Sequencing Choices and Real-World Clinical Management in Advanced Grade 2/3 GEP-NET Treatment: The Emerging Role of PRRT
Simple Summary
Abstract
1. GEP-NETs Overview
1.1. GEP-NET Background Information and GEP-NET World Health Organization Classification
1.2. High-Grade GEP-NET Current Treatment Landscape Overview
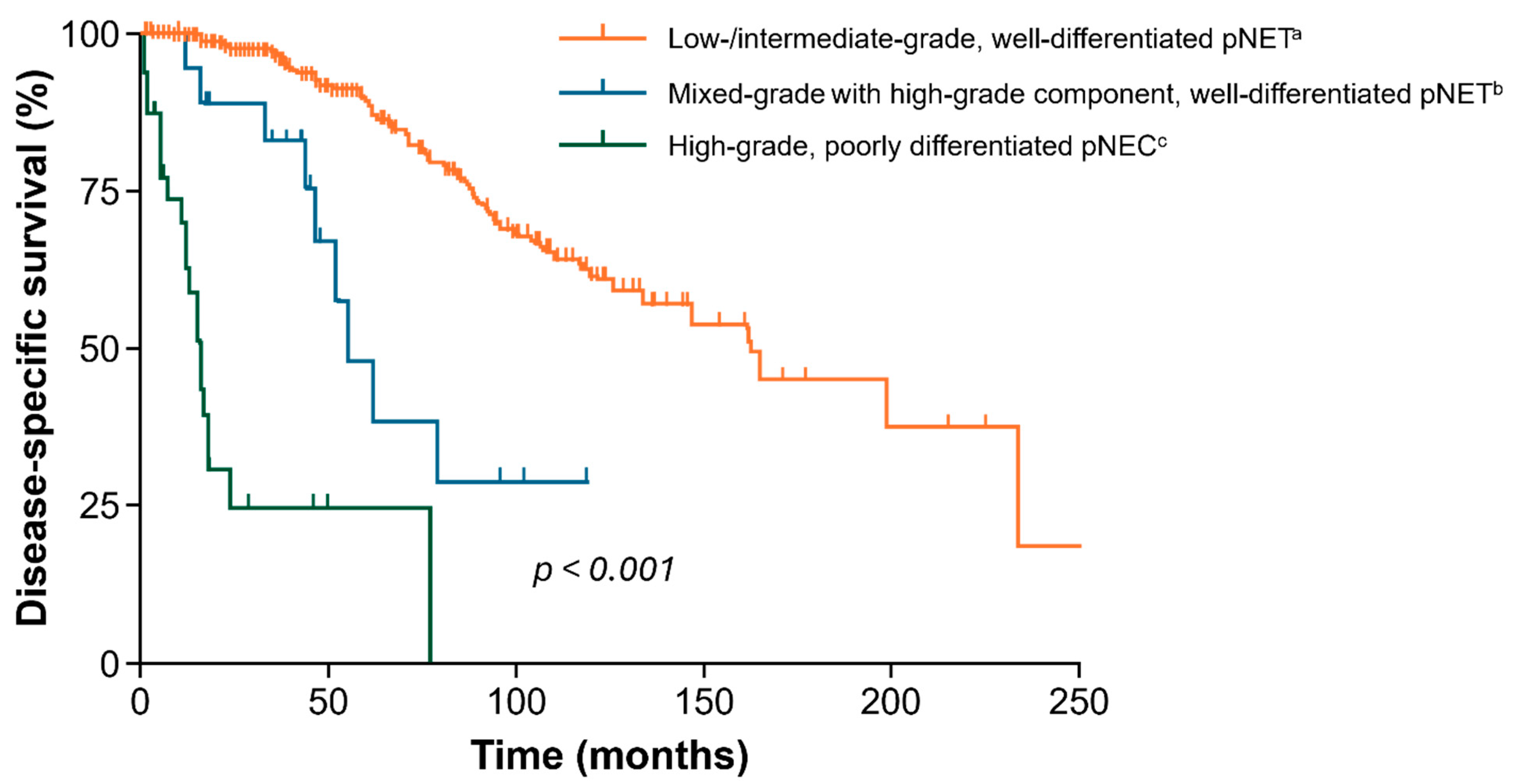
1.3. Outcomes from Current Treatments for Patients with G2/G3 GEP-NETs
| Trial | Type | Grade | Therapy | Line | mPFS (Months) | ORR (%, 95% CI) | Safety Events |
|---|---|---|---|---|---|---|---|
| SSAs | |||||||
| CLARINET [40] | GEP-NET | G1/G2 | Lanreotide | 1L/2L | NR | NR | Hyperglycemia, cholelithiasis |
| PROMID [39] | Midgut NET | G1/G2 | Octreotide LAR | 1L | 14.3 | NR | GI events, hematologic events, fatigue, fever, bile stones |
| PRRT | |||||||
| NETTER-1 [46] | Midgut NET | G1/G2 | [177Lu]Lu-DOTATATE | 2L | NR | 18 (10–25) | Hematologic toxicity, nausea, vomiting |
| NETTER-2 [31] | GEP-NET | G2/G3 | [177Lu]Lu-DOTATATE | 1L | 22.8 | 43 (35–51) | Hematologic toxicity, nausea, diarrhea, abdominal pain |
| Rotterdam [47] | GEP-NET | G1–G3 | [177Lu]Lu-DOTATATE | 2L | 40 | 46 (NR) | Hematologic toxicities, nausea, vomiting, abdominal cramps |
| COMPETE [48] | GEP-NET | G1/G2 | [177Lu]Lu-edotreotide | 1L/2L | 23.9 | NR | NR |
| OCLURANDOM [49] | pNET | Advanced progressive | [177Lu]Lu-DOTATATE | ≥ 2L | 20.7 | 63 | Hematologic toxicity, GI events, fatigue, hypertension, CKD, second cancers |
| mTOR inhibitors | |||||||
| RADIANT-3 [42] | pNET | G1/G2 | Everolimus | 2L | 11 | 5 (confirmed responses) | Stomatitis, rash, diarrhea, fatigue |
| RADIANT-4 [50] | GEP-NET | G1–G3 | Everolimus | 2L | 11 | 2 (confirmed responses) | Stomatitis, diarrhea, infections, anemia, fatigue |
| TKIs | |||||||
| NCT00428597 [43] | pNET | G1/G2 | Sunitinib | 2L | 11.4 | 9.3 | Diarrhea, nausea, vomiting, asthenia |
| CABINET [27] | NETs | G1–G3 | Cabozantinib | 3L | 8.4 (extrapancreatic) 13.8 (pancreatic) | 5 (extrapancreatic) 19 (pancreatic) | Hypertension, fatigue, diarrhea, thromboembolic events |
2. Treatment Sequencing Strategies for G2/G3 GEP-NETs
2.1. Role of Imaging and Biomarkers in G2/G3 GEP-NET Treatment Planning
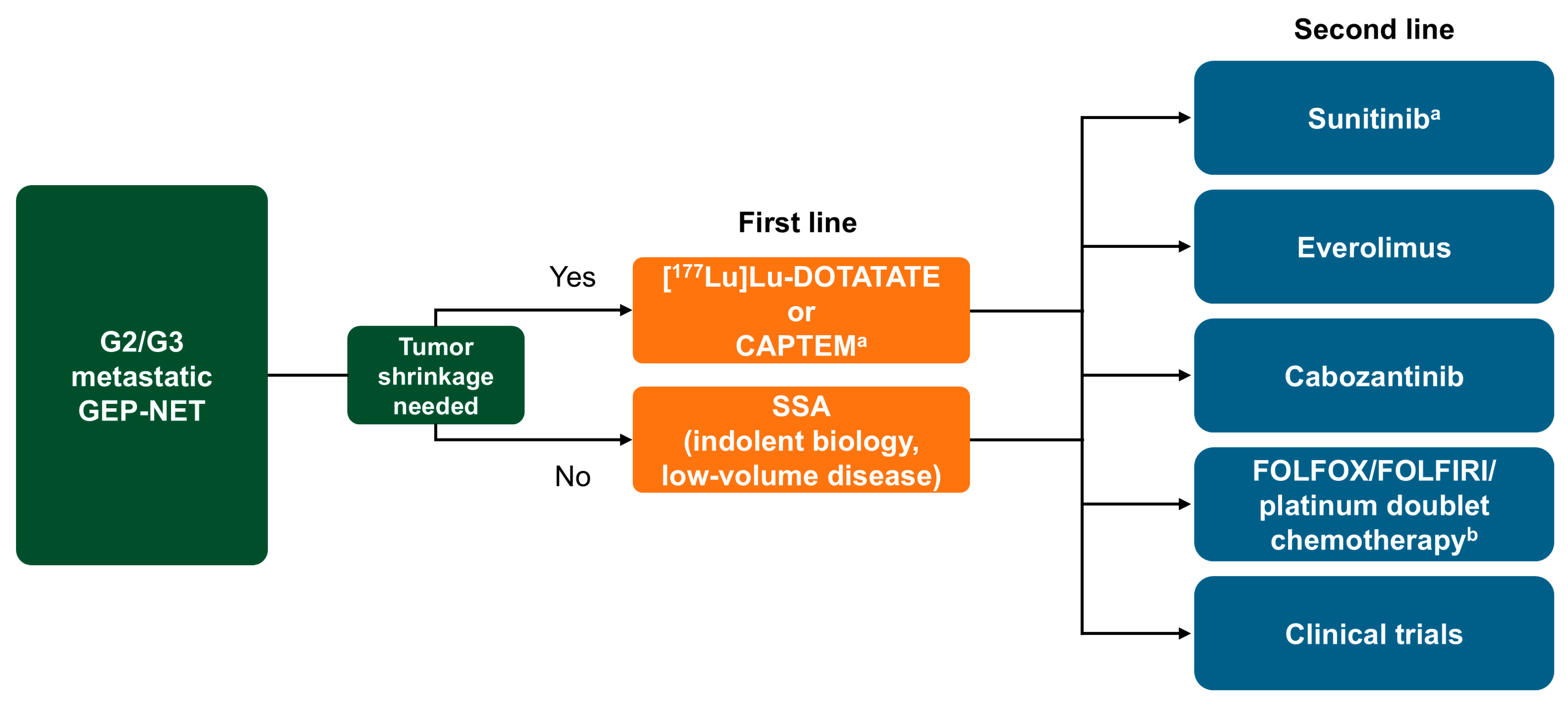
2.2. Treatment Sequence Practices
2.2.1. Existing Guidelines
| Society | Midgut NETs | pNETs | ||
|---|---|---|---|---|
| G2 | G3 | G2 | G3 | |
| American Society of Clinical Oncology (ASCO), 2023 [53] | 1L: Octreotide/lanreotide 2L: PRRT (SSTR-positive) or everolimus (non-functional tumors) | 1L: Octreotide/lanreotide (SSTR-positive, low volume) 2L: PRRT (SSTR-positive), everolimus (non-functional tumors), or chemotherapy (high Ki-67) | 1L: Octreotide/lanreotide (SSTR-positive) or CAPTEM (higher-volume or SSTR-negative tumors) or everolimus or sunitinib 2L: PRRT (SSTR-positive), CAPTEM, everolimus, or sunitinib | 1L: Octreotide/lanreotide (SSTR-positive, low volume) 2L: PRRT (SSTR-positive), everolimus (non-functional tumors), or chemotherapy (high Ki-67) |
| European Society for Medical Oncology (ESMO), 2020 [69] | 1L: SSA or everolimus (Ki-67 > 10%) 2L: PRRT or everolimus (SSTR-negative) 3L: Everolimus (Ki-67 < 10%) FOLFOX, temozolomide (Ki-67 > 10%) | PRRT may be considered | 1L: SSA (Ki-67 < 10%) or streptozotocin/5-FU/ CAPTEM/everolimus/ sunitinib (Ki-67 > 10%) 2L: Streptozotocin/5-FU/ CAPTEM/everolimus/sunitinib (Ki-67 < 10%) or PRRT (Ki-67 > 10%) | 1L: CAPTEM, streptozotocin/5-FU 2L: Everolimus or sunitinib 3L: PRRT |
| North American Neuroendocrine Tumor Society (NANETS), 2017 [73], 2020 [70], 2023 [17] | 1L: SSA (octreotide/lanreotide) 2L: [177Lu]Lu-DOTATATE or everolimus (SSTR-negative) | 1L: Favorable disease: SSA (SSTR-positive), liver-directed therapy (if Ki-67 < 55%), PRRT (high SSTR expression), everolimus (if bowel origin) Aggressive disease: CAPTEM, FOLFOX/CAPEOX 2L: CAPTEM (if not used in 1L), FOLFOX, FOLFIRI | 1L: SSA (octreotide/lanreotide) or chemotherapy, liver-directed therapy, everolimus, and/or sunitinib (SSTR-negative) 2L: Everolimus, sunitinib, or PRRT (SSTR-positive) Consider chemotherapy for progressive disease | 1L: Favorable disease: SSA (SSTR-positive), liver-directed therapy, everolimus, sunitinib Aggressive disease: CAPTEM, FOLFOX/CAPEOX 2L: CAPTEM, FOLFOX/CAPEOX |
| National Comprehensive Cancer Network (NCCN), 2025 [56] | Preferred regimens: Cabozantinib, everolimus, [177Lu]Lu-DOTATATE (SSTR-positive and progression on SSAs), 1L [177Lu]Lu-DOTATATE (SSTR-positive, Ki-67 ≥ 10%), or SSAs (octreotide/lanreotide) | Preferred: Clinical trial Alternatives: Cabozantinib, chemotherapy, everolimus, SSAs (octreotide/lanreotide), pembrolizumab, [177Lu]Lu-DOTATATE (SSTR-positive) | Preferred regimens: Cabozantinib, everolimus, sunitinib, SSAs (octreotide/lanreotide), 1L [177Lu]Lu-DOTATATE (SSTR-positive, Ki-67 ≥ 10%), [177Lu]Lu-DOTATATE (SSTR-positive and progression on SSAs), or CAPTEM | Preferred: Clinical trial Alternatives: Cabozantinib, chemotherapy, everolimus, SSAs (octreotide/lanreotide), pembrolizumab, [177Lu]Lu-DOTATATE (SSTR-positive), sunitinib |
2.2.2. Factors That Influence Sequence of Treatment
- a.
- Clinical Evidence
- b.
- Patient Characteristics and Preferences
2.2.3. Multidisciplinary Approach
2.2.4. Beyond Progression Treatment
3. PRRT in Clinical Practice
3.1. Patient Selection Criteria
3.2. PRRT as 1L in G2/G3 GEP-NETs (10% ≤ Ki-67 ≤ 55%)
3.2.1. Rationale
3.2.2. Safety
3.2.3. Efficacy
3.2.4. PRRT Retreatment Practices
4. Future Directions
4.1. Investigation of New Isotopes in PRRT
4.2. Other Ongoing PRRT Studies
4.2.1. COMPOSE ([177Lu]Lu-Edotreotide vs. CAPTEM, Everolimus, FOLFOX)
4.2.2. NCT04525638 ([177Lu]Lu-DOTATATE + Nivolumab)
4.2.3. NCT05724108 ([177Lu]Lu-DOTATATE + Triapine)
4.2.4. [177Lu]Lu-DOTATATE + Peposertib
4.2.5. PRRT + Chemotherapy Combination
5. Conclusions
5.1. How Can the Data from Recent 1L Studies of PRRT Impact the Treatment Sequencing Guidelines?
5.2. Clinical Practice Recommendations/Expert Opinions from the Authors
5.3. Further Research Needed for G2/G3 GEP-NET Management
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chauhan, A.; Chan, K.; Halfdanarson, T.R.; Bellizzi, A.M.; Rindi, G.; O’Toole, D.; Ge, P.S.; Jain, D.; Dasari, A.; Anaya, D.A.; et al. Critical updates in neuroendocrine tumors: Version 9 American Joint Committee on Cancer staging system for gastroenteropancreatic neuroendocrine tumors. CA Cancer J. Clin. 2024, 74, 359–367. [Google Scholar] [CrossRef]
- McMurry, H.S.; Rivero, J.D.; Chen, E.Y.; Kardosh, A.; Lopez, C.D.; Pegna, G.J. Gastroenteropancreatic neuroendocrine tumors: Epigenetic landscape and clinical implications. Curr. Probl. Cancer 2024, 52, 101131. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing but NET: A review of neuroendocrine tumors and carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Mazza, G.L.; Mi, L.; Oliver, T.; Starr, J.; Gudmundsdottir, H.; Cleary, S.P.; Hobday, T.; Halfdanarson, T.R. Survival and incidence patterns of pancreatic neuroendocrine tumors over the last 2 decades: A SEER database analysis. Oncologist 2022, 27, 573–578. [Google Scholar] [CrossRef]
- Stang, A.; Wellmann, I.; Holleczek, B.; Kim-Wanner, S.Z.; Müller-Nordhorn, J.; Sirri, E.; Wittenberg, I.; Siveke, J.T.; Kajüter, H. Incidence and survival of patients with malignant pancreatic neuroendocrine neoplasms in Germany, 2009–2021. Cancer Epidemiol. 2024, 93, 102659. [Google Scholar] [CrossRef]
- Hoogenkamp, D.S.; de Wit-van der Veen, L.J.; Huizing, D.M.V.; Tesselaar, M.E.T.; van Leeuwaarde, R.S.; Stokkel, M.P.M.; Lam, M.; Braat, A. Advances in radionuclide therapies for patients with neuro-endocrine tumors. Curr. Oncol. Rep. 2024, 26, 551–561. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- White, B.E.; Rous, B.; Chandrakumaran, K.; Wong, K.; Bouvier, C.; Van Hemelrijck, M.; George, G.; Russell, B.; Srirajaskanthan, R.; Ramage, J.K. Incidence and survival of neuroendocrine neoplasia in England 1995–2018: A retrospective, population-based study. Lancet Reg. Health Eur. 2022, 23, 100510. [Google Scholar] [CrossRef]
- Thiis-Evensen, E.; Boyar Cetinkaya, R. Incidence and prevalence of neuroendocrine neoplasms in Norway 1993–2021. J. Neuroendocrinol. 2023, 35, e13264. [Google Scholar] [CrossRef]
- Pathak, S.; Starr, J.S.; Halfdanarson, T.; Sonbol, M.B. Understanding the increasing incidence of neuroendocrine tumors. Expert. Rev. Endocrinol. Metab. 2023, 18, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Wallace, K.; Halperin, D.M.; Maxwell, J.; Kunz, P.; Singh, S.; Chasen, B.; Yao, J.C. Epidemiology of Neuroendocrine Neoplasms in the US. JAMA Netw. Open 2025, 8, e2515798. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Sultana, Q.; Kar, J.; Verma, A.; Sanghvi, S.; Kaka, N.; Patel, N.; Sethi, Y.; Chopra, H.; Kamal, M.A.; Greig, N.H. A comprehensive review on neuroendocrine neoplasms: Presentation, pathophysiology and management. J. Clin. Med. 2023, 12, 5138. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Anton-Pascual, B.; Modrego, A.; Del Carmen Riesco-Martinez, M.; Lens-Pardo, A.; Carretero-Puche, C.; Rubio-Cuesta, B.; Soldevilla, B. Advances in the treatment of gastroenteropancreatic neuroendocrine carcinomas: Are we moving forward? Endocr. Rev. 2023, 44, 724–736. [Google Scholar] [CrossRef]
- Sorbye, H.; Grande, E.; Pavel, M.; Tesselaar, M.; Fazio, N.; Reed, N.S.; Knigge, U.; Christ, E.; Ambrosini, V.; Couvelard, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J. Neuroendocrinol. 2023, 35, e13249. [Google Scholar] [CrossRef]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S.; Strosberg, J. PRRT in high-grade digestive neuroendocrine neoplasms (NET G3 and NEC). J. Neuroendocrinol. 2024, 37, e13443. [Google Scholar] [CrossRef]
- Eads, J.R.; Halfdanarson, T.R.; Asmis, T.; Bellizzi, A.M.; Bergsland, E.K.; Dasari, A.; El-Haddad, G.; Frumovitz, M.; Meyer, J.; Mittra, E.; et al. Expert consensus practice recommendations of the North American Neuroendocrine Tumor Society for the management of high grade gastroenteropancreatic and gynecologic neuroendocrine neoplasms. Endocr. Relat. Cancer 2023, 30, e220206. [Google Scholar] [CrossRef]
- Sigel, C.S.; Krauss Silva, V.W.; Reid, M.D.; Chhieng, D.; Basturk, O.; Sigel, K.M.; Daniel, T.D.; Klimstra, D.S.; Tang, L.H. Assessment of cytologic differentiation in high-grade pancreatic neuroendocrine neoplasms: A multi-institutional study. Cancer Cytopathol. 2018, 126, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Basturk, O.; Sue, J.J.; Klimstra, D.S. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am. J. Surg. Pathol. 2016, 40, 1192–1202. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Dai, S.; Chen, M.; Li, F.; Sun, J.; Luo, F. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA Netw. Open 2021, 4, e2124750. [Google Scholar] [CrossRef]
- Laffi, A.; Spada, F.; Bagnardi, V.; Frassoni, S.; Pisa, E.; Rubino, M.; Barberis, M.; Fazio, N. Gastroenteropancreatic grade 3 neuroendocrine tumors: A single entity or a heterogeneous group? A retrospective analysis. J. Endocrinol. Investig. 2022, 45, 317–325. [Google Scholar] [CrossRef]
- Tang, L.H.; Untch, B.R.; Reidy, D.L.; O’Reilly, E.; Dhall, D.; Jih, L.; Basturk, O.; Allen, P.J.; Klimstra, D.S. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: A pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin. Cancer Res. 2016, 22, 1011–1017. [Google Scholar] [CrossRef]
- Melhorn, P.; Raderer, M.; Mazal, P.; Kozakowski, N.; Kiesewetter, B. NEC versus NET G3—Is there a grey zone? Case report of pancreatic NET G3 with rapid disease progression. memo Mag. Eur. Med. Oncol. 2024, 17, 310–314. [Google Scholar] [CrossRef]
- Pellat, A.; Cottereau, A.S.; Palmieri, L.J.; Soyer, P.; Marchese, U.; Brezault, C.; Coriat, R. Digestive well-differentiated grade 3 neuroendocrine tumors: Current management and future directions. Cancers 2021, 13, 2448. [Google Scholar] [CrossRef]
- Zhang, X.B.; Fan, Y.B.; Jing, R.; Getu, M.A.; Chen, W.Y.; Zhang, W.; Dong, H.X.; Dakal, T.C.; Hayat, A.; Cai, H.J.; et al. Gastroenteropancreatic neuroendocrine neoplasms: Current development, challenges, and clinical perspectives. Mil. Med. Res. 2024, 11, 35. [Google Scholar] [CrossRef]
- Kulke, M.H.; Benson, A.B.; Dasari, A.; Huynh, L.; Cai, B.; Totev, T.; Roesner, N.; Duh, M.S.; Neary, M.P.; Maurer, V.E.; et al. Real-world treatment patterns and clinical outcomes in advanced gastrointestinal neuroendocrine tumors (GI NET): A multicenter retrospective chart review study. Oncologist 2019, 24, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Geyer, S.; Zemla, T.; Knopp, M.V.; Behr, S.; Pulsipher, S.; Ou, F.S.; Dueck, A.C.; Acoba, J.; Shergill, A.; et al. Phase 3 Trial of Cabozantinib to Treat Advanced Neuroendocrine Tumors. N. Engl. J. Med. 2025, 392, 653–665. [Google Scholar] [CrossRef]
- Fjällskog, M.L.; Ludvigsen, E.; Stridsberg, M.; Oberg, K.; Eriksson, B.; Janson, E.T. Expression of somatostatin receptor subtypes 1 to 5 in tumor tissue and intratumoral vessels in malignant endocrine pancreatic tumors. Med. Oncol. 2003, 20, 59–67. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Approves Lutetium Lu 177 Dotatate for Treatment of GEP-NETS. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lutetium-lu-177-dotatate-treatment-gep-nets (accessed on 9 April 2025).
- European Medicines Agency. Authorization Details for Lutathera® in Europe. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera#product-info (accessed on 9 April 2025).
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2–3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef]
- Ammann, M.; Gudmundsdottir, H.; Antwi, S.K.A.; Santol, J.; Podrascanin, V.; Thiels, C.A.; Warner, S.G.; Truty, M.J.; Kendrick, M.L.; Smoot, R.L.; et al. Long-term outcome of cytoreductive hepatectomy in metastatic neuroendocrine neoplasia G3: A single center retrospective analysis. Eur. J. Surg. Oncol. 2025, 51, 109678. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, L.; Dal Buono, A.; Merola, E.; Jann, H.; Jäger, D.; Wiedenmann, B.; Winkler, E.C.; Pavel, M. Multicenter analysis of treatment outcomes for systemic therapy in well differentiated grade 3 neuroendocrine tumors (NET G3). Cancers 2021, 13, 1936. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Bergsland, E.K.; Chan, J.A.; Gadgil, R.; Halfdanarson, T.R.; Hornbacker, K.; Kelly, V.; Kunz, P.L.; McGarrah, P.W.; Raj, N.P.; et al. Temozolomide in grade 3 gastroenteropancreatic neuroendocrine neoplasms: A multicenter retrospective review. Oncologist 2021, 26, 950–955. [Google Scholar] [CrossRef]
- de Mestier, L.; Lamarca, A.; Hernando, J.; Zandee, W.; Alonso-Gordoa, T.; Perrier, M.; Walenkamp, A.M.; Chakrabarty, B.; Landolfi, S.; Van Velthuysen, M.F.; et al. Treatment outcomes of advanced digestive well-differentiated grade 3 NETs. Endocr. Relat. Cancer 2021, 28, 549–561. [Google Scholar] [CrossRef]
- Lithgow, K.; Venkataraman, H.; Hughes, S.; Shah, H.; Kemp-Blake, J.; Vickrage, S.; Smith, S.; Humphries, S.; Elshafie, M.; Taniere, P.; et al. Well-differentiated gastroenteropancreatic G3 NET: Findings from a large single centre cohort. Sci. Rep. 2021, 11, 17947. [Google Scholar] [CrossRef]
- Liu, A.J.; Ueberroth, B.E.; McGarrah, P.W.; Buckner Petty, S.A.; Kendi, A.T.; Starr, J.; Hobday, T.J.; Halfdanarson, T.R.; Sonbol, M.B. Treatment outcomes of well-differentiated high-grade neuroendocrine tumors. Oncologist 2021, 26, 383–388. [Google Scholar] [CrossRef]
- Boutin, M.; Mathews, A.; Badesha, J.; Paul, A.; Safro, M.; Gill, S.; Stuart, H.C.; Schaeffer, D.; Farnell, D.; Loree, J.M. Well-differentiated grade 3 neuroendocrine tumors: Characteristics, treatments, and outcomes from a population-based study. Pancreas 2022, 51, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Müller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Merola, E.; Alonso Gordoa, T.; Zhang, P.; Al-Toubah, T.; Pellè, E.; Kolasińska-Ćwikła, A.; Zandee, W.; Laskaratos, F.; de Mestier, L.; Lamarca, A.; et al. Somatostatin analogs for pancreatic neuroendocrine tumors: Any benefit when Ki-67 is ≥10%? Oncologist 2021, 26, 294–301. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, J.; Jeong, J.H.; Kim, K.P.; Hong, S.M.; Kim, Y.I.; Ryu, J.S.; Ryoo, B.Y.; Yoo, C. Capecitabine plus temozolomide in patients with grade 3 unresectable or metastatic gastroenteropancreatic neuroendocrine neoplasms with Ki-67 index <55%: Single-arm phase II study. ESMO Open 2021, 6, 100119. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.A.H.; Ansquer, C.; Deshayes, E.; Garcia-Carbonero, R.; TeuléVega, A.; Wilmink, J.; Cwikla, J.B.; Srirajaskanthan, R.; Buck, A.; Grana, C.M.; et al. Efficacy and safety of [177Lu]Lu-edotreotide vs everolimus in patients with grade 1 or grade 2 gastroenteropancreaticneuroendocrine tumours: COMPETE Phase 3 trial. In Proceedings of the European Neuroendocrine Tumor Society (ENETS), Krakow, Poland, 5–7 March 2025. [Google Scholar]
- Baudin, E.W.T.; Beron, A.; Smith, D.; Deandreis, D.; Taieb, D.; Ansquer, C.; Diericks, L.; de Mestier, L.; Assenat, E.; Hadoux, J.; et al. Secondary endpoint results of the first academic multicentric randomized phase II trial investigating the antitumor efficacy of 177Lutetium-DOTA-Octreotate(OCLU) in advanced progressive neuroendocrine pancreatic tumour: The OCLURANDOM trial. In Proceedings of the European Neuroendocrine Tumor Society (ENETS), Krakow, Poland, 5–7 March 2025. [Google Scholar]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Pusceddu, S.; Barretta, F.; Trama, A.; Botta, L.; Milione, M.; Buzzoni, R.; De Braud, F.; Mazzaferro, V.; Pastorino, U.; Seregni, E.; et al. A classification prognostic score to predict OS in stage IV well-differentiated neuroendocrine tumors. Endocr. Relat. Cancer 2018, 25, 607–618. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Al-Toubah, T.; El-Haddad, G.; Reidy Lagunes, D.; Bodei, L. Sequencing of somatostatin-receptor-based therapies in neuroendocrine tumor patients. J. Nucl. Med. 2024, 65, 340–348. [Google Scholar] [CrossRef]
- Del Rivero, J.; Perez, K.; Kennedy, E.B.; Mittra, E.S.; Vijayvergia, N.; Arshad, J.; Basu, S.; Chauhan, A.; Dasari, A.N.; Bellizzi, A.M.; et al. Systemic therapy for tumor control in metastatic well-differentiated gastroenteropancreatic neuroendocrine tumors: ASCO guideline. J. Clin. Oncol. 2023, 41, 5049–5067. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Boehm, E.; Prall, O.; Murray, W.K.; Tothill, R.W.; Michael, M. Integrating functional imaging and molecular profiling for optimal treatment selection in neuroendocrine neoplasms (NEN). Curr. Oncol. Rep. 2023, 25, 465–478. [Google Scholar] [CrossRef]
- Bartolomei, M.; Berruti, A.; Falconi, M.; Fazio, N.; Ferone, D.; Lastoria, S.; Pappagallo, G.; Seregni, E.; Versari, A. Clinical management of neuroendocrine neoplasms in clinical practice: A formal consensus exercise. Cancers 2022, 14, 2501. [Google Scholar] [CrossRef]
- NCCN Guidelines®. In Neuroendocrine and Adrenal Tumors; Version 2.2025; NCCN: Plymouth Meeting, PA, USA, 28 May 2025.
- Albertelli, M.; Dotto, A.; Di Dato, C.; Malandrino, P.; Modica, R.; Versari, A.; Colao, A.; Ferone, D.; Faggiano, A. PRRT: Identikit of the perfect patient. Rev. Endocr. Metab. Disord. 2021, 22, 563–579. [Google Scholar] [CrossRef]
- Donadio, M.D.; Brito, Â.B.; Riechelmann, R.P. A systematic review of therapeutic strategies in gastroenteropancreatic grade 3 neuroendocrine tumors. Ther. Adv. Med. Oncol. 2023, 15, 17588359231156218. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, H.S.; Woo, S.; Kim, T.H.; Yoo, C.; Ryoo, B.Y.; Ryu, J.S. Prognostic value of 18F-FDG PET in neuroendocrine neoplasm: A systematic review and meta-analysis. Clin. Nucl. Med. 2021, 46, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Hayes, A.R.; Karfis, I.; Conner, A.; Furtado O’Mahony, L.; Mileva, M.; Bernard, E.; Roach, P.; Marin, G.; Pavlakis, N.; et al. Dual [68Ga]DOTATATE and [18F]FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasms: A multicentre validation of the NETPET score. Br. J. Cancer 2023, 128, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Hayes, A.R.; Karfis, I.; Conner, A.; Mileva, M.; Bernard, E.; Schembri, G.; Navalkissoor, S.; Gnanasegaran, G.; Pavlakis, N.; et al. [18F]FDG PET/CT-avid discordant volume as a biomarker in patients with gastroenteropancreatic neuroendocrine neoplasms: A multicenter study. J. Nucl. Med. 2024, 65, 185–191. [Google Scholar] [CrossRef]
- Hayes, A.R.; Furtado O’Mahony, L.; Quigley, A.M.; Gnanasegaran, G.; Caplin, M.E.; Navalkissoor, S.; Toumpanakis, C. The combined interpretation of 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT in metastatic gastroenteropancreatic neuroendocrine tumors: A classification system with prognostic impact. Clin. Nucl. Med. 2022, 47, 26–35. [Google Scholar] [CrossRef]
- Metser, U.; Nunez, J.E.; Chan, D.; Kulanthaivelu, R.; Murad, V.; Santiago, A.T.; Singh, S. Dual somatostatin receptor/18F-FDG PET/CT imaging in patients with well-differentiated, grade 2 and 3 gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2024, 65, 1591–1596. [Google Scholar] [CrossRef]
- Paiella, S.; Landoni, L.; Tebaldi, S.; Zuffante, M.; Salgarello, M.; Cingarlini, S.; D’Onofrio, M.; Parisi, A.; Deiro, G.; Manfrin, E.; et al. Dual-tracer (68Ga-DOTATOC and 18F-FDG-)-PET/CT scan and G1-G2 nonfunctioning pancreatic neuroendocrine tumors: A single-center retrospective evaluation of 124 nonmetastatic resected cases. Neuroendocrinology 2022, 112, 143–152. [Google Scholar] [CrossRef]
- Pattison, D.A.; Hofman, M.S. Role of Fluorodeoxyglucose PET/Computed Tomography in Targeted Radionuclide Therapy for Endocrine Malignancies. PET Clin. 2015, 10, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Caplin, M.; Castaño, J.P.; Christ, E.; Denecke, T.; Deroose, C.M.; Dromain, C.; Falconi, M.; Grozinsky-Glasberg, S.; Hicks, R.J.; et al. Use and perceived utility of [18F]FDG PET/CT in neuroendocrine neoplasms: A consensus report from the European Neuroendocrine Tumor Society (ENETS) Advisory Board Meeting 2022. J. Neuroendocrinol. 2024, 36, e13359. [Google Scholar] [CrossRef] [PubMed]
- Severi, S.; Nanni, O.; Bodei, L.; Sansovini, M.; Ianniello, A.; Nicoletti, S.; Scarpi, E.; Matteucci, F.; Gilardi, L.; Paganelli, G. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Panzuto, F.; Ramage, J.; Pritchard, D.M.; van Velthuysen, M.F.; Schrader, J.; Begum, N.; Sundin, A.; Falconi, M.; O’Toole, D. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for gastroduodenal neuroendocrine tumours (NETs) G1–G3. J. Neuroendocrinol. 2023, 35, e13306. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of pancreatic neuroendocrine tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Hope, T.A.; Pavel, M.; Bergsland, E.K. Neuroendocrine tumors and peptide receptor radionuclide therapy: When is the right time? J. Clin. Oncol. 2022, 40, 2818–2829. [Google Scholar] [CrossRef]
- Aalbersberg, E.A.; Huizing, D.M.V.; Walraven, I.; de Wit-van der Veen, B.J.; Kulkarni, H.R.; Singh, A.; Stokkel, M.P.M.; Baum, R.P. Parameters to predict progression-free and overall survival after peptide receptor radionuclide therapy: A multivariate analysis in 782 patients. J. Nucl. Med. 2019, 60, 1259–1265. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Halfdanarson, T.R.; Bellizzi, A.M.; Chan, J.A.; Dillon, J.S.; Heaney, A.P.; Kunz, P.L.; O’Dorisio, T.M.; Salem, R.; Segelov, E.; et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 2017, 46, 707–714. [Google Scholar] [CrossRef]
- Chauhan, A.; Del Rivero, J.; Ramirez, R.A.; Soares, H.P.; Li, D. Treatment sequencing strategies in advanced neuroendocrine tumors: A review. Cancers 2022, 14, 5248. [Google Scholar] [CrossRef]
- Naraev, B.G.; Mailman, J.; Halfdanarson, T.R.; Soares, H.P.; Mittra, E.S.; Hallet, J. Consideration of quality of life in the treatment decision-making for patients with advanced gastroenteropancreatic neuroendocrine tumors. Expert. Rev. Anticancer Ther. 2023, 23, 601–615. [Google Scholar] [CrossRef]
- Becx, M.N.; Minczeles, N.S.; Brabander, T.; de Herder, W.W.; Nonnekens, J.; Hofland, J. A clinical guide to peptide receptor radionuclide therapy with 177Lu-DOTATATE in neuroendocrine tumor patients. Cancers 2022, 14, 5792. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the Phase III NETTER-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Edfeldt, K.; Hellman, P.; Granberg, D.; Lagergren, P.; Thiis-Evensen, E.; Sundin, A.; Andersson, C. Improved health-related quality of life during peptide receptor radionuclide therapy in patients with neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13342. [Google Scholar] [CrossRef]
- Martini, C.; Buxbaum, S.; Rodrigues, M.; Nilica, B.; Scarpa, L.; Holzner, B.; Virgolini, I.; Gamper, E.M. Quality of life in patients with metastatic gastroenteropancreatic neuroendocrine tumors receiving peptide receptor radionuclide therapy: Information from a monitoring program in clinical routine. J. Nucl. Med. 2018, 59, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Burkett, B.J.; Dundar, A.; Young, J.R.; Packard, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Eiring, R.A.; Gansen, D.N.; Patton, C.M.; Kendi, A.T. How we do it: A multidisciplinary approach to 177Lu DOTATATE peptide receptor radionuclide therapy. Radiology 2021, 298, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Merola, E.; Michielan, A.; Rozzanigo, U.; Erini, M.; Sferrazza, S.; Marcucci, S.; Sartori, C.; Trentin, C.; de Pretis, G.; Chierichetti, F. Therapeutic strategies for gastroenteropancreatic neuroendocrine neoplasms: State-of-the-art and future perspectives. World J. Gastrointest. Surg. 2022, 14, 78–106. [Google Scholar] [CrossRef]
- Alsadik, S.; Gnanasegaran, G.; Chen, L.; Mandair, D.; Toumpanakis, C.; Caplin, M.; Navalkissoor, S. Safety of peptide receptor radionuclide therapy with 177Lu-DOTATATE in neuroendocrine tumor patients with chronic kidney disease. J. Nucl. Med. 2022, 63, 1503–1508. [Google Scholar] [CrossRef]
- Baum, R.P.; Fan, X.; Jakobsson, V.; Yu, F.; Schuchardt, C.; Chen, X.; Zhang, J. Long-term nephrotoxicity after PRRT: Myth or reality. Theranostics 2024, 14, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Al-Toubah, T.; Pellè, E.; Smith, J.; Haider, M.; Hutchinson, T.; Fleming, J.B.; El-Haddad, G. Risk of bowel obstruction in patients with mesenteric or peritoneal disease receiving peptide receptor radionuclide therapy. J. Nucl. Med. 2021, 62, 69–72. [Google Scholar] [CrossRef]
- Fernandez, C.J.; Agarwal, M.; Pottakkat, B.; Haroon, N.N.; George, A.S.; Pappachan, J.M. Gastroenteropancreatic neuroendocrine neoplasms: A clinical snapshot. World J. Gastrointest. Surg. 2021, 13, 231–255. [Google Scholar] [CrossRef]
- Raymond, L.M.; Korzun, T.; Kardosh, A.; Kolbeck, K.J.; Pommier, R.; Mittra, E.S. The state of peptide receptor radionuclide therapy and its sequencing among current therapeutic options for gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology 2021, 111, 1086–1098. [Google Scholar] [CrossRef]
- Hope, T.A.; Abbott, A.; Colucci, K.; Bushnell, D.L.; Gardner, L.; Graham, W.S.; Lindsay, S.; Metz, D.C.; Pryma, D.A.; Stabin, M.G.; et al. NANETS/SNMMI Procedure Standard for Somatostatin Receptor-Based Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. J. Nucl. Med. 2019, 60, 937–943. [Google Scholar] [CrossRef]
- Padilla-Morales, E.; Tan, A.; Hope, T.; Bergsland, E. Comparison of Patient Experience with Commercial Versus Compounded Amino Acid Infusion for 177Lu-DOTATATE Therapy. J. Nucl. Med. 2019, 60, 2015. [Google Scholar]
- Al-Toubah, T.E.; Pelle, E.; Strosberg, J.R. Risk of myelodysplastic syndrome/acute leukemia with sequential capecitabine/temozolomide and 177Lu-dotatate. J. Clin. Oncol. 2022, 40, 513. [Google Scholar] [CrossRef]
- Alonzo, N.; Seyer, M.; Kim, E.-J.; Keshavarzi, R.; Yee, K.; Kunz, P.L. Evaluation of the incidence of acute nausea and vomiting after administration of an amino acid solution containing only arginine and lysine with lutetium Lu-177 dotatate. J. Clin. Oncol. 2020, 38, 12113. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Fazio, N.; Granberg, D.; Grozinsky-Glasberg, S.; Ahmadzadehfar, H.; Grana, C.M.; Zandee, W.T.; Cwikla, J.; Walter, M.A.; Oturai, P.S.; et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr. Relat. Cancer 2019, 26, 227–239. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef]
- Pritzl, S.L.; Kusne, Y.; Halfdanarson, T.R.; Hobday, T.; Sonbol, M.B.; Kendi, A.T.; Mangaonkar, A.A.; Gangat, N.; Shah, M.; Patnaik, M.M. Spectrum of therapy-related clonal cytopenias and neoplasms after exposure to Lutetium-177-Dotatate. Leuk. Res. 2024, 136, 107434. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Halfdanarson, T.R.; Hilal, T. Assessment of therapy-related myeloid neoplasms in patients with neuroendocrine tumors after peptide receptor radionuclide therapy: A systematic review. JAMA Oncol. 2020, 6, 1086–1092. [Google Scholar] [CrossRef]
- Brieau, B.; Hentic, O.; Lebtahi, R.; Palazzo, M.; Ben Reguiga, M.; Rebours, V.; Maire, F.; Hammel, P.; Ruszniewski, P.; Fenaux, P. High risk of myelodysplastic syndrome and acute myeloid leukemia after 177Lu-octreotate PRRT in NET patients heavily pretreated with alkylating chemotherapy. Endocr. Relat. Cancer 2016, 23, L17-23. [Google Scholar] [CrossRef]
- Morton, L.M.; Dores, G.M.; Schonfeld, S.J.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Curtis, R.E. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019, 5, 318–325. [Google Scholar] [CrossRef]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.E.; Kunz, P.L.; Chasen, B.; Capdevila, J.; Tafuto, S.; Oh, D.Y.; et al. 211MO First-line efficacy of [177Lu]Lu-DOTA-TATE in patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors by tumor grade and primary origin: Subgroup analysis of the phase III NETTER-2 study. Ann. Oncol. 2024, 35, S92–S93. [Google Scholar] [CrossRef]
- Kunz, P.L.; Ferone, D.; Halperin, D.M.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Chasen, B.; Capdevila, J.; Tafuto, S.; Oh, D.-Y.; et al. Safety and time to response of [177Lu]Lu-DOTATATE in patients with newly diagnosed advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors: Sub-analysis of the phase 3 randomized NETTER-2 study. J. Clin. Oncol. 2024, 42, 4131. [Google Scholar] [CrossRef]
- Singh, S.; Hope, T.A.; Bergsland, E.B.; Bodei, L.; Bushnell, D.L.; Chan, J.A.; Chasen, B.R.; Chauhan, A.; Das, S.; Dasari, A.; et al. Consensus report of the 2021 National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J. Natl. Cancer Inst. 2023, 115, 1001–1010. [Google Scholar] [CrossRef]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef]
- Strosberg, J.; Leeuwenkamp, O.; Siddiqui, M.K. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 93, 102141. [Google Scholar] [CrossRef]
- van der Zwan, W.A.; Brabander, T.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; Krenning, E.P.; de Herder, W.W. Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 704–717. [Google Scholar] [CrossRef]
- Chauhan, A.; O’Callaghan, C.; Myrehaug, S.; Bodei, L.; Kunz, P.; Dasari, A.; Strosberg, J.; Alexander, S.; Cheung, W.; Singh, S. NET RETREAT: A Phase II study of 177Lutetium-Dotatate retreatment vs. everolimus in metastatic/unresectable midgut NET. Endocr. Abstr. 2023, 98, 23777. [Google Scholar] [CrossRef]
- Halperin, D.M.; Morris, M.; Ulaner, G.A.; Strosberg, J.R.; Mehr, S.H.; Li, D.; Soares, H.P.; Anthony, L.B.; Kotiah, S.D.; Jacene, H.; et al. Phase Ib portion of the ACTION-1 phase Ib/3 trial of RYZ101 in gastroenteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177Lu somatostatin analogue (SSA) therapy: Safety and efficacy findings. J. Clin. Oncol. 2024, 42, 3091. [Google Scholar] [CrossRef]
- Urso, L.; Panareo, S.; Castello, A.; Ambrosio, M.R.; Zatelli, M.C.; Caracciolo, M.; Tonini, E.; Valpiani, G.; Boschi, A.; Uccelli, L.; et al. Glucose Metabolism Modification Induced by Radioligand Therapy with [177Lu]Lu/[90Y]Y-DOTATOC in Advanced Neuroendocrine Neoplasms: A Prospective Pilot Study within FENET-2016 Trial. Pharmaceutics 2022, 14, 2009. [Google Scholar] [CrossRef]
- Harris, P.E.; Zhernosekov, K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front. Endocrinol. 2022, 13, 941832. [Google Scholar] [CrossRef]
- Prasad, V.; Trikalinos, N.; Hanna, A.; Johnson, F.; Puhlmann, M.; Wahl, R. A Phase I/IIa of [212Pb]VMT-α-NET targeted alpha-particle therapy for advanced SSTR2 positive neuroendocrine tumors. J. Nucl. Med. 2024, 65, 242430. [Google Scholar]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: First-in-humans dose-escalation clinical trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef]
- Santo, G.; di Santo, G.; Cicone, F.; Virgolini, I. Peptide receptor radionuclide therapy with somatostatin analogs beyond gastroenteropancreatic neuroendocrine tumors. J. Neuroendocrinol. 2025, 37, e70013. [Google Scholar] [CrossRef]
- Chow, Z.; Johnson, J.; Chauhan, A.; Jeong, J.C.; Castle, J.T.; Izumi, T.; Weiss, H.; Townsend, C.M., Jr.; Schrader, J.; Anthony, L.; et al. Inhibition of ribonucleotide reductase subunit M2 enhances the radiosensitivity of metastatic pancreatic neuroendocrine tumor. Cancer Lett. 2024, 596, 216993. [Google Scholar] [CrossRef]
- Chauhan, A.; Arnold, S.; Kolesar, J.; Carson, W.; Weiss, H.; Jayswal, R.; Yan, D.; Khouli, R.E.; Khurana, A.; Beumer, J.; et al. Abstract CT194: ETCTN 10388: A first in human phase I trial of triapine and lutetium Lu 177 DOTATATE in well-differentiated somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Cancer Res. 2023, 83, CT194. [Google Scholar] [CrossRef]
- Chauhan, A.; Kolesar, J.; Yan, D.; Li, D.; Khurana, A.; Carson, W.E.; Arnold, S.M.; Gore, S.; Rubinstein, L.; Kohn, E.C.; et al. ETCTN 10450: A phase I trial of peposertib and lutetium 177 DOTATATE in well-differentiated somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol. 2023, 41, TPS658. [Google Scholar] [CrossRef]
- Parghane, R.V.; Ostwal, V.; Ramaswamy, A.; Bhandare, M.; Chaudhari, V.; Talole, S.; Shrikhande, S.V.; Basu, S. Long-term outcome of “Sandwich” chemo-PRRT: A novel treatment strategy for metastatic neuroendocrine tumors with both FDG- and SSTR-avid aggressive disease. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 913–923. [Google Scholar] [CrossRef]
- Nicolini, S.; Bodei, L.; Bongiovanni, A.; Sansovini, M.; Grassi, I.; Ibrahim, T.; Monti, M.; Caroli, P.; Sarnelli, A.; Diano, D.; et al. Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3260–3267. [Google Scholar] [CrossRef]
- Calomino, N.; Poto, G.E.; Carbone, L.; Bagnacci, G.; Piccioni, S.; Andreucci, E.; Nenci, L.; Marano, L.; Verre, L.; Petrioli, R.; et al. Neuroendocrine tumors’ patients treated with somatostatin analogue could complicate with emergency cholecystectomy. Ann. Ital. Chir. 2023, 94, 518–522. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, A.; Halfdanarson, T.R.; Vijayvergia, N. Sequencing Choices and Real-World Clinical Management in Advanced Grade 2/3 GEP-NET Treatment: The Emerging Role of PRRT. Cancers 2025, 17, 3008. https://doi.org/10.3390/cancers17183008
Chauhan A, Halfdanarson TR, Vijayvergia N. Sequencing Choices and Real-World Clinical Management in Advanced Grade 2/3 GEP-NET Treatment: The Emerging Role of PRRT. Cancers. 2025; 17(18):3008. https://doi.org/10.3390/cancers17183008
Chicago/Turabian StyleChauhan, Aman, Thorvardur R. Halfdanarson, and Namrata Vijayvergia. 2025. "Sequencing Choices and Real-World Clinical Management in Advanced Grade 2/3 GEP-NET Treatment: The Emerging Role of PRRT" Cancers 17, no. 18: 3008. https://doi.org/10.3390/cancers17183008
APA StyleChauhan, A., Halfdanarson, T. R., & Vijayvergia, N. (2025). Sequencing Choices and Real-World Clinical Management in Advanced Grade 2/3 GEP-NET Treatment: The Emerging Role of PRRT. Cancers, 17(18), 3008. https://doi.org/10.3390/cancers17183008








