Molecular Testing and Surgical Outcomes in Bethesda III and IV Thyroid Nodules: A Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Data Collection
2.3. Definition of Optimal Management
2.4. Statistical Analysis
2.5. Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aribon, P.A.; Teope, E.; Corneja-Egwolf, A.L.; Maningat, M.P.D. Diagnostic Accuracy of American College of Radiology Thyroid Imaging Reporting Data System: A Single-Center Cross-Sectional Study. JAFES 2024, 39, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Davis, A.; Verma, V. Thyroid Nodules: Advances in Evaluation and Management. Am. Fam. Physician 2020, 102, 298–304. [Google Scholar]
- Rajabzadeh, F.; Hassannejad, E.; Akhlaghipour, I.; Imen, M.J.; Babazadeh Baghan, A.; Goshayeshi, L.; Taghavi, S.M.; Vojouhi, S.; Payandeh, A.; Moodi Ghalibaf, A. Differentiating Benign and Malignant Thyroid Nodules: A Cross-Sectional Study on the Comparison of Diagnostic Value of Ultrasound Elastography and Fine Needle Aspiration Biopsy. Health Sci. Rep. 2023, 6, e1619. [Google Scholar] [CrossRef]
- Bailey, S.; Wallwork, B. Differentiating Between Benign and Malignant Thyroid Nodules: An Evidence-Based Approach in General Practice. Aust. J. Gen. Pract. 2018, 47, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Almahari, S.A.; Harb, Z.; Alshaikh, S. Evaluation of Thyroid Nodules Classified as Bethesda Category III on Cytology and Their Malignancy Rate: An Institutional Experience. CytoJournal 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.; Ramdas, A.; Ambroise, M.M.; Kumar, N.P. The Bethesda System for Reporting Thyroid Cytopathology: A Cytohistological Study. J. Thyroid. Res. 2020, 2020, 8095378. [Google Scholar] [CrossRef]
- Dhyani, M.; Faquin, W.; Lubitz, C.C.; Daniels, G.H.; Samir, A.E. How to Interpret Thyroid Fine-Needle Aspiration Biopsy Reports: A Guide for the Busy Radiologist in the Era of the Bethesda Classification System. Am. J. Roentgenol. 2013, 201, 1335–1339. [Google Scholar] [CrossRef]
- Ali, S.Z.; VanderLaan, P.A. The Bethesda System for Reporting Thyroid Cytopathology: Definitions, Criteria, and Explanatory Notes, 3rd ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Kuta, V.; Forner, D.; Azzi, J.; Curry, D.; Noel, C.W.; Munroe, K.; Bullock, M.; McDonald, T.; Taylor, S.M.; Rigby, M.H.; et al. Treatment Choices in Managing Bethesda III and IV Thyroid Nodules: A Canadian Multi-institutional Study. OTO Open 2021, 5, 2473974X211015937. [Google Scholar] [CrossRef]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204. [Google Scholar] [CrossRef]
- Alzumaili, B.; Sadow, P.M. Update on Molecular Diagnostics in Thyroid Pathology: A Review. Genes 2023, 14, 1314. [Google Scholar] [CrossRef] [PubMed]
- Hlozek, J.; Pekova, B.; Rotnágl, J.; Holý, R.; Astl, J. Genetic Changes in Thyroid Cancers and the Importance of Their Preoperative Detection in Relation to the General Treatment and Determination of the Extent of Surgical Intervention—A Review. Biomedicines 2022, 10, 1515. [Google Scholar] [CrossRef]
- Ringel, M.D.; Sosa, J.A.; Baloch, Z.; Bischoff, L.; Bloom, G.; Brent, G.A.; Brock, P.L.; Chou, R.; Flavell, R.R.; Goldner, W.; et al. 2025 American Thyroid Association Management Guidelines for Adult Patients with Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2025, 35, 841–985. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma; NCCN: Plymouth Meeting, PA, USA, 2023. [Google Scholar]
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for Thyroid Nodule Management. Eur. Thyroid J. 2023, 12, e230067. [Google Scholar] [CrossRef]
- Hall, E.A.; Hartzband, P.; VanderLaan, P.A.; Nishino, M. Risk Stratification of Cytologically Indeterminate Thyroid Nodules with Nondiagnostic or Benign Cytology on Repeat FNA: Implications for Molecular Testing and Surveillance. Cancer Cytopathol. 2023, 131, 313–324. [Google Scholar] [CrossRef]
- Connelly, C.F.; Smithgall, M.C.; Desai, N.; Cimic, A.; Baskota, S.U. Performance Characteristics of ThyroSeq, ThyGeNEXT/ThyraMIR, and Afirma Molecular Platforms in Evaluation of 1252 Cytologically-Indeterminate Thyroid Nodules. J. Am. Soc. Cytopathol. 2025, 14, 243–254. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Endocrine and Neuroendocrine Tumours, WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2022; Volume 10, ISBN 978-92-832-4508-3. [Google Scholar]
- Sadowski, S.M.; Petrenko, V.; Meyer, P.; Pusztaszeri, M.; Brulhart-Meynet, M.-C.; Heddad Masson, M.; Triponez, F.; Philippe, J.; Dibner, C. Validation of Molecular Biomarkers for Preoperative Diagnostics of Human Papillary Thyroid Carcinoma in Fine Needle Aspirates. Gland. Surg. 2019, 8, S62–S76. [Google Scholar] [CrossRef]
- Almquist, M.; Muth, A. Surgical Management of Cytologically Indeterminate Thyroid Nodules. Gland. Surg. 2019, 8, S105–S111. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Klopper, J.; Cottrill, E.E. Molecular Diagnostics in the Evaluation of Thyroid Nodules: Current Use and Prospective Opportunities. Front. Endocrinol. 2023, 14, 1101410. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Hier, J.; Payne, K.E.; Abdulhaleem, M.; Dimitstein, O.; Eisenbach, N.; Forest, V.-I.; Payne, R.J. Impact of Molecular Testing on Surgical Decision-Making in Indeterminate Thyroid Nodules: A Systematic Review and Meta-Analysis of Recent Advancements. Cancers 2025, 17, 1156. [Google Scholar] [CrossRef]
- Jin, S.; Sugitani, I. Narrative Review of Management of Thyroid Surgery Complications. Gland. Surg. 2021, 10, 1135–1146. [Google Scholar] [CrossRef]
- Lukinović, J. Overview of Thyroid Surgery Complications. ACC 2020, 59, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Uppal, N.; Collins, R.; James, B. Thyroid Nodules: Global, Economic, and Personal Burdens. Front. Endocrinol. 2023, 14, 1113977. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, F.; Payne, R.J.; Beaudoin, D.; Boucher, A.; Fortier, P.-H.; Massicotte, M.-H.; Pusztaszeri, M.; Rondeau, G.; Corriveau, E.; El Malt, F.; et al. Publicly Funded Molecular Testing of Indeterminate Thyroid Nodules: Canada’s Experience. J. Clin. Endocrinol. Metab. 2025, 110, e1031–e1037. [Google Scholar] [CrossRef] [PubMed]
- Medas, F.; Tuveri, M.; Canu, G.L.; Erdas, E.; Calò, P.G. Complications after Reoperative Thyroid Surgery: Retrospective Evaluation of 152 Consecutive Cases. Updates Surg. 2019, 71, 705–710. [Google Scholar] [CrossRef]
- Turkdogan, S.; Pusztaszeri, M.; Forest, V.-I.; Hier, M.P.; Payne, R.J. Are Bethesda III Thyroid Nodules More Aggressive than Bethesda IV Thyroid Nodules When Found to Be Malignant? Cancers 2020, 12, 2563. [Google Scholar] [CrossRef]
- Bourque, C.; Savoia, G.; Noik, M.; Florianova, L.; Bandargal, S.; Da Silva, S.D.; Payne, R.; Pusztaszeri, M.P. Malignancy Risk, Molecular Mutations, and Surgical Outcomes of Thyroid Nodules Classified as Atypia of Undetermined Significance in the Bethesda System: A Comprehensive Analysis. Cytopathology 2025, ahead of print. [Google Scholar] [CrossRef]
- Dell’Aquila, M.; Fiorentino, V.; Martini, M.; Capodimonti, S.; Cenci, T.; Lombardi, C.P.; Raffaelli, M.; Pontecorvi, A.; Fadda, G.; Pantanowitz, L.; et al. How Limited Molecular Testing Can Also Offer Diagnostic and Prognostic Evaluation of Thyroid Nodules Processed with Liquid-Based Cytology: Role of TERT Promoter and BRAF V600E Mutation Analysis. Cancer Cytopathol. 2021, 129, 819–829. [Google Scholar] [CrossRef]
- Payne, A.E.; Lefebvre, C.; Minello, M.; Rajab, M.; Da Silva, S.D.; Pusztaszeri, M.; Hier, M.P.; Forest, V.-I. Molecular Mutations and Clinical Behavior in Bethesda III and IV Thyroid Nodules: A Comparative Study. Cancers 2024, 16, 4249. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Huston-Paterson, H.H.; Schumm, M.A.; Longstaff, X.R.; Hughes, E.G.; Tseng, C.-H.; Cheung, D.S.; Yeh, M.W.; Wu, J.X.; Livhits, M.J. Histopathologic and Clinical Features of Bethesda III-VI Nodules Harboring Isolated RAS Mutations. Endocr. Pract. 2025, 31, 366–372. [Google Scholar] [CrossRef]
- Addasi, N.; Fingeret, A.; Goldner, W. Hemithyroidectomy for Thyroid Cancer: A Review. Medicina 2020, 56, 586. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.J.; Pitt, S.C.; Liu, J.; Schaefer, S.; Sippel, R.S.; Chen, H. Thyroid Hormone Replacement After Thyroid Lobectomy. Surgery 2009, 146, 554–558; discussion 558–560. [Google Scholar] [CrossRef] [PubMed]
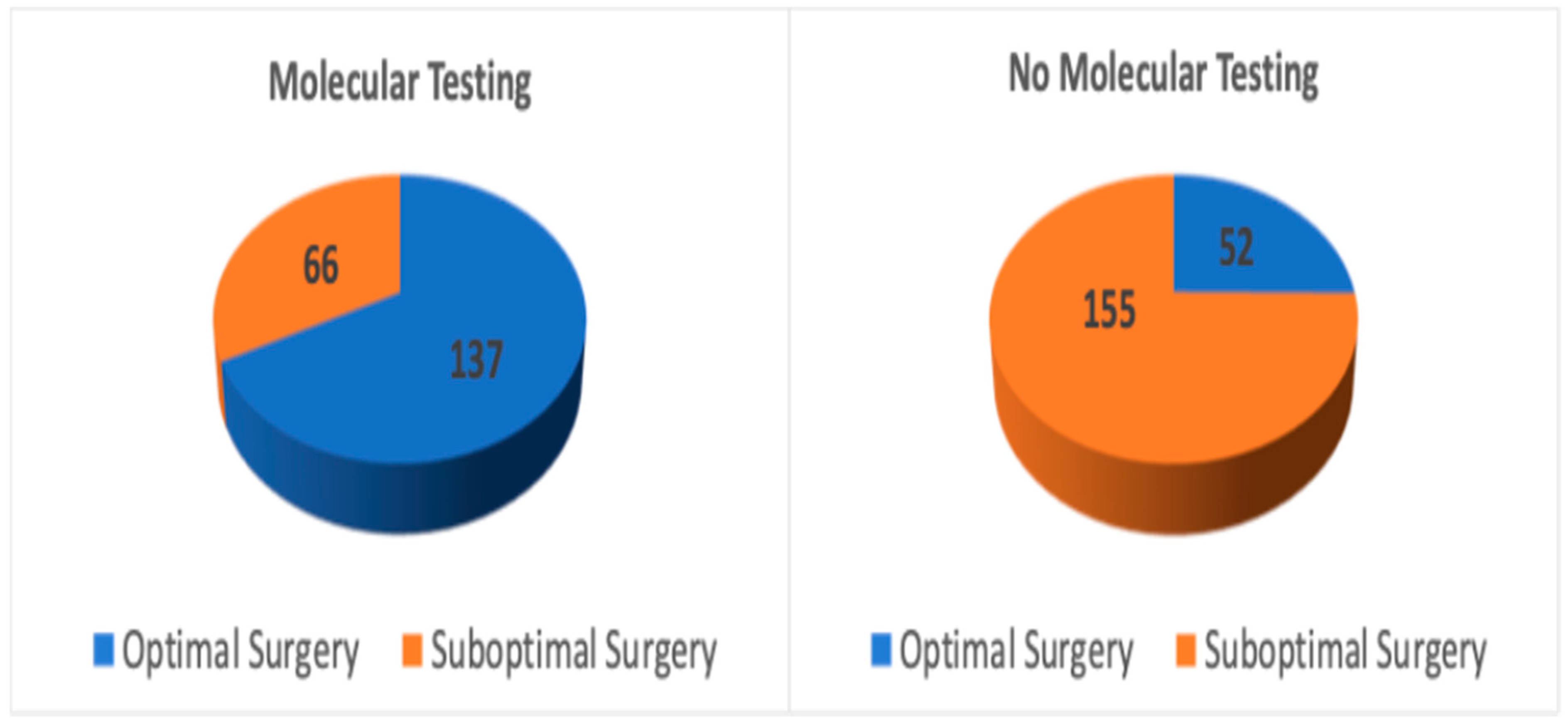
| Group | Total Patients (n) | Optimal Surgery (n, %) | Suboptimal Surgery (n, %) | p-Value | |
|---|---|---|---|---|---|
| 1 | Molecular testing | 203 | 137 (67.5%) | 66 (32.5%) | p < 0.001 |
| 2 | No molecular testing | 207 | 52 (25.1%) | 155 (74.9%) |
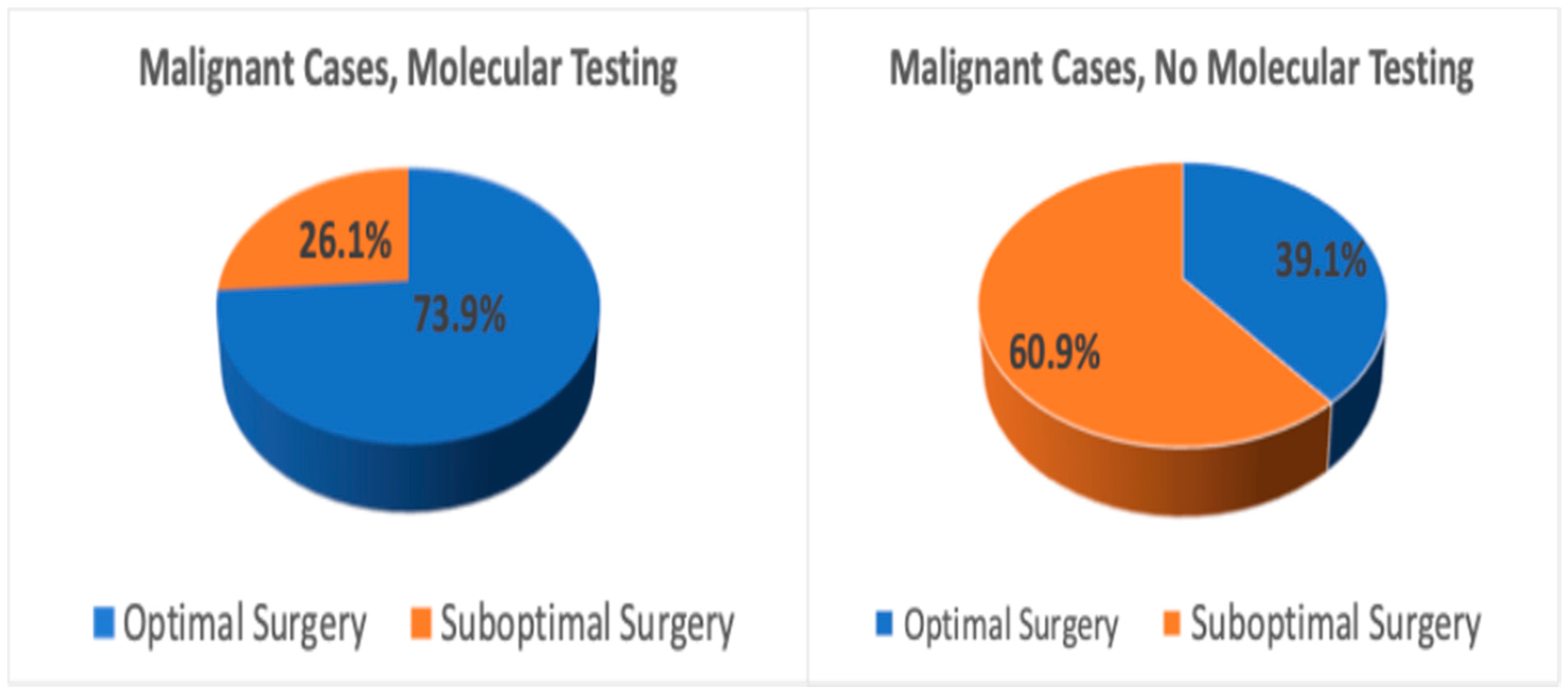
| Group | Total Patients (n) | Optimal Surgery (n, %) | Suboptimal Surgery (n, %) | Total Thyroidectomies (n) | Hemi-Thyroidectomies (n) | Benign Hemi-Thyroidectomies (n) | p-Value |
|---|---|---|---|---|---|---|---|
| No molecular testing | 129 | 27 (20.9%) | 102 (79.1%) | 30 | 72 | 64 (88.9%) | p < 0.001 |
| Molecular testing | 117 | 72 (61.5%) | 45 (38.5%) | 4 | 41 | 36 (87.8%) |
| Group | Total Patients (n) | Optimal Surgery (n, %) | Suboptimal Surgery (n, %) | Total Thyroidectomies (n) | Hemi-Thyroidectomies(n) | Benign Hemi-Thyroidectomies (n) | p-Value |
|---|---|---|---|---|---|---|---|
| No molecular testing | 78 | 25 (32.1%) | 53 (67.9%) | 14 | 39 | 30 (76.9%) | p < 0.001 |
| Molecular testing | 86 | 65 (75.6%) | 21 (24.4%) | 1 | 20 | 14 (70.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payne, A.E.; Gobeil, L.; Pusztaszeri, M.P.; Bannister, I.; Bandargal, S.; da Silva, S.D.; Forest, V.-I. Molecular Testing and Surgical Outcomes in Bethesda III and IV Thyroid Nodules: A Retrospective Cohort Study. Cancers 2025, 17, 3376. https://doi.org/10.3390/cancers17203376
Payne AE, Gobeil L, Pusztaszeri MP, Bannister I, Bandargal S, da Silva SD, Forest V-I. Molecular Testing and Surgical Outcomes in Bethesda III and IV Thyroid Nodules: A Retrospective Cohort Study. Cancers. 2025; 17(20):3376. https://doi.org/10.3390/cancers17203376
Chicago/Turabian StylePayne, Alexandra E., Layla Gobeil, Marc P. Pusztaszeri, Isabelle Bannister, Saruchi Bandargal, Sabrina Daniela da Silva, and Veronique-Isabelle Forest. 2025. "Molecular Testing and Surgical Outcomes in Bethesda III and IV Thyroid Nodules: A Retrospective Cohort Study" Cancers 17, no. 20: 3376. https://doi.org/10.3390/cancers17203376
APA StylePayne, A. E., Gobeil, L., Pusztaszeri, M. P., Bannister, I., Bandargal, S., da Silva, S. D., & Forest, V.-I. (2025). Molecular Testing and Surgical Outcomes in Bethesda III and IV Thyroid Nodules: A Retrospective Cohort Study. Cancers, 17(20), 3376. https://doi.org/10.3390/cancers17203376








