Latest Advancements and Future Directions in Prostate Cancer Surgery: Reducing Invasiveness and Expanding Indications
Simple Summary
Abstract
1. Introduction
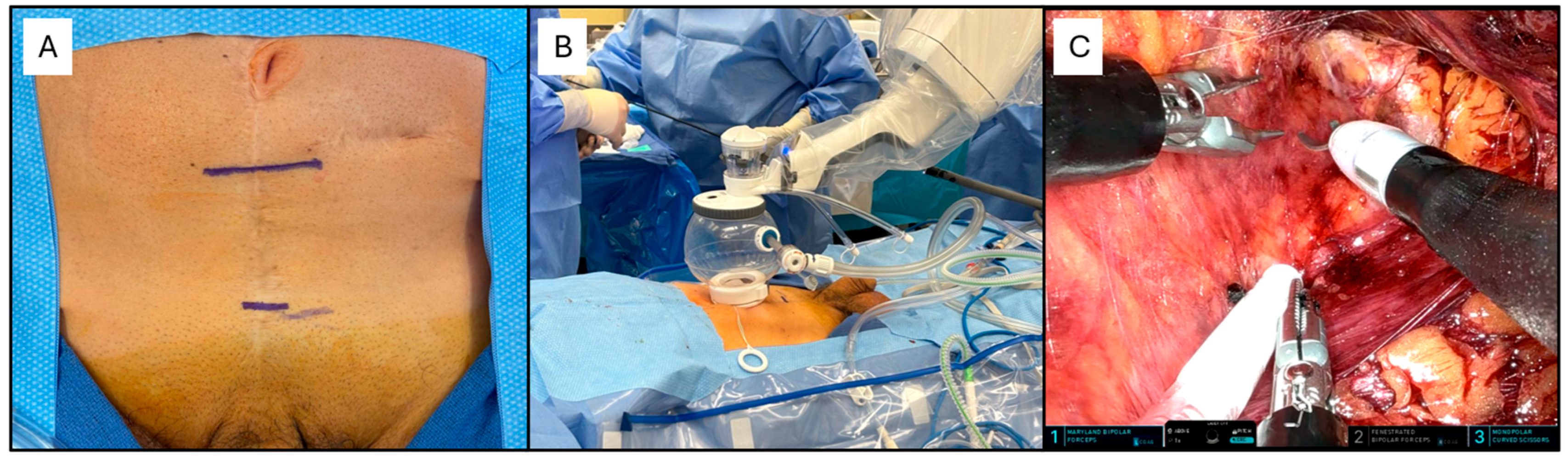
2. Single Port Robotic-Assisted Radical Prostatectomy
2.1. The Procedure
2.2. Literature Synthesis
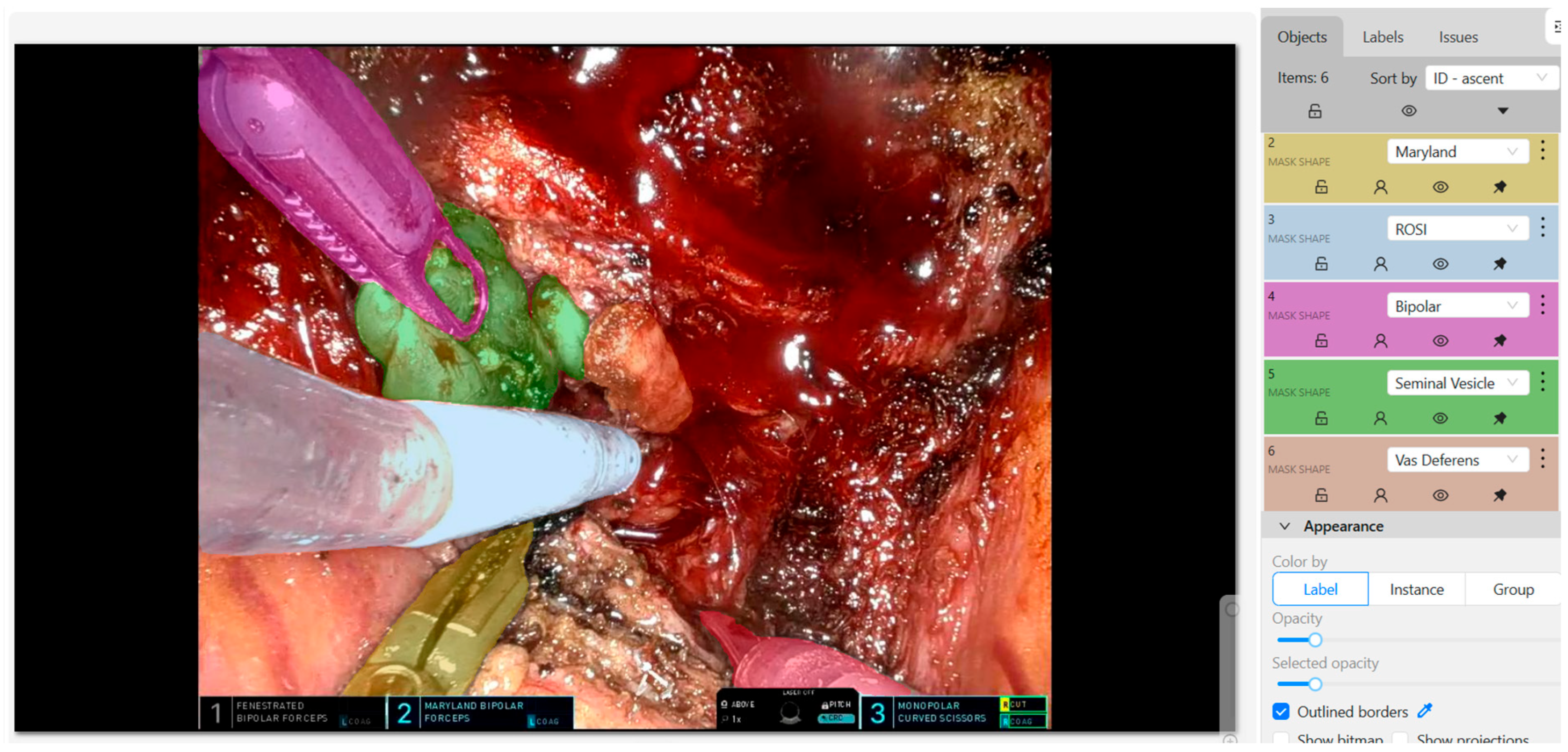
3. Role of Artificial Intelligence (AI) in Prostate Cancer Surgery
3.1. Key Surgical Steps Identification and Error Feedback
3.2. Recognition of Relevant Anatomy
3.3. Future Directions: Interdisciplinary/Interinstitutional Collaborations and Integration in the Robotic Console
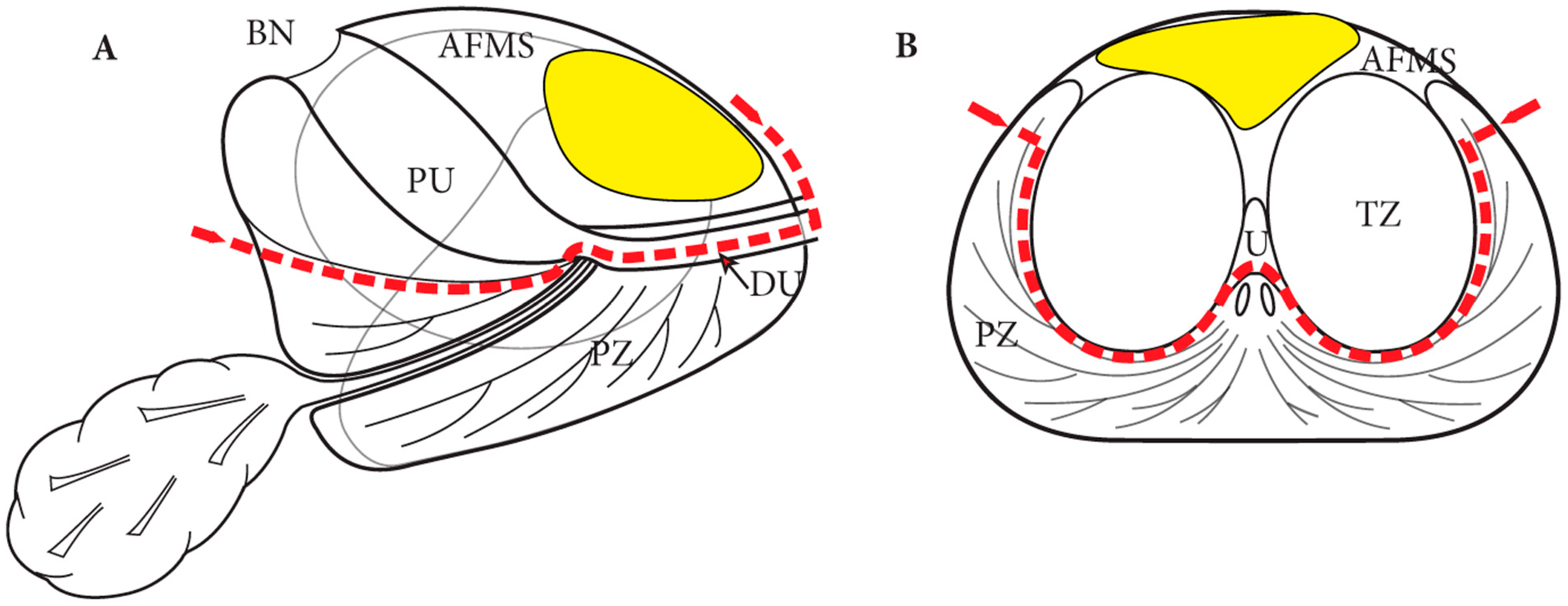
4. Partial Prostatectomy
4.1. Anterior Prostatectomy and Precision Prostatectomy
4.2. Single Port Transvesical Partial Prostatectomy
4.3. Future Prospectives
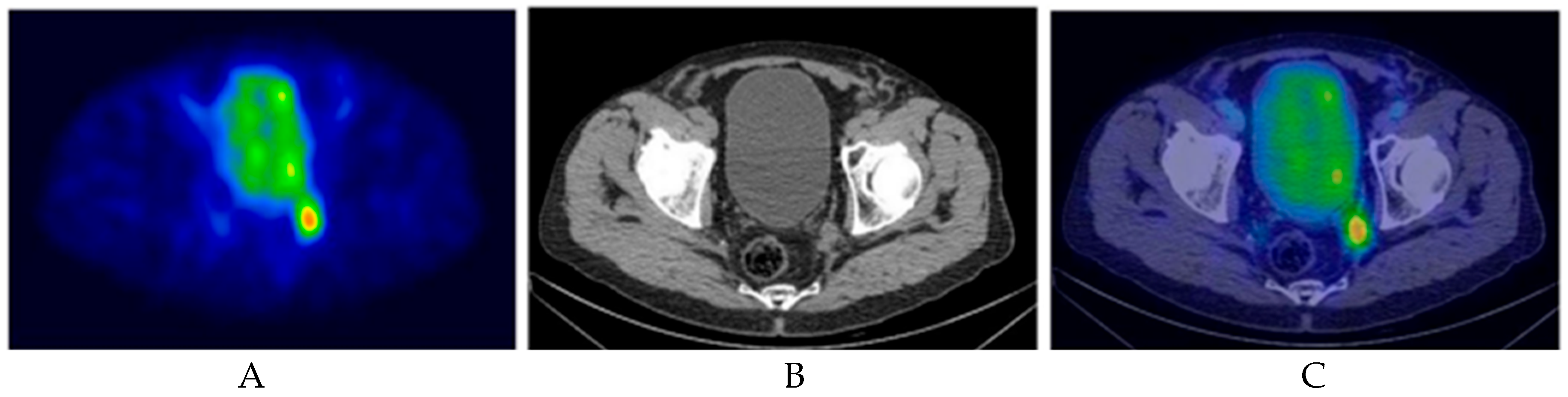
5. Radioguided Surgery (RGS)
5.1. Sentinel Node Biopsy (SNB) for Primary Staging
5.2. PSMA-RGS
5.2.1. Recurrent Prostate Cancer Setting
5.2.2. Nodal Staging During RARP with ePLND Setting
5.3. Fluorescence Guided Surgery (FGS) Using PSMA-Based Tracers
6. Role of Targeting Primary Tumor in Oligometastatic PCa
6.1. Current Standard of Care (SOC) and Based on Treatment Strategies
6.1.1. Systemic Therapy
6.1.2. Cytoreduction
6.2. Radical Prostatectomy in OMPC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Achard, V.; Panje, C.M.; Engeler, D.; Zilli, T.; Putora, P.M. Localized and Locally Advanced Prostate Cancer: Treatment Options. Oncology 2021, 99, 413–421. [Google Scholar] [CrossRef]
- Herranz-Amo, F. Radical retropubic prostatectomy: Origins and evolution of the technique. Actas Urol. Esp. (Engl. Ed.) 2020, 44, 408–416. [Google Scholar] [CrossRef]
- Hammad, F.T. Radical prostatectomy. Ann. N. Y. Acad. Sci. 2008, 1138, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Redondo, C.; Rozet, F.; Velilla, G.; Sánchez-Salas, R.; Cathelineau, X. Complications of radical prostatectomy. Arch. Esp. Urol. 2017, 70, 766–776. [Google Scholar]
- Wang, J.; Hu, K.; Wang, Y.; Wu, Y.; Bao, E.; Wang, J. Robot-assisted versus open radical prostatectomy: A systematic review and meta-analysis of prospective studies. J. Robot. Surg. 2023, 17, 2617–2631. [Google Scholar] [CrossRef]
- Bhayani, S.B.; Pavlovich, C.P.; Strup, S.E.; Dahl, D.M.; Landman, J.; Fabrizio, M.D. Laparoscopic radical prostatectomy: A multi-institutional study of conversion to open surgery. Urology 2004, 63, 99–102. [Google Scholar] [CrossRef]
- Stolzenburg, J.-U.; Holze, S.; Arthanareeswaran, V.-K.-A.; Neuhaus, P.; Do, H.M.; Haney, C.M. Robotic-assisted Versus Laparoscopic Radical Prostatectomy: 12-month Outcomes of the Multicentre Randomised Controlled LAP-01 Trial. Eur. Urol. Focus 2022, 8, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, W.; Chen, R.; Zhu, Y.; Wang, Y.; Cao, W.; Ju, G.; Ren, J.; Ye, X.; He, Q.; et al. Robotic-assisted versus laparoscopic radical prostatectomy for prostate cancer: The first separate systematic review and meta-analysis of randomised controlled trials and non-randomised studies. Int. J. Surg. 2023, 109, 1350–1359. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; Mottrie, A.; Mazzone, E. Robot-assisted radical prostatectomy vs. open radical prostatectomy: Latest evidences on perioperative, functional and oncological outcomes. Curr. Opin. Urol. 2020, 30, 73–78. [Google Scholar] [CrossRef]
- Gacci, M.; Artibani, W.; Bassi, P.; Bertoni, F.; Bracarda, S.; Briganti, A.; Carmignani, G.; Carmignani, L.; Conti, G.; Corvò, R.; et al. How radical prostatectomy procedures have changed over the last 10 years in Italy: A comparative analysis based on more than 1500 patients participating in the MIRROR-SIU/LUNA and the Pros-IT CNR study. World J. Urol. 2021, 39, 1445–1452. [Google Scholar] [CrossRef]
- Salciccia, S.; Santarelli, V.; Di Pierro, G.B.; Del Giudice, F.; Bevilacqua, G.; Di Lascio, G.; Gentilucci, A.; Corvino, R.; Brunelli, V.; Basile, G.; et al. Real-Life Comparative Analysis of Robotic-Assisted Versus Laparoscopic Radical Prostatectomy in a Single Centre Experience. Cancers 2024, 16, 3604. [Google Scholar] [CrossRef]
- Santarelli, V.; Carino, D.; Corvino, R.; Salciccia, S.; De Berardinis, E.; Krajewski, W.; Nowak, Ł.; Łaszkiewicz, J.; Szydełko, T.; Nair, R.; et al. Surgical technique and perioperative outcomes of the “Sapienza” urology residency program’s trocar placement configuration during robotic-assisted radical prostatectomy (RARP): A retrospective, single-centre observational study comparing experienced attendings vs. Post-graduate Year I-III residents as bedside assistants. Cancers 2025, 17, 20. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Zheng, X.; Wang, X. Comparison of perioperative, functional, and oncologic outcomes between standard laparoscopic and robotic-assisted radical prostatectomy: A systemic review and meta-analysis. Surg. Endosc. 2017, 31, 1045–1060. [Google Scholar] [CrossRef]
- Lepor, H. A review of surgical techniques for radical prostatectomy. Rev. Urol. 2005, 7 (Suppl. S2), S11–S17. [Google Scholar]
- Walsh, P.C. Anatomic radical prostatectomy: Evolution of the surgical technique. J. Urol. 1998, 160, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Soeterik, T.F.W.; van Melick, H.H.E.; Dijksman, L.M.; Stomps, S.; Witjes, J.A.; van Basten, J.P.A. Nerve Sparing during Robot-Assisted Radical Prostatectomy Increases the Risk of Ipsilateral Positive Surgical Margins. J. Urol. 2020, 204, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Reale, G.; Castelli, T.; Favilla, V.; Giardina, R.; Russo, G.I.; Privitera, S.; Morgia, G. Comparison between Briganti, Partin and MSKCC tools in predicting positive lymph nodes in prostate cancer: A systematic review and meta-analysis. Scand. J. Urol. 2017, 51, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Lucciola, S.; Pisciotti, M.L.; Frisenda, M.; Magliocca, F.; Gentilucci, A.; Del Giudice, F.; Canale, V.; Scarrone, E.; Busetto, G.M.; Carrieri, G.; et al. Predictive role of node-rads score in patients with prostate cancer candidates for radical prostatectomy with extended lymph node dissection: Comparative analysis with validated nomograms. Prostate Cancer Prostatic Dis. 2023, 26, 379–387. [Google Scholar] [CrossRef]
- Cornford, P.; Bergh, R.C.v.D.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Agarwal, D.K.; Sharma, V.; Toussi, A.; Viers, B.R.; Tollefson, M.K.; Gettman, M.T.; Frank, I. Initial Experience with da Vinci Single-port Robot-assisted Radical Prostatectomies. Eur. Urol. 2020, 77, 373–379. [Google Scholar] [CrossRef]
- Koukourikis, P.; Alqahtani, A.A.; Han, W.K.; Rha, K.H. Pure single-port retzius-sparing robot-assisted radical prostatectomy with the da Vinci SP: Initial experience and technique description. BJUI Compass 2022, 3, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Lenfant, L.; Garisto, J.; Sawczyn, G.; Wilson, C.A.; Aminsharifi, A.; Kim, S.; Schwen, Z.; Bertolo, R.; Kaouk, J. Robot-assisted Radical Prostatectomy Using Single-port Perineal Approach: Technique and Single-surgeon Matched-paired Comparative Outcomes. Eur. Urol. 2021, 79, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Nathan, S.; Pavan, N.; Gu, D.; Sridhar, A.; Autorino, R. Robot-Assisted Radical Prostatectomy: Advanced Surgical Techniques; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- Kaouk, J.; Valero, R.; Sawczyn, G.; Garisto, J. Extraperitoneal single-port robot-assisted radical prostatectomy: Initial experience and description of technique. BJU Int. 2020, 125, 182–189. [Google Scholar] [CrossRef]
- Dobbs, R.W.; Halgrimson, W.R.; Madueke, I.; Vigneswaran, H.T.; Wilson, J.O.; Crivellaro, S. Single-port robot-assisted laparoscopic radical prostatectomy: Initial experience and technique with the da Vinci SP platform. BJU Int. 2019, 124, 1022–1027. [Google Scholar]
- Zeinab, M.A.; Beksac, A.T.; Ferguson, E.; Kaviani, A.; Moschovas, M.C.; Joseph, J.; Kim, M.; Crivellaro, S.; Nix, J.; Patel, V.; et al. Single-port Extraperitoneal and Transperitoneal Radical Prostatectomy: A Multi-Institutional Propensity-Score Matched Study. Urology 2023, 171, 140–145. [Google Scholar]
- Jiang, Y.; Liu, Y.; Qin, S.; Zhong, S.; Huang, X. Perioperative, function, and positive surgical margin in extraperitoneal versus transperitoneal single port robot-assisted radical prostatectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2023, 21, 383. [Google Scholar] [CrossRef]
- Yuan, J.; He, Q.; Zheng, Y.; Lv, Q.; Hu, X.; Wang, D.; Tian, J.; Ren, S. Early outcomes of single-site versus multi-port robotic-assisted radical prostatectomy: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2024, 50, 107263. [Google Scholar] [CrossRef]
- Pellegrino, A.A.; Pellegrino, F.; Cannoletta, D.; Calvo, R.S.; Anguiano, J.T.; Morgantini, L.; Briganti, A.; Montorsi, F.; Crivellaro, S. Learning Curve for Single-port Robot-assisted Urological Surgery: Single-center Experience and Implications for Adoption. Eur. Urol. Focus 2025, 11, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, V.; Valenzi, F.M.; Aljoulani, M.; Haberal, H.B.; Morgantini, L.A.; Biasatti, A.; Salciccia, S.; Di Pierro, G.B.; Franco, G.; Autorino, R.; et al. Learning Curve of Single-Port Robotic-Assisted Extraperitoneal Radical Prostatectomy: A CUSUM-Based Analysis. J. Laparoendosc. Adv. Surg. Tech. A 2025, 35, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Kaul, V.; Enslin, S.; Gross, S.A. History of artificial intelligence in medicine. Gastrointest. Endosc. 2020, 92, 807–812. [Google Scholar] [CrossRef]
- Abraham, A.; Jose, R.; Ahmad, J.; Joshi, J.; Jacob, T.; Khalid, A.-U.; Ali, H.; Patel, P.; Singh, J.; Toma, M. Comparative Analysis of Machine Learning Models for Image Detection of Colonic Polyps vs. Resected Polyps. J. Imaging 2023, 9, 215. [Google Scholar] [CrossRef]
- Aydin, F.; Yildirim, Ö.T.; Aydin, A.H.; Murat, B.; Basaran, C.H. Comparison of artificial intelligence-assisted informed consent obtained before coronary angiography with the conventional method: Medical competence and ethical assessment. Digit. Health 2023, 9, 20552076231218141. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Antolin, A.; Bar, O.; Ben-Ayoun, D.; Zohar, M.; Boorjian, S.A.; Frank, I.; Shah, P.; Sharma, V.; Thompson, R.H.; et al. Automated Identification of Key Steps in Robotic-Assisted Radical Prostatectomy Using Artificial Intelligence. J. Urol. 2024, 211, 575–584. [Google Scholar] [CrossRef]
- Sirajudeen, N.; Boal, M.; Anastasiou, D.; Xu, J.; Stoyanov, D.; Kelly, J.; Collins, J.W.; Sridhar, A.; Mazomenos, E.; Francis, N.K. Deep learning prediction of error and skill in robotic prostatectomy suturing. Surg. Endosc. 2024, 38, 7663–7671. [Google Scholar] [CrossRef] [PubMed]
- OpenCV. Computer Vision Annotation Tool (CVAT) [Computer Software]. GitHub. 2024. Available online: https://github.com/opencv/cvat (accessed on 1 June 2025).
- Beser, B.; Reis, T.; Berber, M.N.; Topaloglu, E.; Gungor, E.; Kılıc, M.C.; Duman, S.; Çelik, Ö.; Kuran, A.; Bayrakdar, I.S. YOLO-V5 based deep learning approach for tooth detection and segmentation on pediatric panoramic radiographs in mixed dentition. BMC Med. Imaging 2024, 24, 172. [Google Scholar] [CrossRef]
- Yang, T.; Lu, X.; Yang, L.; Yang, M.; Chen, J.; Zhao, H. Application of MRI image segmentation algorithm for brain tumors based on improved YOLO. Front. Neurosci. 2024, 18, 1510175. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Park, J.; Choi, H.R.; Lee, J.H.; Cho, S.T.; Lee, Y.G.; Ahn, H.; Pak, S. Deep Learning Model for Real-time Semantic Segmentation During Intraoperative Robotic Prostatectomy. Eur. Urol. Open Sci. 2024, 62, 47–53. [Google Scholar] [CrossRef]
- Takahashi, S.; Sakaguchi, Y.; Kouno, N.; Takasawa, K.; Ishizu, K.; Akagi, Y.; Aoyama, R.; Teraya, N.; Bolatkan, A.; Shinkai, N.; et al. Comparison of Vision Transformers and Convolutional Neural Networks in Medical Image Analysis: A Systematic Review. J. Med. Syst. 2024, 48, 84. [Google Scholar] [CrossRef]
- Waqas, M.; Humphries, U.W. A critical review of RNN and LSTM variants in hydrological time series predictions. MethodsX 2024, 13, 102946. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.intuitive.com/en-us/products-and-services/da-vinci/5 (accessed on 9 June 2025).
- Bratu, O.; Oprea, I.; Marcu, D.; Spinu, D.; Niculae, A.; Geavlete, B.; Mischianu, D. Erectile dysfunction post-radical prostatectomy—A challenge for both patient and physician. J. Med. Life 2017, 10, 13–18. [Google Scholar]
- Geraghty, K.; Keane, K.; Davis, N. Systematic review on urinary continence rates after robot-assisted laparoscopic radical prostatectomy. Ir. J. Med. Sci. 2024, 193, 1603–1612. [Google Scholar] [CrossRef]
- Gacci, M.; De Nunzio, C.; Sakalis, V.; Rieken, M.; Cornu, J.-N.; Gravas, S. Latest Evidence on Post-Prostatectomy Urinary Incontinence. J. Clin. Med. 2023, 12, 1190. [Google Scholar] [CrossRef]
- van Velthoven, R.; Aoun, F.; Marcelis, Q.; Albisinni, S.; Zanaty, M.; Lemort, M.; Peltier, A.; Limani, K. A prospective clinical trial of HIFU hemiablation for clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2016, 19, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Polascik, T.J.; de la Rosette, J.; Sanchez-Salas, R.; Rastinehad, A.R. Imaging and Focal Therapy of Early Prostate Cancer; Springer Nature: Cham, Switzerland, 2024. [Google Scholar]
- Guillaumier, S.; Peters, M.; Arya, M.; Afzal, N.; Charman, S.; Dudderidge, T.; Hosking-Jervis, F.; Hindley, R.G.; Lewi, H.; McCartan, N.; et al. A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur. Urol. 2018, 74, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Bass, R.; Fleshner, N.; Finelli, A.; Barkin, J.; Zhang, L.; Klotz, L. Oncologic and Functional Outcomes of Partial Gland Ablation with High Intensity Focused Ultrasound for Localized Prostate Cancer. J. Urol. 2019, 201, 113–119. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Long, X.; Wang, T.; Wang, Z.; Yang, C.; Wang, S. Partial prostatectomy for localized prostate cancer. World J. Urol. 2024, 42, 543. [Google Scholar] [CrossRef]
- Sivaraman, A.; Barret, E. Focal Therapy for Prostate Cancer: An “À la Carte” Approach. Eur. Urol. 2016, 69, 973–975. [Google Scholar] [CrossRef]
- Huber, P.M.; Afzal, N.; Arya, M.; Boxler, S.; Dudderidge, T.; Emberton, M.; Guillaumier, S.; Hindley, R.G.; Hosking-Jervis, F.; Leemann, L.; et al. Focal HIFU therapy for anterior compared to posterior prostate cancer lesions. World J. Urol. 2021, 39, 1115–1119. [Google Scholar] [CrossRef]
- Villers, A.; Flamand, V.; Arquímedes, R.-C.; Puech, P.; Haber, G.-P.; Desai, M.M.; Crouzet, S.; Ouzzane, A.; Gill, I.S. Robot-assisted partial prostatectomy for anterior prostate cancer: A step-by-step guide. BJU Int. 2017, 119, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Villers, A.; Seguier, D.; Puech, P.; Haber, G.-P.; Desai, M.M.; Crouzet, S.; Leroy, X.; Labreuche, J.; Gill, I.S.; Olivier, J. Robot Partial Prostatectomy for Anterior Cancer: Long-term Functional and Oncological Outcomes at 7 Years. Eur. Urol. Open Sci. 2023, 55, 11–14. [Google Scholar] [CrossRef]
- Sood, A.; Jeong, W.; Taneja, K.; Abdollah, F.; Palma-Zamora, I.; Arora, S.; Gupta, N.; Menon, M. The Precision Prostatectomy: An IDEAL Stage 0, 1 and 2a Study. BMJ Surg. Interv. Health Technol. 2019, 1, e000002. [Google Scholar] [CrossRef]
- Sood, A.; Jeong, W.; Palma-Zamora, I.; Abdollah, F.; Butaney, M.; Corsi, N.; Wurst, H.; Arora, S.; Kachroo, N.; Hassan, O.; et al. Description of Surgical Technique and Oncologic and Functional Outcomes of the Precision Prostatectomy Procedure (IDEAL Stage 1-2b Study). Eur. Urol. 2022, 81, 396–406. [Google Scholar] [CrossRef]
- Zeinab, M.A.; Kaviani, A.; Ferguson, E.; Beksac, A.T.; Kaouk, J. Single-port transvesical robotic radical prostatectomy: Description of technique. Urol. Video J. 2022, 15, 100172. [Google Scholar] [CrossRef]
- Kaouk, J.H.; Ferguson, E.L.; Beksac, A.T.; Zeinab, M.A.; Kaviani, A.; Weight, C.; Haywood, S.; Eltemamy, M.; Purysko, A.; McKenney, J.K.; et al. Single-port Robotic Transvesical Partial Prostatectomy for Localized Prostate Cancer: Initial Series and Description of Technique. Eur. Urol. 2022, 82, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, A.M.; Ferguson, E.L.; Ramos-Carpinteyro, R.; Soputro, N.; Chavali, J.S.; Mikesell, C.; Nguyen, J.K.; Kaouk, J. Single-Port Robotic Transvesical Partial Prostatectomy: A Novel Approach for Focal Treatment in Prostate Cancer. J. Endourol. 2025, 39, 261–270. [Google Scholar] [CrossRef]
- Quarta, L.; Cannoletta, D.; Pellegrino, F.; Barletta, F.; Scuderi, S.; Mazzone, E.; Stabile, A.; Montorsi, F.; Gandaglia, G.; Briganti, A. The Role of Robot-Assisted, Imaging-Guided Surgery in Prostate Cancer Patients. Cancers 2025, 17, 1401. [Google Scholar] [CrossRef]
- Mazzone, E.; Dell’oGlio, P.; Grivas, N.; Wit, E.; Donswijk, M.; Briganti, A.; Van Leeuwen, F.; van der Poel, H. Diagnostic Value, Oncologic Outcomes, and Safety Profile of Image-Guided Surgery Technologies During Robot-Assisted Lymph Node Dissection with Sentinel Node Biopsy for Prostate Cancer. J. Nucl. Med. 2021, 62, 1363–1371. [Google Scholar] [CrossRef]
- Lannes, F.; Baboudjian, M.; Ruffion, A.; Rouy, M.; Giammarile, F.; Rousseau, T.; Kraeber-Bodéré, F.; Rousseau, C.; Rusu, D.; Colombié, M.; et al. Radioisotope-guided Lymphadenectomy for Pelvic Lymph Node Staging in Patients With Intermediate- and High-risk Prostate Cancer (The Prospective SENTINELLE Study). J. Urol. 2023, 209, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Quarta, L.; Stabile, A.; Chiti, A.; Montorsi, F.; Briganti, A.; Gandaglia, G. Radioguided Surgery for Prostate Cancer. Eur. Urol. Focus 2025, 11, 29–32. [Google Scholar] [CrossRef]
- Quarta, L.; Mazzone, E.; Cannoletta, D.; Stabile, A.; Scuderi, S.; Barletta, F.; Cucchiara, V.; Nocera, L.; Pellegrino, A.; Robesti, D.; et al. Defining the optimal target-to-background ratio to identify positive lymph nodes in prostate cancer patients undergoing robot-assisted [Tc]Tc-PSMA radioguided surgery: Updated results and ad interim analyses of a prospective phase II study. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3789–3798. [Google Scholar] [CrossRef]
- Sciarra, A.; Santarelli, V.; Salciccia, S.; Moriconi, M.; Basile, G.; Santodirocco, L.; Carino, D.; Frisenda, M.; Di Pierro, G.; Del Giudice, F.; et al. How the Management of Biochemical Recurrence in Prostate Cancer Will Be Modified by the Concept of Anticipation and Incrementation of Therapy. Cancers 2024, 16, 764. [Google Scholar] [CrossRef]
- Bravi, C.A.; Fossati, N.; Gandaglia, G.; Suardi, N.; Mazzone, E.; Robesti, D.; Osmonov, D.; Juenemann, K.-P.; Boeri, L.; Karnes, R.J.; et al. Long-term Outcomes of Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer After Radical Prostatectomy: Not as Good as Previously Thought. Eur. Urol. 2020, 78, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Thompson, R.H.; Tollefson, M.K.; Rangel, L.J.; Bergstralh, E.J.; Blute, M.L.; Karnes, R.J. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: The impact of time from surgery to recurrence. Eur. Urol. 2011, 59, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Weirich, G.; Schottelius, M.; Weineisen, M.; Frisch, B.; Okur, A.; Kübler, H.; Thalgott, M.; Navab, N.; Schwaiger, M.; et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 2015, 68, 530–534. [Google Scholar] [CrossRef]
- de Barros, H.A.; van Oosterom, M.N.; Donswijk, M.L.; Hendrikx, J.J.; Vis, A.N.; Maurer, T.; van Leeuwen, F.W.; van der Poel, H.G.; van Leeuwen, P.J. Robot-assisted Prostate-specific Membrane Antigen-radioguided Salvage Surgery in Recurrent Prostate Cancer Using a DROP-IN Gamma Probe: The First Prospective Feasibility Study. Eur. Urol. 2022, 82, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Knipper, S.; Lischewski, F.; Koehler, D.; Eiber, M.; van Leeuwen, F.W.; de Barros, H.; Berrens, A.-C.; Zuur, L.; van Leeuwen, P.J.; van der Poel, H.; et al. Biochemical Response of < 0.1 ng/ml Predicts Therapy-free Survival of Prostate Cancer Patients following Prostate-specific Membrane Antigen-targeted Salvage Surgery. Eur. Urol. Oncol. 2025, 8, 270–277. [Google Scholar]
- Berrens, A.-C.; Sorbi, M.A.; Donswijk, M.L.; de Barros, H.A.; Azargoshasb, S.; van Oosterom, M.N.; Rietbergen, D.D.; Bekers, E.M.; van der Poel, H.G.; van Leeuwen, F.W.; et al. Strong Correlation Between SUV on PSMA PET/CT and Numeric Drop-In γ-Probe Signal for Intraoperative Identification of Prostate Cancer Lesions. J. Nucl. Med. 2024, 65, 548–554. [Google Scholar] [CrossRef]
- Gondoputro, W.; Scheltema, M.J.; Blazevski, A.; Doan, P.; Thompson, J.E.; Amin, A.; Geboers, B.; Agrawal, S.; Siriwardana, A.R.; van Leeuwen, P.J.; et al. Robot-Assisted Prostate-Specific Membrane Antigen-Radioguided Surgery in Primary Diagnosed Prostate Cancer. J. Nucl. Med. 2022, 63, 1659–1664. [Google Scholar] [CrossRef]
- Gandaglia, G.; Mazzone, E.; Stabile, A.; Pellegrino, A.; Cucchiara, V.; Barletta, F.; Scuderi, S.; Robesti, D.; Leni, R.; Gajate, A.M.S.; et al. Prostate-specific membrane antigen Radioguided Surgery to Detect Nodal Metastases in Primary Prostate Cancer Patients Undergoing Robot-assisted Radical Prostatectomy and Extended Pelvic Lymph Node Dissection: Results of a Planned Interim Analysis of a Prospective Phase 2 Study. Eur. Urol. 2022, 82, 411–418. [Google Scholar]
- Yılmaz, B.; Şahin, S.; Ergül, N.; Çolakoğlu, Y.; Baytekin, H.F.; Sökmen, D.; Tuğcu, V.; Taşçı, A.İ.; Çermik, T.F. Tc-PSMA targeted robot-assisted radioguided surgery during radical prostatectomy and extended lymph node dissection of prostate cancer patients. Ann. Nucl. Med. 2022, 36, 597–609. [Google Scholar] [CrossRef]
- Schilham, M.G.; Somford, D.M.; Küsters-Vandevelde, H.V.; Hermsen, R.; van Basten, J.P.A.; Hoekstra, R.J.; Scheenen, T.W.; Gotthardt, M.; Sedelaar, J.M.; Rijpkema, M. Prostate-Specific Membrane Antigen-Targeted Radioguided Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients with a Suspicion of Locoregional Lymph Node Metastases: The DETECT Trial. J. Nucl. Med. 2024, 65, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.A.; Thomas, M.; Myers, C.H.; Gagare, P.; Kanduluru, A.K.; Crian, C.J.; Cichocki, B.N. Evaluation of Novel Prostate-Specific Membrane Antigen-Targeted Near-Infrared Imaging Agent for Fluorescence-Guided Surgery of Prostate Cancer. Clin. Cancer Res. 2019, 25, 177–187. [Google Scholar] [CrossRef]
- A Stibbe, J.; A de Barros, H.; Linders, D.G.J.; Bhairosingh, S.S.; Bekers, E.M.; van Leeuwen, P.J.; Low, P.S.; A Kularatne, S.; Vahrmeijer, A.L.; Burggraaf, J.; et al. First-in-patient study of OTL78 for intraoperative fluorescence imaging of prostate-specific membrane antigen-positive prostate cancer: A single-arm, phase 2a, feasibility trial. Lancet Oncol. 2023, 24, 457–467. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Berg, N.S.v.D.; Antaris, A.L.; Xue, L.; Greenberg, S.; Rosenthal, J.W.; Muchnik, A.; Klaassen, A.; Simko, J.P.; Dutta, S.; et al. First-in-human Evaluation of a Prostate-specific Membrane Antigen-targeted Near-infrared Fluorescent Small Molecule for Fluorescence-based Identification of Prostate Cancer in Patients with High-risk Prostate Cancer Undergoing Robotic-assisted Prostatectomy. Eur. Urol. Oncol. 2024, 7, 63–72. [Google Scholar]
- Gong, J.; Janes, J.L.; Eve, C.T.; Stock, S.; Waller, J.; De Hoedt, A.M.; Kim, J.; Ghate, S.R.; Shui, I.M.; Freedland, S.J. Epidemiology, treatment patterns, and clinical outcomes in de novo oligometastatic hormone-sensitive prostate cancer. Cancer 2024, 130, 3815–3825. [Google Scholar] [CrossRef]
- Virgo, K.S.; Rumble, R.B.; Talcott, J.A. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update: ASCO Guideline Q and A. JCO Oncol. Pract. 2023, 19, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Boevé, L.M.; Hulshof, M.C.; Vis, A.N.; Zwinderman, A.H.; Twisk, J.W.; Witjes, W.P.; Delaere, K.P.; van Moorselaar, R.J.A.; Verhagen, P.C.; van Andel, G. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur. Urol. 2019, 75, 410–418. [Google Scholar]
- Bossi, A.; Foulon, S.; Maldonado, X.; Sargos, P.; MacDermott, R.; Kelly, P.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; et al. Efficacy and safety of prostate radiotherapy in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2024, 404, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Zilli, T.; Achard, V.; Pra, A.D.; Schmidt-Hegemann, N.; Jereczek-Fossa, B.A.; Lancia, A.; Ingrosso, G.; Alongi, F.; Aluwini, S.; Arcangeli, S.; et al. Recommendations for radiation therapy in oligometastatic prostate cancer: An ESTRO-ACROP Delphi consensus. Radiother. Oncol. 2022, 176, 199–207. [Google Scholar] [CrossRef]
- Ost, P.; Bossi, A.; Decaestecker, K.; De Meerleer, G.; Giannarini, G.; Karnes, R.J.; Roach, M., 3rd; Briganti, A. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: A systematic review of the literature. Eur. Urol. 2015, 67, 852–863. [Google Scholar] [CrossRef]
- Steuber, T.; Jilg, C.; Tennstedt, P.; De Bruycker, A.; Tilki, D.; Decaestecker, K.; Zilli, T.; Jereczek-Fossa, B.; Wetterauer, U.; Grosu, A.; et al. Standard of Care Versus Metastases-directed Therapy for PET-detected Nodal Oligorecurrent Prostate Cancer Following Multimodality Treatment: A Multi-institutional Case-control Study. Eur. Urol. Focus 2019, 5, 1007–1013. [Google Scholar] [CrossRef]
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomäcker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.D.; Krapf, P.; et al. Comparison of [(18)F]DCFPyL and [(68)Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Supiot, S.; Vaugier, L.; Pasquier, D.; Buthaud, X.; Magné, N.; Peiffert, D.; Sargos, P.; Crehange, G.; Pommier, P.; Loos, G.; et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur. Urol. 2021, 80, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.J.; Shah, T.T.; Horan, G.; Bevan, C.L.; Winkler, M.; Ahmed, H.U. Cytoreductive treatment strategies for de novo metastatic prostate cancer. Nat. Rev. Clin. Oncol. 2020, 17, 168–182. [Google Scholar] [CrossRef]
- Nickols, N.G.; Tsai, S.; Kane, N.; Tran, S.; Ghayouri, L.; Diaz-Perez, S.; Thein, M.; Anderson-Berman, N.; Eason, J.; Kishan, A.U.; et al. Systemic and Tumor-directed Therapy for Oligometastatic Prostate Cancer: The SOLAR Phase 2 Trial in De Novo Oligometastatic Prostate Cancer. Eur. Urol. 2024, 86, 190–193. [Google Scholar] [CrossRef]
- Gratzke, C.; Engel, J.; Stief, C.G. Role of radical prostatectomy in metastatic prostate cancer: Data from the Munich Cancer Registry. Eur. Urol. 2014, 66, 602–603. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Karnes, J.; Stief, C.; Copsey, B.; Montorsi, F.; Hammerer, P.; Beyer, B.; Moschini, M.; Gratzke, C.; Steuber, T.; et al. A Multi-institutional Analysis of Perioperative Outcomes in 106 Men Who Underwent Radical Prostatectomy for Distant Metastatic Prostate Cancer at Presentation. Eur. Urol. 2016, 69, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Fossati, N.; Pfister, D.; Suardi, N.; Montorsi, F.; Shariat, S.; Grubmüller, B.; Gandaglia, G.; Briganti, A.; Karnes, R.J. Cytoreductive Radical Prostatectomy in Men with Prostate Cancer and Skeletal Metastases. Eur. Urol. Oncol. 2018, 1, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Multimodal Approach in Patients with mHSPC. Randomized Trial of APA+ADT vs APA-ADT and Local Treatment (APPROACH). Available online: https://clinicaltrials.gov/study/NCT05649943?term=APPROACH&cond=Prostate%20Cancer&aggFilters=status:rec&rank=1 (accessed on 20 August 2025).
| Author and Year | Intervention | Comparator | Included Studies | Outcomes |
|---|---|---|---|---|
| A. Jiang et al., 2023 [29] | Extraperitoneal SP RARP | Transperitoneal SP RARP | 5 studies and 883 patients | Intervention:
|
| B. Yuan et al., 2024 [30] | SP RARP | / | 24 studies and 1385 patients | Intervention:
|
| SP RARP | MP RARP | 11 studies and 1553 patients | Intervention:
|
| Author and Year | Cohort of Patients | Tracer Used | Main Goals | Outcomes |
|---|---|---|---|---|
| Gondoputro et al., 2022, [74] prospective | 12 HR-PCa patients with a LNI risk > 10% | [99mTc]TcPSMA-I&S | evaluating the safety and feasibility of PSMA-RGS to guide the intraoperative detection of LNMs during RARP with ePLND |
|
| Gandaglia et al., 2022, [75] prospective | 12 IR- or HRcN0cM0 PCa patients at CIM with a LNI risk > 5% | [99mTc]TcPSMA-I&S | reporting the planned interim analyses of a phase 2 prospective study aimed at describing PSMA-RGS during RARP with ePLND |
|
| Schilham et al., 2024, [77] prospective | 20 PCa patients with positive preoperative PSMA PET | [111In]InPSMA-I&T | evaluating the safety and feasibility of PSMA-RGS during RARP with ePLND |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santarelli, V.; Corvino, R.; Bevilacqua, G.; Salciccia, S.; Di Lascio, G.; Del Giudice, F.; Di Pierro, G.B.; Franco, G.; Crivellaro, S.; Sciarra, A. Latest Advancements and Future Directions in Prostate Cancer Surgery: Reducing Invasiveness and Expanding Indications. Cancers 2025, 17, 3053. https://doi.org/10.3390/cancers17183053
Santarelli V, Corvino R, Bevilacqua G, Salciccia S, Di Lascio G, Del Giudice F, Di Pierro GB, Franco G, Crivellaro S, Sciarra A. Latest Advancements and Future Directions in Prostate Cancer Surgery: Reducing Invasiveness and Expanding Indications. Cancers. 2025; 17(18):3053. https://doi.org/10.3390/cancers17183053
Chicago/Turabian StyleSantarelli, Valerio, Roberta Corvino, Giulio Bevilacqua, Stefano Salciccia, Giovanni Di Lascio, Francesco Del Giudice, Giovanni Battista Di Pierro, Giorgio Franco, Simone Crivellaro, and Alessandro Sciarra. 2025. "Latest Advancements and Future Directions in Prostate Cancer Surgery: Reducing Invasiveness and Expanding Indications" Cancers 17, no. 18: 3053. https://doi.org/10.3390/cancers17183053
APA StyleSantarelli, V., Corvino, R., Bevilacqua, G., Salciccia, S., Di Lascio, G., Del Giudice, F., Di Pierro, G. B., Franco, G., Crivellaro, S., & Sciarra, A. (2025). Latest Advancements and Future Directions in Prostate Cancer Surgery: Reducing Invasiveness and Expanding Indications. Cancers, 17(18), 3053. https://doi.org/10.3390/cancers17183053






