. Healthc. 2021, 14, 1053–1061. [Google Scholar] [CrossRef]
- Kelley, S.; Beck, A.C.; Weigel, R.J.; Howe, J.R.; Sugg, S.L.; Lal, G. Influence of endocrine multidisciplinary tumor board on patient management and treatment decision making. Am. J. Surg. 2022, 223, 76–80. [Google Scholar] [CrossRef]
- Alansari, A.N.; Zaazouee, M.S.; Najar, S.; Elshanbary, A.A. Quality-of-Life Outcomes Following Thyroid Surgery in Pediatric Patients: A Systematic Review of Physical, Emotional, and Social Dimensions. Children 2025, 12, 891. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I. Thyroid carcinoma. Lancet 2003, 361, 501–511. [Google Scholar] [CrossRef] [PubMed]
| Thyroid Surgery | Control | p-Value | Std Diff. | |||
|---|---|---|---|---|---|---|
| (N = 49,219) | (N = 49,219) | |||||
| Patients | % | Patients | % | |||
| Demographics | ||||||
| Age at index (Mean ± SD) | 51.6 ± 15.1 | 51.8 ± 15.3 | 0.125 | 0.01 | ||
| Female | 36,507 | 74.20% | 36,430 | 74.00% | 0.575 | 0.004 |
| Male | 12,712 | 25.80% | 12,789 | 26.00% | 0.575 | 0.004 |
| Race | ||||||
| White | 28,906 | 58.70% | 29,502 | 59.90% | <0.001 | 0.025 |
| Black or African American | 3224 | 6.60% | 3169 | 6.40% | 0.477 | 0.005 |
| Asian | 3591 | 7.30% | 3683 | 7.50% | 0.262 | 0.007 |
| Unknown race | 3791 | 7.70% | 3030 | 6.20% | <0.001 | 0.061 |
| Ethnicity | ||||||
| Hispanic or Latino | 3291 | 6.70% | 3317 | 6.70% | 0.741 | 0.002 |
| Not Hispanic or Latino | 27,523 | 55.90% | 27,528 | 55.90% | 0.974 | <0.001 |
| Unknown ethnicity | 18,405 | 37.40% | 18,374 | 37.30% | 0.838 | 0.001 |
| Diagnosis | ||||||
| Cardiovascular diseases | 13,516 | 27.50% | 13,654 | 27.70% | 0.325 | 0.006 |
| Osteoporosis or bone disorders | 1902 | 3.90% | 1911 | 3.90% | 0.882 | 0.001 |
| Hypothyroidism | 5493 | 11.20% | 5463 | 11.10% | 0.761 | 0.002 |
| Hyperthyroidism | 1600 | 3.30% | 1536 | 3.10% | 0.245 | 0.007 |
| Metabolic syndrome | 143 | 0.30% | 142 | 0.30% | 0.953 | <0.001 |
| Diabetes | 4649 | 9.40% | 4697 | 9.50% | 0.602 | 0.003 |
| Immune-related disorders | 49 | 0.10% | 52 | 0.10% | 0.765 | 0.002 |
| Hypertension | 10,397 | 21.10% | 10,555 | 21.40% | 0.219 | 0.008 |
| Breast cancer | 1102 | 2.20% | 1128 | 2.30% | 0.578 | 0.004 |
| Vocal cord paralysis | 740 | 1.50% | 708 | 1.40% | 0.397 | 0.005 |
| Hypoparathyroidism | 304 | 0.60% | 282 | 0.60% | 0.362 | 0.006 |
| Procedure | ||||||
| Oral radiopharmaceutical therapy | 278 | 0.60% | 276 | 0.60% | 0.932 | 0.001 |
| Intra-articular injection (percutaneous) | 20 | 0.00% | 18 | 0.00% | 0.746 | 0.002 |
| Medication | ||||||
| Levothyroxine | 11,030 | 22.40% | 11,284 | 22.90% | 0.053 | 0.012 |
| Sorafenib | 10 | 0.00% | 14 | 0.00% | 0.414 | 0.005 |
| Cabozantinib | 10 | 0.00% | 12 | 0.00% | 0.670 | 0.003 |
| Vandetanib | 10 | 0.00% | 10 | 0.00% | 1.000 | <0.001 |
| Platinum compounds | 279 | 0.60% | 273 | 0.60% | 0.798 | 0.002 |
| Monoclonal antibodies | 332 | 0.70% | 345 | 0.70% | 0.616 | 0.003 |
| Methylhydrazines | 10 | 0.00% | 10 | 0.00% | 1.000 | <0.001 |
| Pembrolizumab | 67 | 0.10% | 71 | 0.10% | 0.733 | 0.002 |
| Laboratory (Mean ± SD) | ||||||
| TSH (thyroid stimulating hormone) | 4.2 ± 24.2 | 8.3 ± 33.3 | <0.001 | 0.141 | ||
| Free T4 (free thyroxine) | 1.3 ± 0.8 | 1.3 ± 0.7 | <0.001 | 0.074 | ||
| Serum calcium | 9.3 ± 0.8 | 9.2 ± 0.9 | <0.001 | 0.087 | ||
| Serum creatinine | 1.0 ± 2.0 | 1.0 ± 2.2 | 0.109 | 0.015 | ||
| eGFR (estimated GFR, MDRD) | 83.2 ± 26.6 | 82.2 ± 29.2 | <0.001 | 0.036 | ||
| Serum albumin | 4.1 ± 0.5 | 4.1 ± 0.5 | <0.001 | 0.133 | ||
| Hemoglobin | 13.2 ± 1.8 | 13.0 ± 1.9 | <0.001 | 0.157 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.-W.; Chang, M.-H.; Lin, L.; Lin, F.C.-F.; Chen, S.-W.; Kuan, Y.-H.; Tsai, P.-C.; Yu, J.-K.; Tsai, S.C.-S. Long-Term Survival After Thyroidectomy for Thyroid Cancer: A Propensity-Matched TriNetX Study with Specialty-Stratified Analyses. Cancers 2025, 17, 3051. https://doi.org/10.3390/cancers17183051
Luo C-W, Chang M-H, Lin L, Lin FC-F, Chen S-W, Kuan Y-H, Tsai P-C, Yu J-K, Tsai SC-S. Long-Term Survival After Thyroidectomy for Thyroid Cancer: A Propensity-Matched TriNetX Study with Specialty-Stratified Analyses. Cancers. 2025; 17(18):3051. https://doi.org/10.3390/cancers17183051
Chicago/Turabian StyleLuo, Ci-Wen, Meng-Hao Chang, Lan Lin, Frank Cheau-Feng Lin, Shih-Wei Chen, Yu-Hsiang Kuan, Pei-Chi Tsai, Ji-Kuen Yu, and Stella Chin-Shaw Tsai. 2025. "Long-Term Survival After Thyroidectomy for Thyroid Cancer: A Propensity-Matched TriNetX Study with Specialty-Stratified Analyses" Cancers 17, no. 18: 3051. https://doi.org/10.3390/cancers17183051
APA StyleLuo, C.-W., Chang, M.-H., Lin, L., Lin, F. C.-F., Chen, S.-W., Kuan, Y.-H., Tsai, P.-C., Yu, J.-K., & Tsai, S. C.-S. (2025). Long-Term Survival After Thyroidectomy for Thyroid Cancer: A Propensity-Matched TriNetX Study with Specialty-Stratified Analyses. Cancers, 17(18), 3051. https://doi.org/10.3390/cancers17183051










