Cytokine Profiling of Children, Adolescents, and Young Adults Newly Diagnosed with Sarcomas Demonstrates the Role of IL-1β in Osteosarcoma Metastasis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Plasma Extraction
2.3. Luminex Assays
2.4. Statistical Analysis
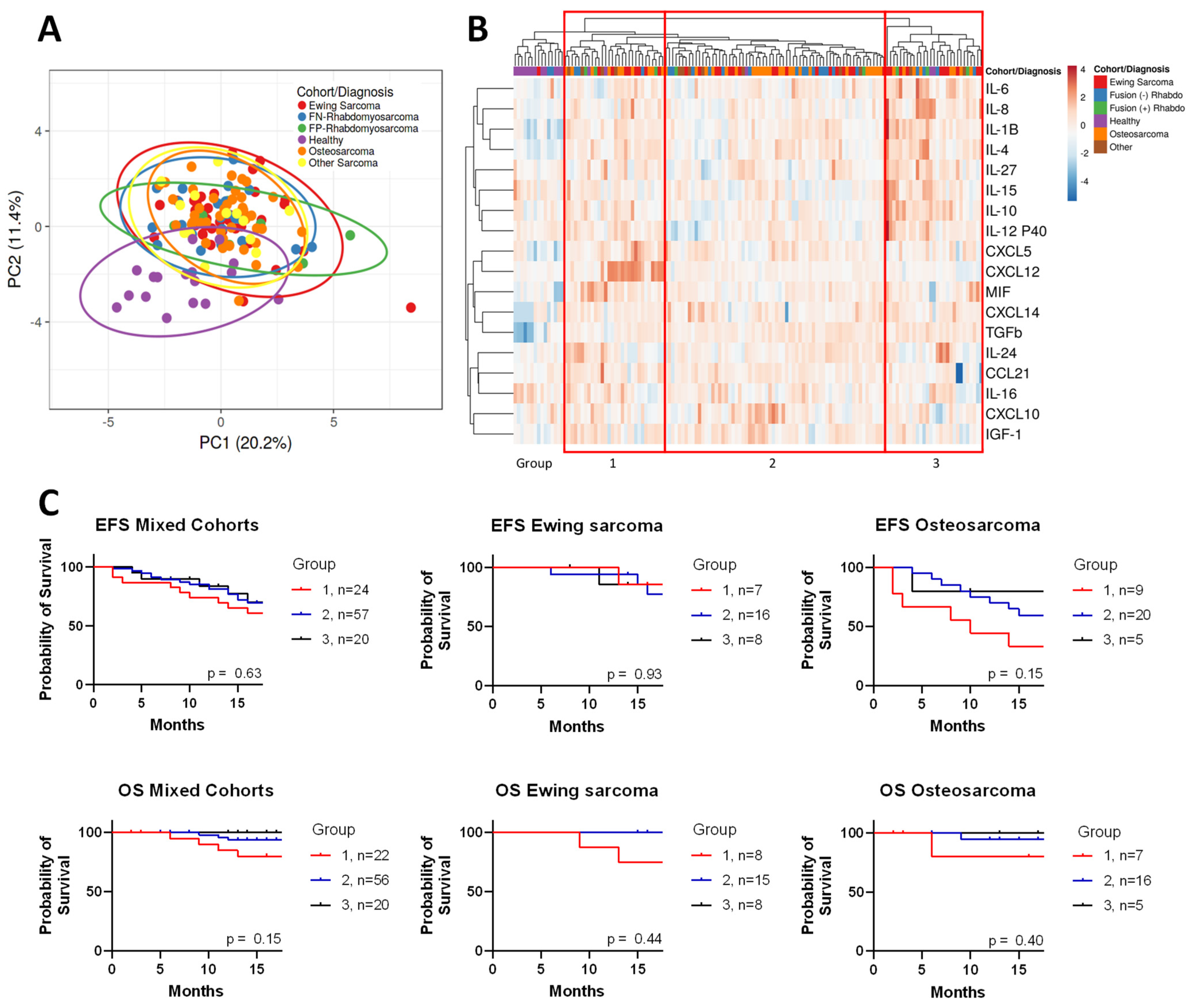
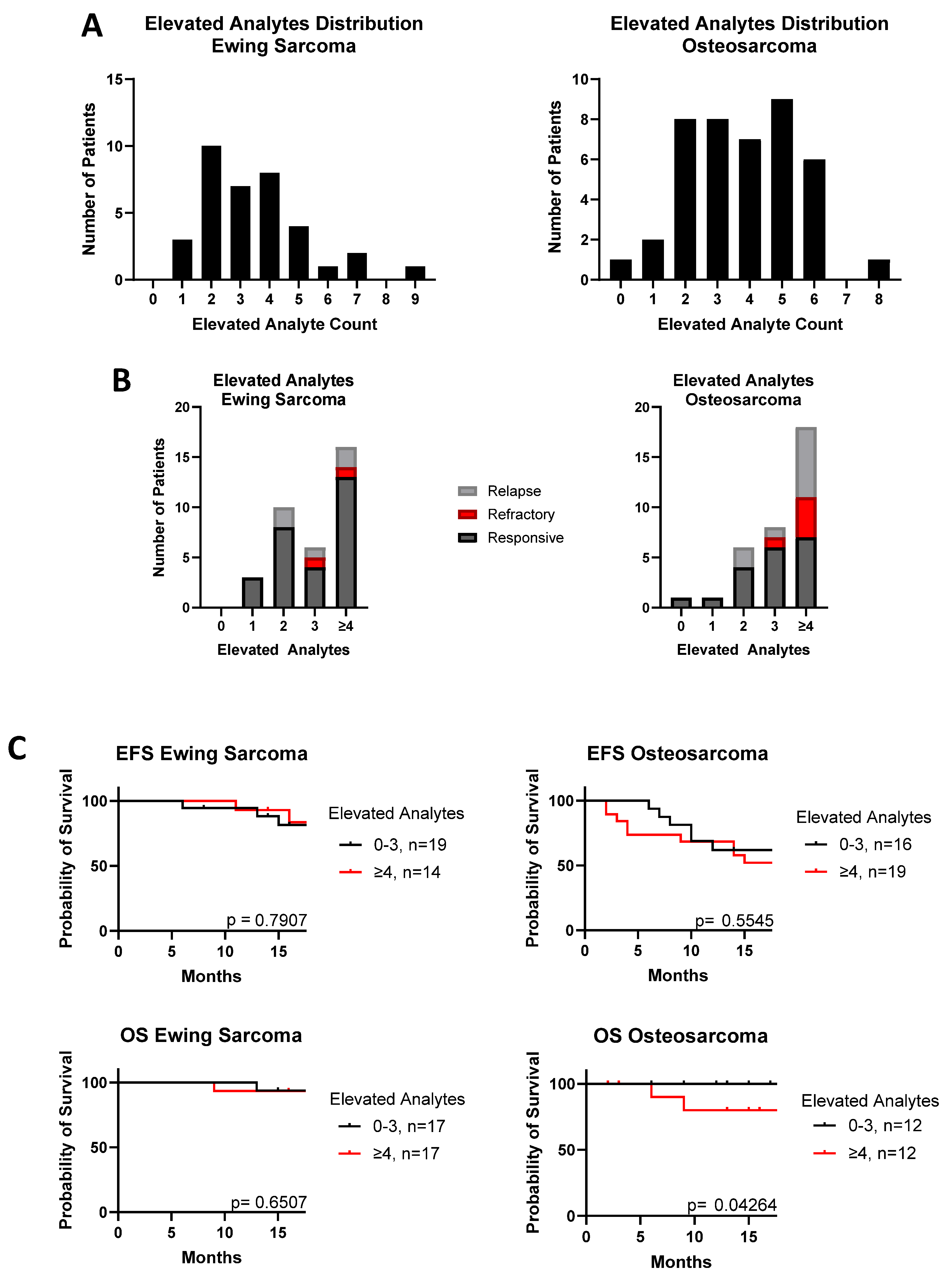
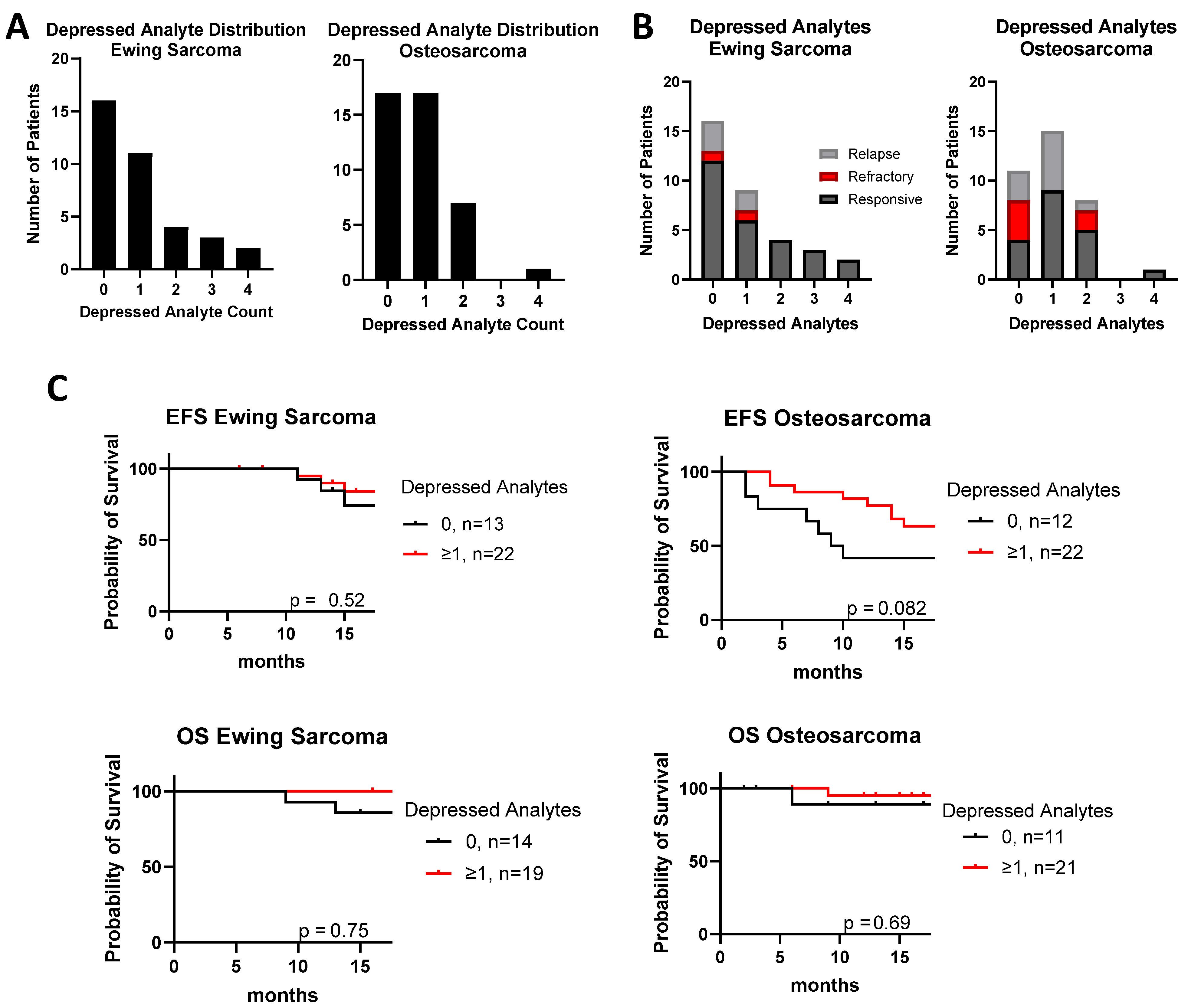
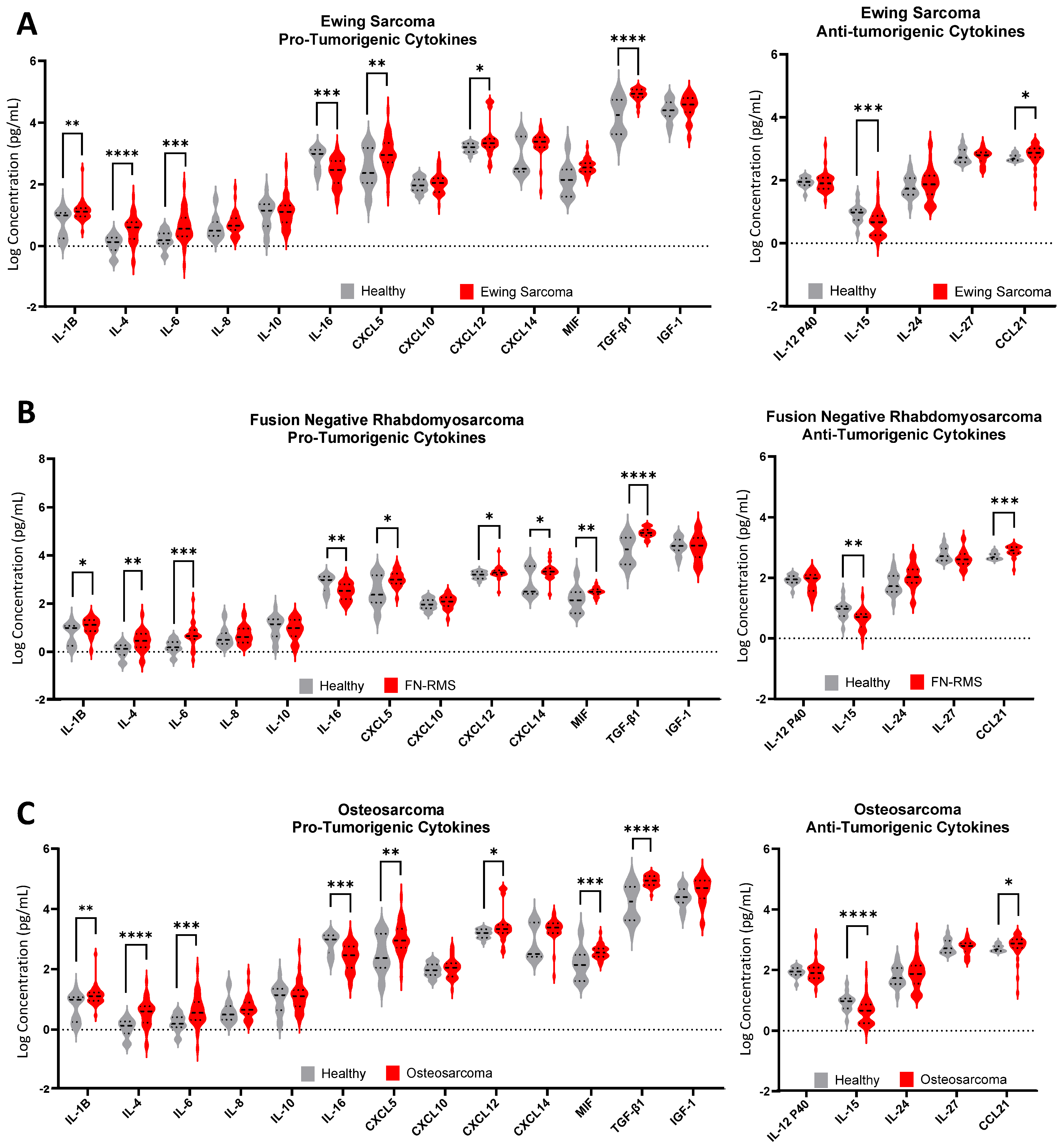
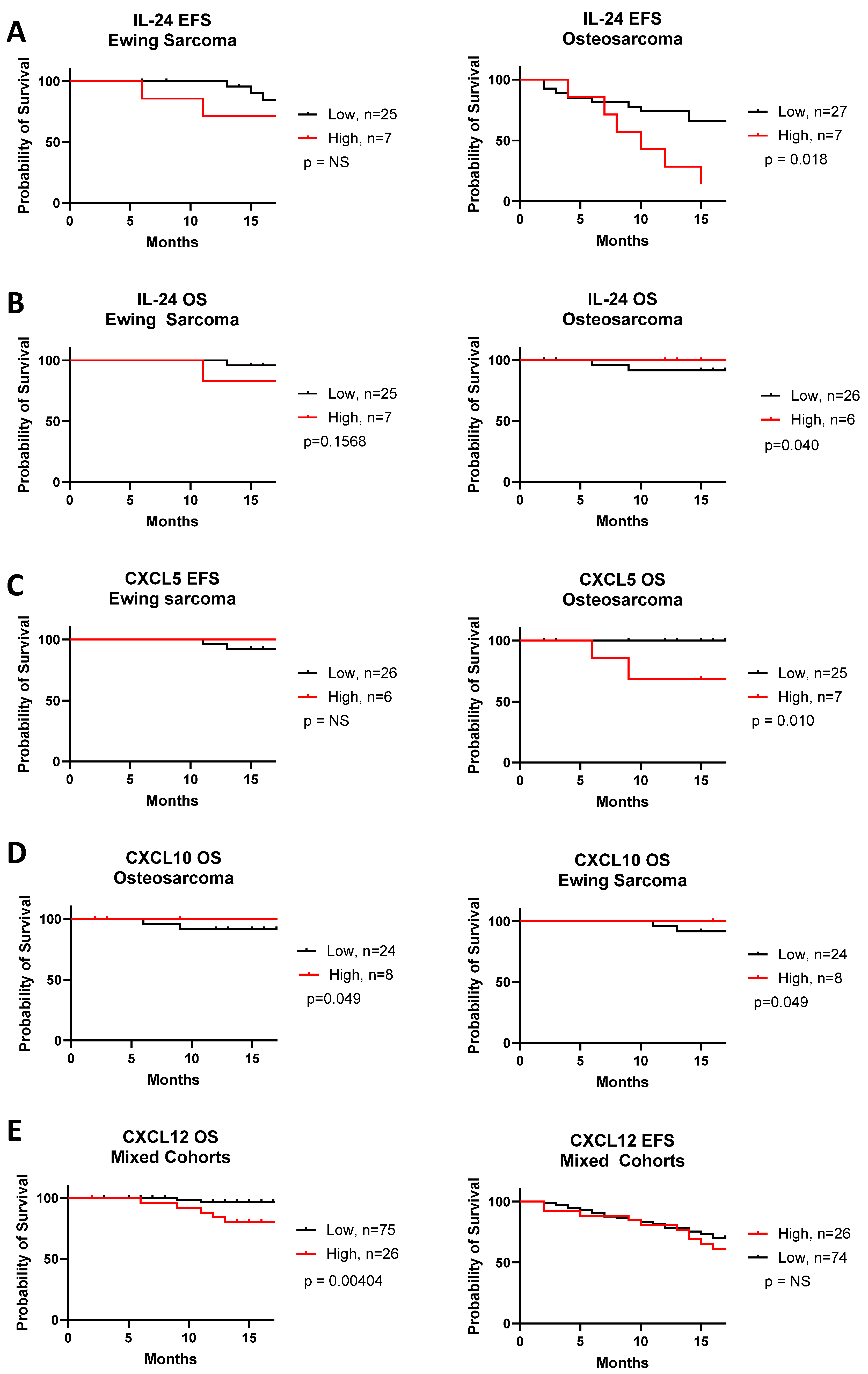
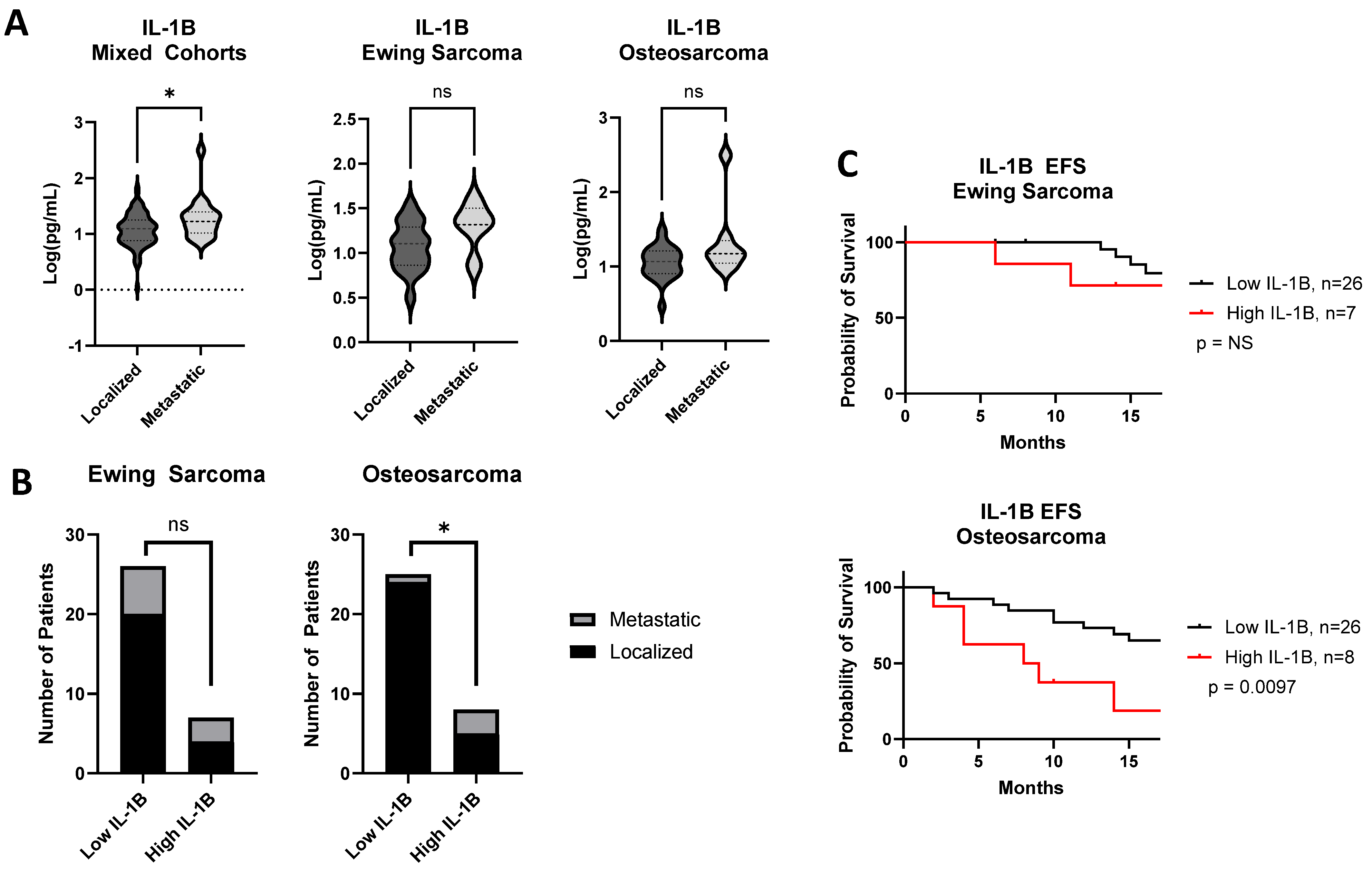
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Conflicts of Interest
References
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-Induced Inflammatory Cytokines and the Emerging Diagnostic Devices for Cancer Detection and Prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Lamot, L.; Niemietz, I.; Brown, K.L. Methods for type I interferon detection and their relevance for clinical utility and improved understanding of rheumatic diseases. Clin. Exp. Rheumatol. 2019, 37, 1077–1083. [Google Scholar]
- Yang, H.; Zhao, L.; Zhang, Y.; Li, F. A comprehensive analysis of immune infiltration in the tumor microenvironment of osteosarcoma. Cancer Med. 2021, 10, 5696–5711. [Google Scholar] [CrossRef]
- Kloen, P.; Gebhardt, M.C.; Perez-Atayde, A.; Rosenberg, A.E.; Springfield, D.S.; Gold, L.I.; Mankin, H.J. Expression of transforming growth factor-β (TGF-beta) isoforms in osteosarcomas: TGF-β3 is related to disease progression. Cancer 1997, 80, 2230–2239. [Google Scholar] [CrossRef]
- Lee, M.H.; Laajala, E.; Kreutzman, A.; Järvinen, P.; Nísen, H.; Mirtti, T.; Hollmén, M.; Mustjoki, S. The tumor and plasma cytokine profiles of renal cell carcinoma patients. Sci. Rep. 2022, 12, 13416. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Zhang, C.Y.; Lu, C.Y.; Guo, G.H.; Tian, Y.P.; Li, Y.L. Serum expression level of cytokine and chemokine correlates with progression of human ovarian cancer. Eur. J. Gynaecol. Oncol. 2017, 38, 33–39. [Google Scholar] [CrossRef]
- Munisamy, S.; Radhakrishnan, A.K.; Ramdas, P.; Samuel, P.J.; Singh, V.A. Immune Biomarkers in Blood from Sarcoma Patients: A Pilot Study. Curr. Oncol. 2022, 29, 5585–5603. [Google Scholar] [CrossRef]
- Helman, L.J.; Meltzer, P. Mechanisms of sarcoma development. Nat. Rev. Cancer 2003, 3, 685–694. [Google Scholar] [CrossRef]
- Grünewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef]
- Wang, M.; Xia, F.; Wei, Y.; Wei, X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 2020, 8, 30. [Google Scholar] [CrossRef]
- Yao, M.; Brummer, G.; Acevedo, D.; Cheng, N. Cytokine Regulation of Metastasis and Tumorigenicity. Adv. Cancer Res. 2016, 132, 265–367. [Google Scholar] [CrossRef]
- Landuzzi, L.; Ruzzi, F.; Pellegrini, E.; Lollini, P.-L.; Scotlandi, K.; Manara, M.C. IL-1 Family Members in Bone Sarcomas. Cells 2024, 13, 233. [Google Scholar] [CrossRef]
- Richmond, J.; Tuzova, M.; Cruikshank, W.; Center, D. Regulation of Cellular Processes by Interleukin-16 in Homeostasis and Cancer. J. Cell Physiol. 2014, 229, 139–147. [Google Scholar] [CrossRef]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Jiang, R.; Meng, E.; Wu, H. CXCL5: A coachman to drive cancer progression. Front. Oncol. 2022, 12, 944494. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.C.G.; De Araújo, J.M.G.; Fernandes, T.A.A.D.M.; Cobucci, R.N.O.; Lanza, D.C.F.; Andrade, V.S.; Fernandes, J.V. Macrophage Migration Inhibitory Factor (MIF): Biological Activities and Relation with Cancer. Pathol. Oncol. Res. 2017, 23, 235–244. [Google Scholar] [CrossRef]
- Liang, W.; Yang, C.; Peng, J.; Qian, Y.; Wang, Z. The Expression of HSPD1, SCUBE3, CXCL14 and Its Relations with the Prognosis in Osteosarcoma. Cell Biochem. Biophys. 2015, 73, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, C.; Morrione, A.; Scotlandi, K. Unraveling the IGF System Interactome in Sarcomas Exploits Novel Therapeutic Options. Cells 2021, 10, 2075. [Google Scholar] [CrossRef]
- Li, Y.; Flores, R.; Yu, A.; Okcu, M.F.; Murray, J.; Chintagumpala, M.; Hicks, J.; Lau, C.C.; Man, T.-K. Elevated expression of CXC chemokines in pediatric osteosarcoma patients. Cancer 2011, 117, 207–217. [Google Scholar] [CrossRef]
- Lu, J.; Song, G.; Tang, Q.; Zou, C.; Han, F.; Zhao, Z.; Yong, B.; Yin, J.; Xu, H.; Xie, X.; et al. IRX1 hypomethylation promotes osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J. Clin. Investig. 2015, 125, 1839–1856. [Google Scholar] [CrossRef]
- Smyth, M.J.; Taniguchi, M.; Street, S.E.A. The Anti-Tumor Activity of IL-12: Mechanisms of Innate Immunity That Are Model and Dose Dependent. J. Immunol. 2000, 165, 2665–2670. [Google Scholar] [CrossRef]
- Fiore, P.F.; Di Matteo, S.; Tumino, N.; Mariotti, F.R.; Pietra, G.; Ottonello, S.; Negrini, S.; Bottazzi, B.; Moretta, L.; Mortier, E.; et al. Interleukin-15 and cancer: Some solved and many unsolved questions. J. Immunother. Cancer 2020, 8, e001428. [Google Scholar] [CrossRef]
- Fisher, P.B. Is mda-7/IL-24 a “Magic Bullet” for Cancer? Cancer Res. 2005, 65, 10128–10138. [Google Scholar] [CrossRef]
- Yoshida, H.; Hunter, C.A. The Immunobiology of Interleukin-27. Annu. Rev. Immunol. 2015, 33, 417–443. [Google Scholar] [CrossRef]
- Lin, Y.; Sharma, S.; John, M. CCL21 Cancer Immunotherapy. Cancers 2014, 6, 1098–1110. [Google Scholar] [CrossRef]
- Ruka, W.; Rutkowski, P.; Kaminska, J.; Rysinska, A.; Steffen, J. Alterations of routine blood tests in adult patients with soft tissue sarcomas: Relationships to cytokine serum levels and prognostic significance. Ann. Oncol. 2001, 12, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.M.; Bharathy, N.; Abraham, J.; Kim, J.-A.; Rudzinski, E.R.; Michalek, J.E.; Keller, C. Interleukin-4 Receptor Inhibition Targeting Metastasis Independent of Macrophages. Mol. Cancer Ther. 2021, 20, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Qin, Z. Paradoxical Roles of IL-4 in Tumor Immunity. Cell Mol. Immunol. 2009, 6, 415–422. [Google Scholar] [CrossRef]
- Allende, C.; Higgins, B.; Johns, J. Comparison of serum cytokine concentrations between healthy dogs and canine osteosarcoma patients at the time of diagnosis. Vet. Immunol. Immunopathol. 2020, 227, 110084. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Kamińska, J.; Kowalska, M.; Ruka, W.; Steffen, J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: Correlations with local tumor extent and prognosis. J. Surg. Oncol. 2003, 84, 151–159. [Google Scholar] [CrossRef]
- Hagi, T.; Nakamura, T.; Iino, T.; Matsubara, T.; Asanuma, K.; Matsumine, A.; Sudo, A. The diagnostic and prognostic value of interleukin-6 in patients with soft tissue sarcomas. Sci. Rep. 2017, 7, 9640. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, M.J.; Cruz, A.; Faber, M.; Oldham, R.A.A.; Wang, D.; Medin, J.A.; Schloemer, N.J. Sarcoma IL-12 overexpression facilitates NK cell immunomodulation. Sci. Rep. 2021, 11, 8321. [Google Scholar] [CrossRef] [PubMed]
- Lollini, P.-L.; Palmieri, G.; De Giovanni, C.; Landuzzi, L.; Nicoletti, G.; Rossi, I.; Griffoni, C.; Frabetti, F.; Scotlandi, K.; Benini, S.; et al. Expression of interleukin 15 (IL-15) in human rhabdomyosarcoma, osteosarcoma and Ewing’s sarcoma. Int. J. Cancer 1997, 71, 732–736. [Google Scholar] [CrossRef]
- Rebhun, R.B.; York, D.; Cruz, S.M.; Judge, S.J.; Razmara, A.M.; Farley, L.E.; Brady, R.V.; Johnson, E.G.; Burton, J.H.; Willcox, J.; et al. Inhaled recombinant human IL-15 in dogs with naturally occurring pulmonary metastases from osteosarcoma or melanoma: A phase 1 study of clinical activity and correlates of response. J. Immunother. Cancer 2022, 10, e004493. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Wang, J.-L.; Xie, K.-G.; Lan, C.-G. Association of interleukin 16 gene polymorphisms and plasma IL16 level with osteosarcoma risk. Sci. Rep. 2016, 6, 34607. [Google Scholar] [CrossRef]
- Zhuo, B.; Wang, X.; Shen, Y.; Li, J.; Li, S.; Li, Y.; Wang, R. Interleukin-24 inhibits the phenotype and tumorigenicity of cancer stem cell in osteosarcoma via downregulation Notch and Wnt/β-catenin signaling. J. Bone Oncol. 2021, 31, 100403. [Google Scholar] [CrossRef]
- Kushlinskii, N.E.; Timofeev, Y.S.; Solov’ev, Y.N.; Gerstein, E.S.; Lyubimova, N.V.; Bulycheva, I.V. Components of the RANK/RANKL/OPG System, IL-6, IL-8, IL-16, MMP-2, and Calcitonin in the Sera of Patients with Bone Tumors. Bull. Exp. Biol. Med. 2014, 157, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, F.; Gao, H.; Xu, Z.; Zhou, Y.; Wang, S.; Lv, Z.; Zhang, Y.; Xu, Z.; Huo, J.; et al. Cytokine concentration in peripheral blood of patients with colorectal cancer. Front. Immunol. 2023, 14, 1175513. [Google Scholar] [CrossRef]
- Khoshroo, M.; Yazdanpanah, M.J.; Yasrebi, S. Serum IL-24 Levels in Gastric and Breast Cancers and Non-cancerous Inflammations. Middle East J. Cancer 2021, 12, 183–189. [Google Scholar] [CrossRef]
- Hayashi, M.; Chu, D.; Meyer, C.F.; Llosa, N.J.; McCarty, G.; Morris, C.D.; Levin, A.S.; Wolinsky, J.-P.; Albert, C.M.; Steppan, D.A.; et al. Highly personalized detection of minimal Ewing sarcoma disease burden from plasma tumor DNA. Cancer 2016, 122, 3015–3023. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Evdokimova, V.; Gassmann, H.; Radvanyi, L.; Burdach, S.E.G. Current State of Immunotherapy and Mechanisms of Immune Evasion in Ewing Sarcoma and Osteosarcoma. Cancers 2022, 15, 272. [Google Scholar] [CrossRef]
- Kelesidis, T.; Yang, O. Good’s syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin. Immunol. 2010, 135, 347–363. [Google Scholar] [CrossRef]
- Marx, A.; Belharazem, D.; Lee, D.-H.; Popovic, Z.V.; Reißfelder, C.; Schalke, B.; Schölch, S.; Ströbel, P.; Weis, C.-A.; Yamada, Y. Molecular pathology of thymomas: Implications for diagnosis and therapy. Virchows Arch. 2021, 478, 101–110. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, C. When the Good Syndrome Goes Bad: A Systematic Literature Review. Front. Immunol. 2021, 12, 679556. [Google Scholar] [CrossRef] [PubMed]
- Kerner, G.; Neehus, A.-L.; Philippot, Q.; Bohlen, J.; Rinchai, D.; Kerrouche, N.; Puel, A.; Zhang, S.-Y.; Boisson-Dupuis, S.; Abel, L.; et al. Genetic adaptation to pathogens and increased risk of inflammatory disorders in post-Neolithic Europe. Cell Genom. 2023, 3, 100248. [Google Scholar] [CrossRef]
- Schnappauf, O.; Chae, J.J.; Kastner, D.L.; Aksentijevich, I. The Pyrin Inflammasome in Health and Disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Wu, W.; Wang, B.; Cui, C.; Niu, J.; Chen, J.; Chen, Z.; Liu, Y. CXCL5 Plays a Promoting Role in Osteosarcoma Cell Migration and Invasion in Autocrine- and Paracrine-Dependent Manners. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 177–186. [Google Scholar] [CrossRef]
- Brylka, L.J.; Schinke, T. Chemokines in Physiological and Pathological Bone Remodeling. Front. Immunol. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Rutkowski, P.; Kaminska, J.; Kowalska, M.; Ruka, W.; Steffen, J. Cytokine serum levels in soft tissue sarcoma patients: Correlations with clinico-pathological features and prognosis. Int. J. Cancer 2002, 100, 463–471. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Li, M.; Bao, Q.; Zhang, Z.; Wang, B.; Liu, Z.; Wen, J.; Wan, R.; Shen, Y.; Zhang, W. Exceptional response to PD-1 inhibition immunotherapy in advanced metastatic osteosarcoma with tumor site infection. J. Immunother. Cancer 2022, 10, e004673. [Google Scholar] [CrossRef]
- Grimes, C.L.; Ariyananda, L.D.Z.; Melnyk, J.E.; O’Shea, E.K. The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J. Am. Chem. Soc. 2012, 134, 13535–13537. [Google Scholar] [CrossRef]
- Cai, M.; Huang, X.; Huang, X.; Ju, D.; Zhu, Y.Z.; Ye, L. Research progress of interleukin-15 in cancer immunotherapy. Front. Pharmacol. 2023, 14, 1184703. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Inui, N.; Karayama, M.; Asada, K.; Fujii, M.; Matsuura, S.; Uto, T.; Hashimoto, D.; Matsui, T.; Ikeda, M.; et al. Cytokine profiling identifies circulating IL-6 and IL-15 as prognostic stratifiers in patients with non-small cell lung cancer receiving anti-PD-1/PD-L1 blockade therapy. Cancer Immunol. Immunother. 2023, 72, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Kumamoto, Y.; Miyao, N.; Masumori, N.; Takahashi, A.; Yanase, M. Interleukin-6 in Renal Cell Carcinoma. J. Urol. 1992, 148, 1778–1781. [Google Scholar] [CrossRef]
- Martín, F.; Santolaria, F.; Batista, N.; Milena, A.; González-Reimers, E.; Brito, M.J.; Oramas, J. CYTOKINE LEVELS (IL-6 AND IFN-γ), ACUTE PHASE RESPONSE AND NUTRITIONAL STATUS AS PROGNOSTIC FACTORS IN LUNG CANCER. Cytokine 1999, 11, 80–86. [Google Scholar] [CrossRef]
- Tartour, E.; Dorval, T.; Mosseri, V.; Deneux, L.; Mathiot, C.; Brailly, H.; Montero, F.; Joyeux, I.; Pouillart, P.; Fridman, W. Serum interleukin 6 and C-reactive protein levels correlate with resistance to IL-2 therapy and poor survival in melanoma patients. Br. J. Cancer 1994, 69, 911–913. [Google Scholar] [CrossRef]
- Han, Z.-P.; Liu, D.-B.; Wu, L.-Q.; Li, Q.; Wang, Z.-G.; Zang, X.-F. IL-1β secreted by macrophage M2 promotes metastasis of osteosarcoma via NF-κB/miR-181α-5p/RASSF1A/Wnt pathway. Transl. Cancer Res. 2020, 9, 2721–2733. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, J.B.; Saraf, A.; Hinckley, J.; Gross, A.C.; Pommellette, H.L.; Garcia, L.J.; Cam, M.; Cannon, M.V.; Vatelle, S.; Gryder, B.E.; et al. Metastasis-initiating osteosarcoma subpopulations establish paracrine interactions with both lung and tumor cells to create a metastatic niche. Cancer Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Eislmayr, K.; Bestehorn, A.; Morelli, L.; Borroni, M.; Walle, L.V.; Lamkanfi, M.; Kovarik, P. Nonredundancy of IL-1α and IL-1β is defined by distinct regulation of tissues orchestrating resistance versus tolerance to infection. Sci. Adv. 2022, 8, eabj7293. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kastner, L.; Kandalaft, W.; Mahant, A.M.; Crimella, J.; Hakim, S.; Peng, X.P.; Isakoff, M.S.; Hayashi, M.; Loeb, D.M. Cytokine Profiling of Children, Adolescents, and Young Adults Newly Diagnosed with Sarcomas Demonstrates the Role of IL-1β in Osteosarcoma Metastasis. Cancers 2025, 17, 3009. https://doi.org/10.3390/cancers17183009
Kastner L, Kandalaft W, Mahant AM, Crimella J, Hakim S, Peng XP, Isakoff MS, Hayashi M, Loeb DM. Cytokine Profiling of Children, Adolescents, and Young Adults Newly Diagnosed with Sarcomas Demonstrates the Role of IL-1β in Osteosarcoma Metastasis. Cancers. 2025; 17(18):3009. https://doi.org/10.3390/cancers17183009
Chicago/Turabian StyleKastner, Laurel, William Kandalaft, Aakash Mahant Mahant, Jessica Crimella, Sydney Hakim, Xiao P. Peng, Michael S. Isakoff, Masanori Hayashi, and David M. Loeb. 2025. "Cytokine Profiling of Children, Adolescents, and Young Adults Newly Diagnosed with Sarcomas Demonstrates the Role of IL-1β in Osteosarcoma Metastasis" Cancers 17, no. 18: 3009. https://doi.org/10.3390/cancers17183009
APA StyleKastner, L., Kandalaft, W., Mahant, A. M., Crimella, J., Hakim, S., Peng, X. P., Isakoff, M. S., Hayashi, M., & Loeb, D. M. (2025). Cytokine Profiling of Children, Adolescents, and Young Adults Newly Diagnosed with Sarcomas Demonstrates the Role of IL-1β in Osteosarcoma Metastasis. Cancers, 17(18), 3009. https://doi.org/10.3390/cancers17183009





