Curcumol Targets the VHL/HIF-1α Axis to Suppress Glycolysis-Driven Progression in Colorectal Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Curcumol Preparation and Administration
2.2. Animal Studies and Treatment
2.3. Transcriptomic Analysis and Correlation Assessment of Mouse Tumor Tissues
2.4. CRC Organoids
2.4.1. Organoid Derivation
2.4.2. Organoid Viability Assay
2.4.3. H&E Staining and Immunohistochemistry (IHC)
2.5. Hypoxia Simulation in Cell Culture
2.6. Hematoxylin and Eosin (H&E) Staining
2.7. Western Blot Analysis
2.8. Seahorse X 96 Metabolic Flux Analysis
2.9. Immunofluorescence Assay
2.10. RT-PCR
2.11. Transwell Invasion Assay
2.12. Wound Healing Assay
2.13. Colony Formation Assay
2.14. Metabolic Analysis
2.15. Co-IP
2.16. Statistical Analysis
3. Results
3.1. Curcumol Inhibits Proliferation and Migration of CRC Cells Under Hypoxia
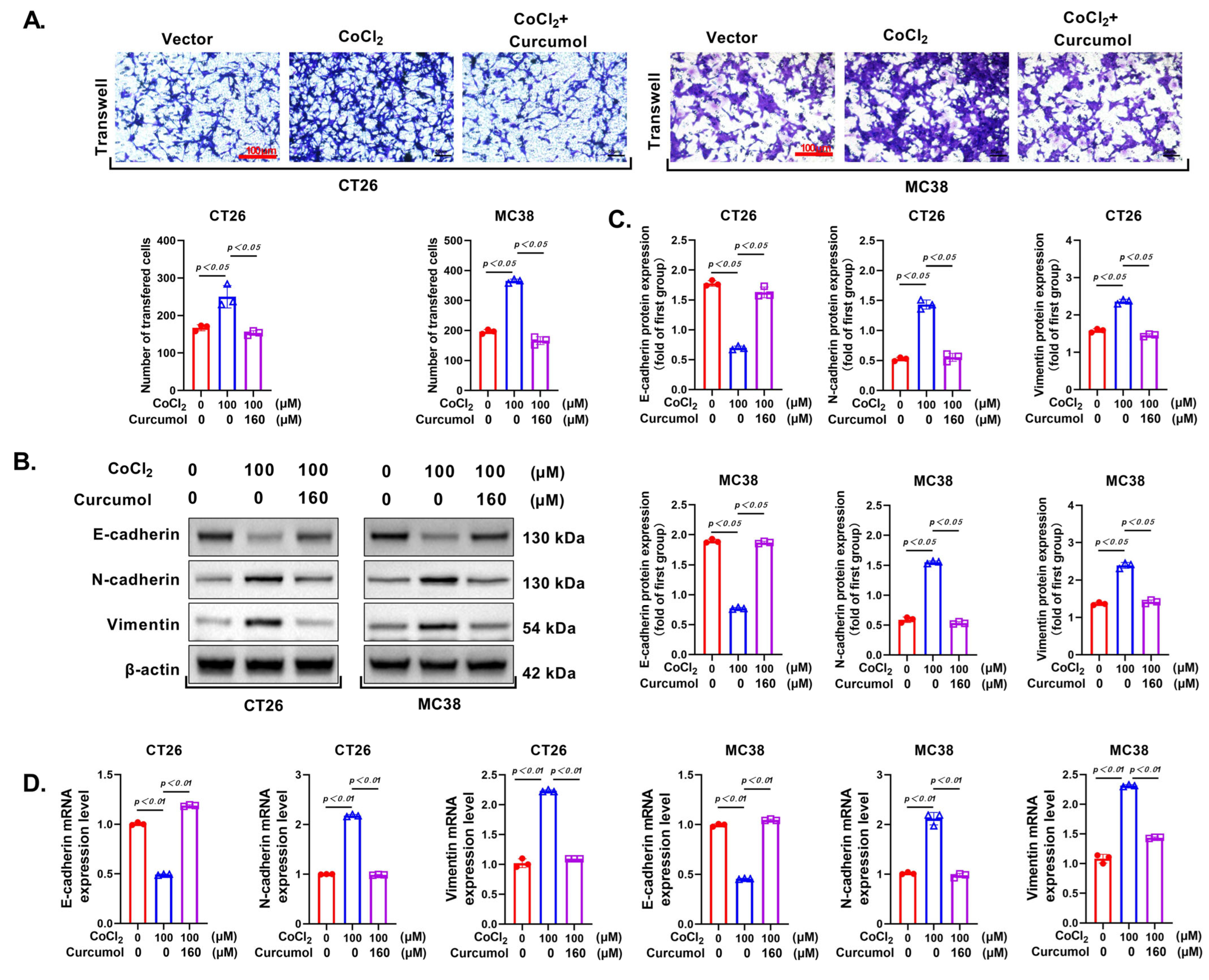
3.2. Curcumol Suppresses the Invasion and Metastasis of CRC Cells
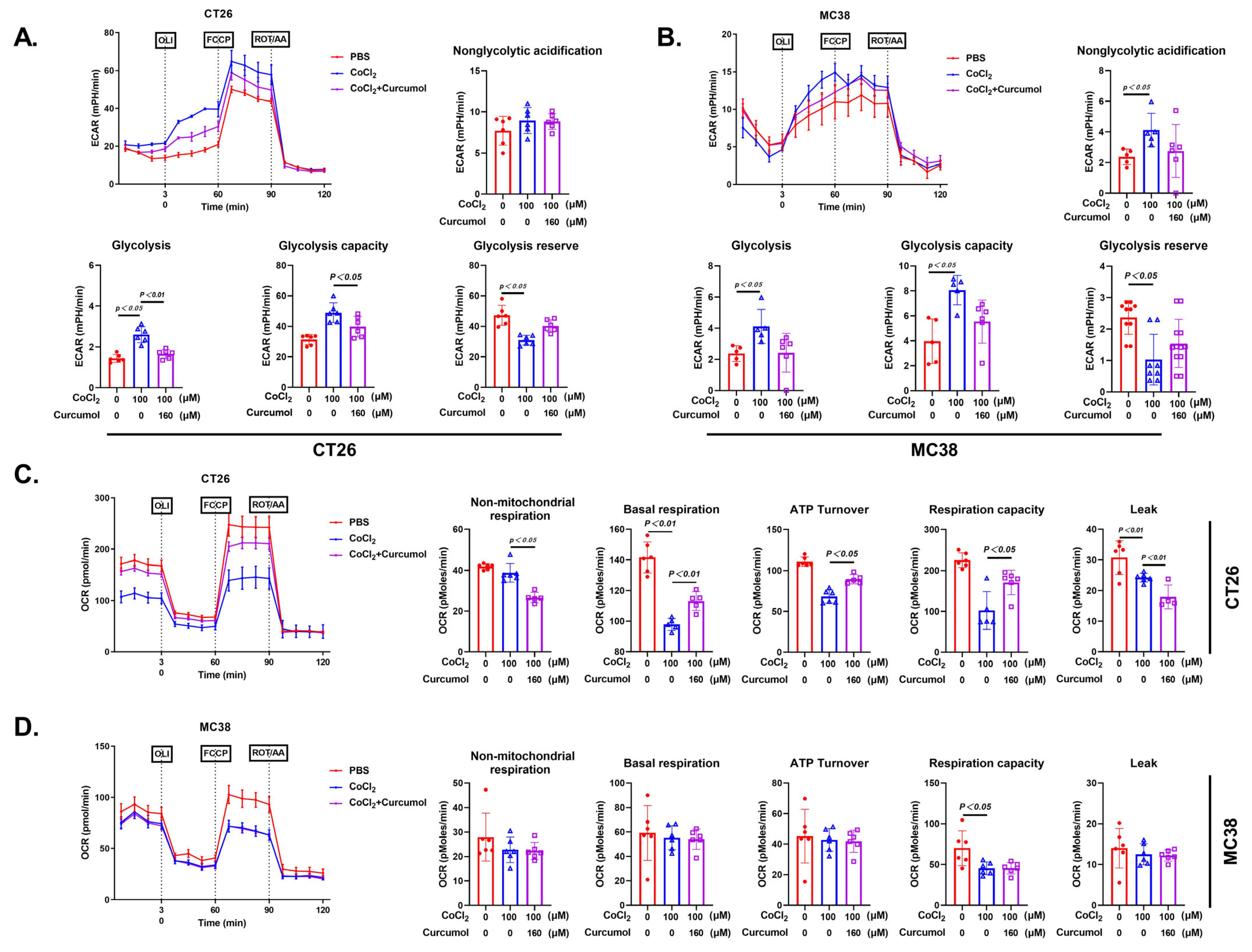
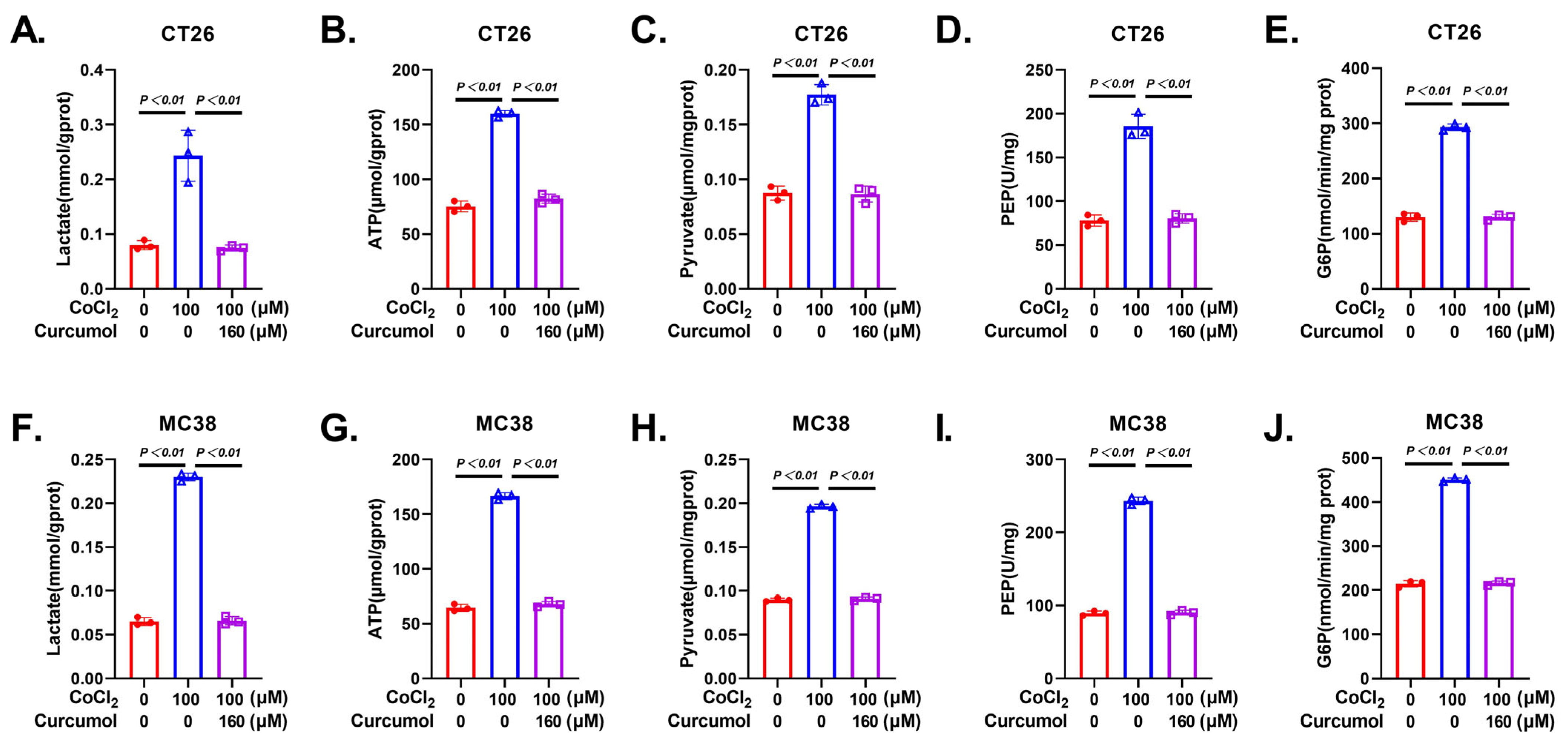
3.3. Curcumol Inhibits Glycolytic Metabolic Pathway
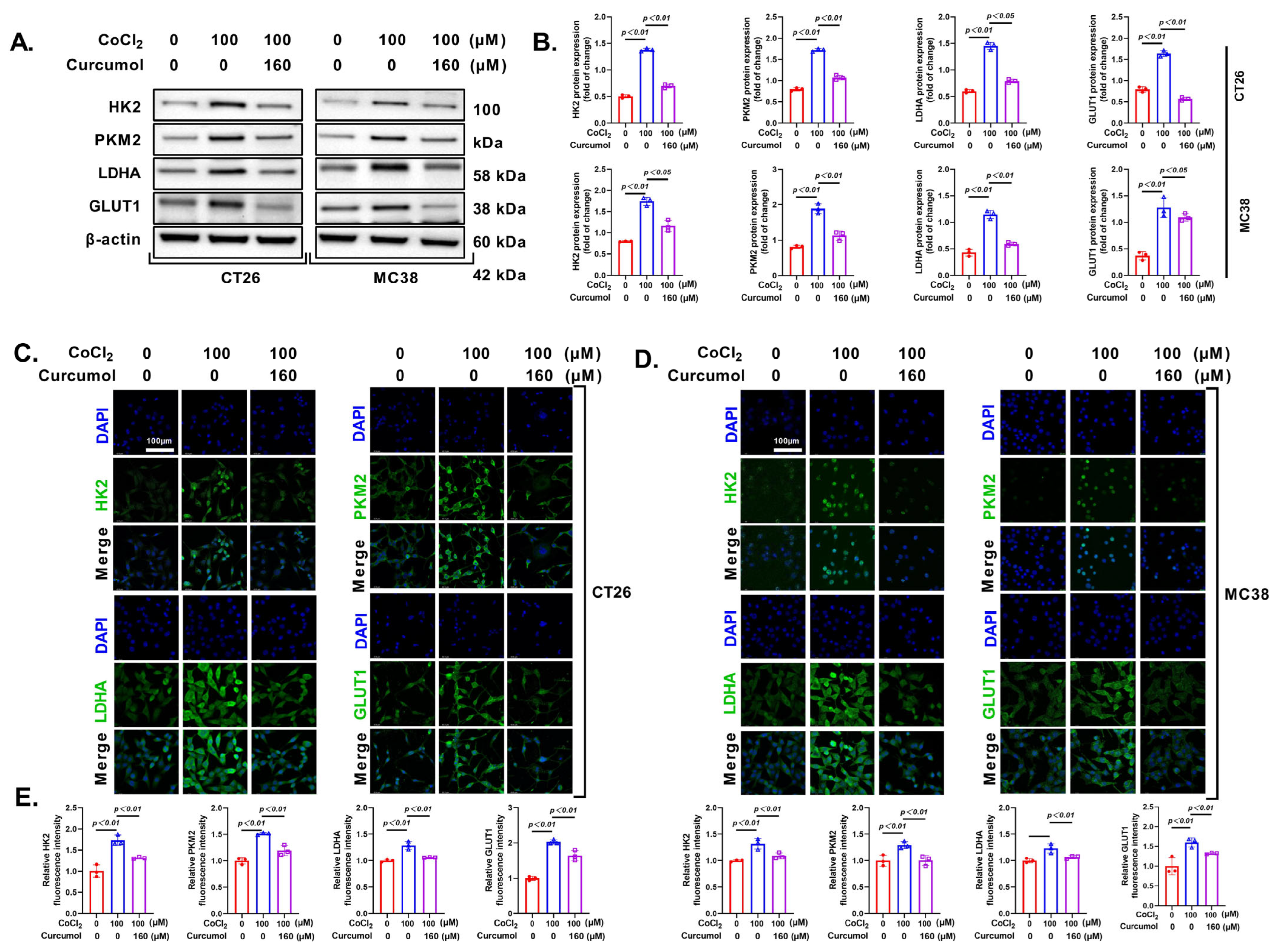
3.4. Curcumol Inhibits Energy Metabolism by Suppressing Glycolysis-Related Enzymes
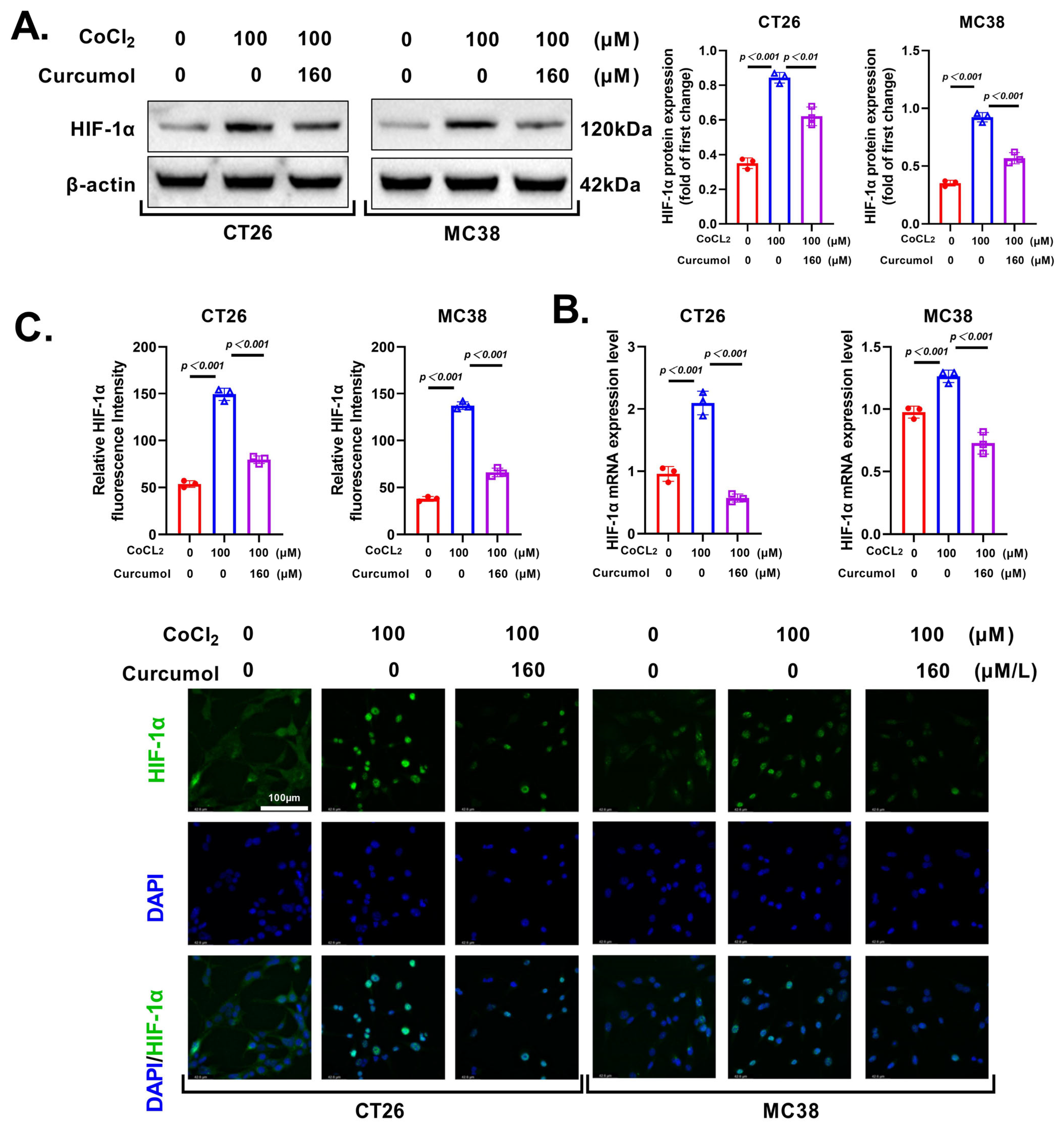
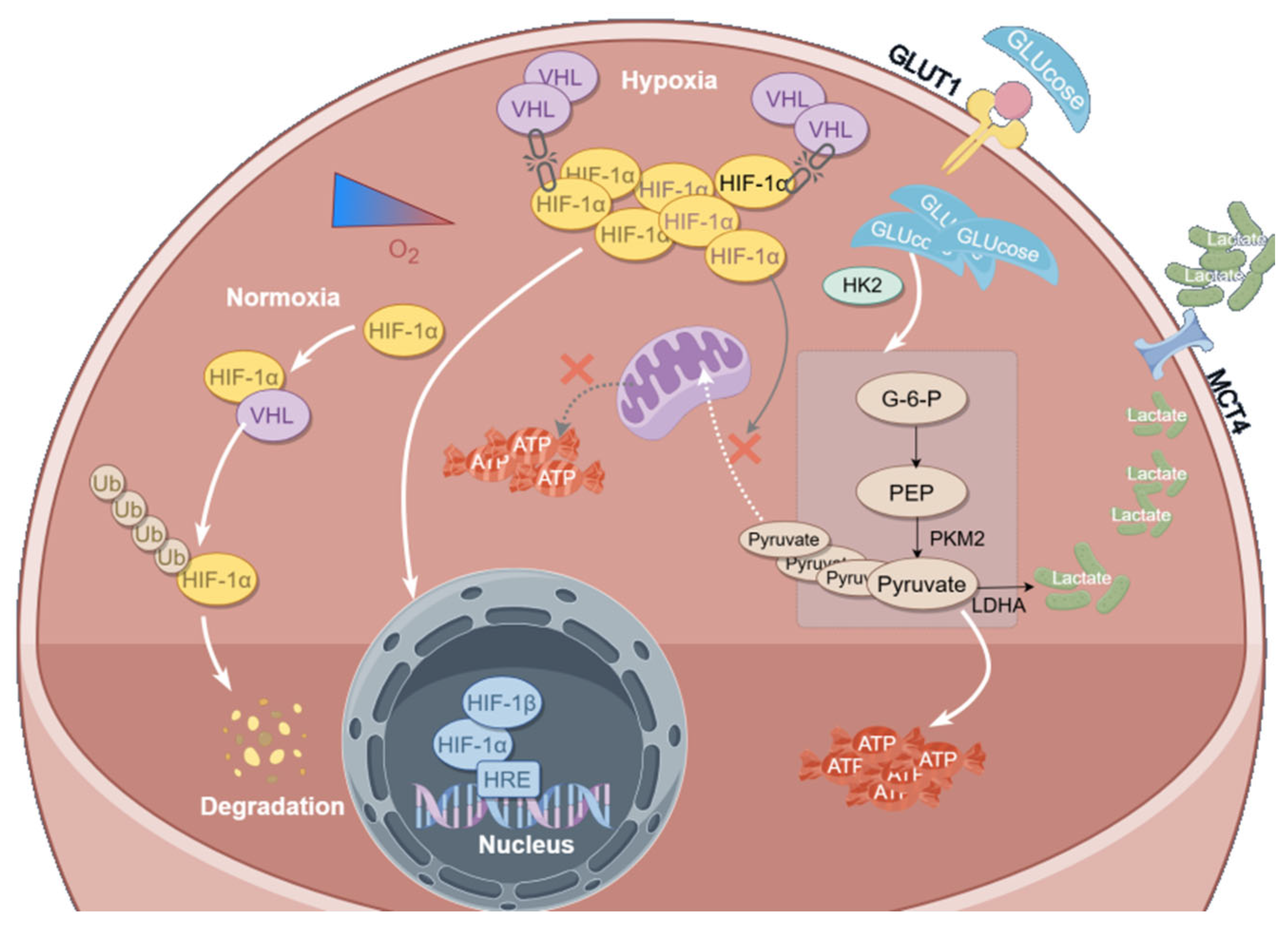
3.5. Curcumol Regulates Glycolytic Metabolism by Inhibiting HIF-1α
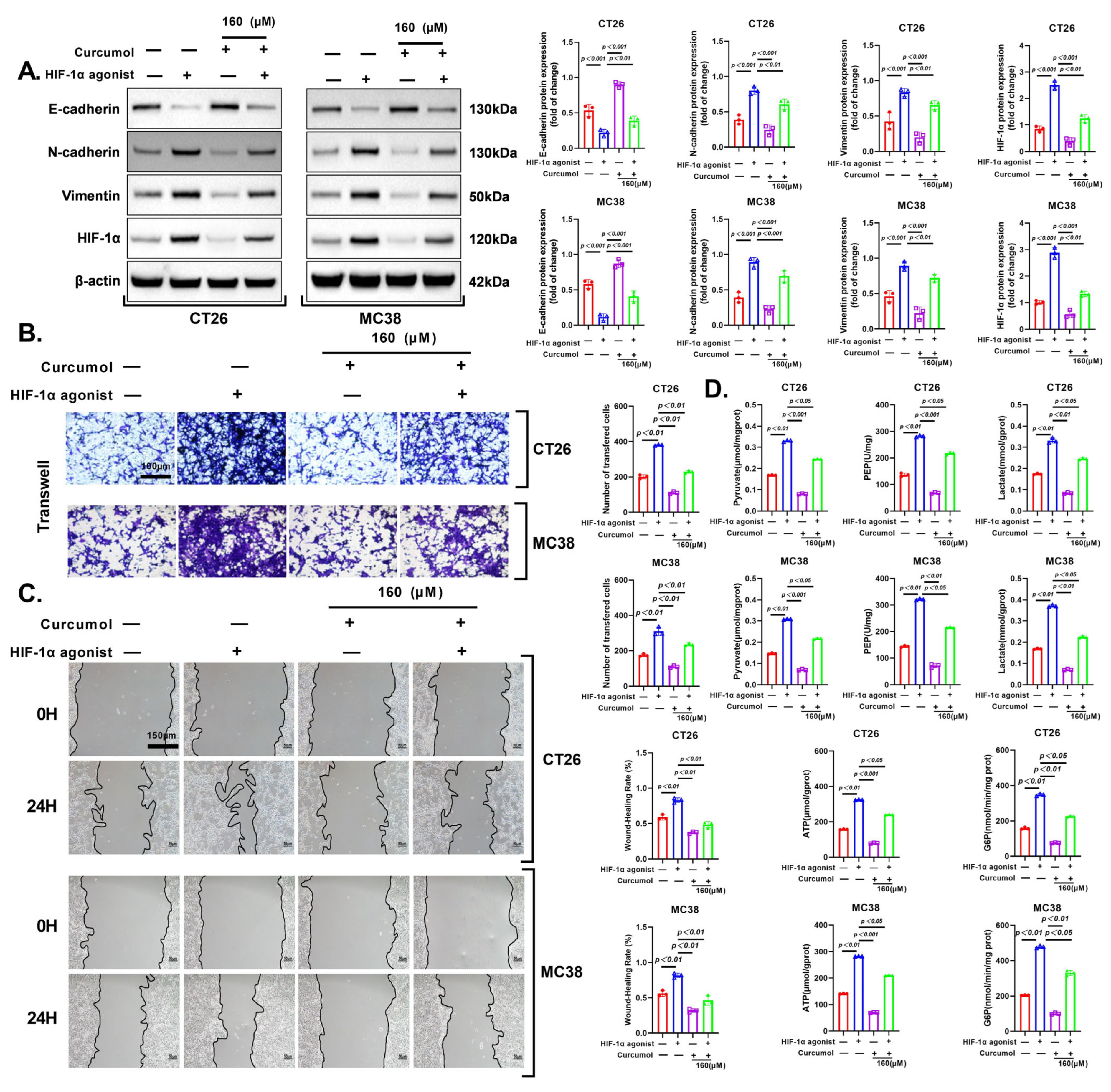
3.6. HIF-1α Activation Rescues Curcumol-Induced Glycolytic Suppression
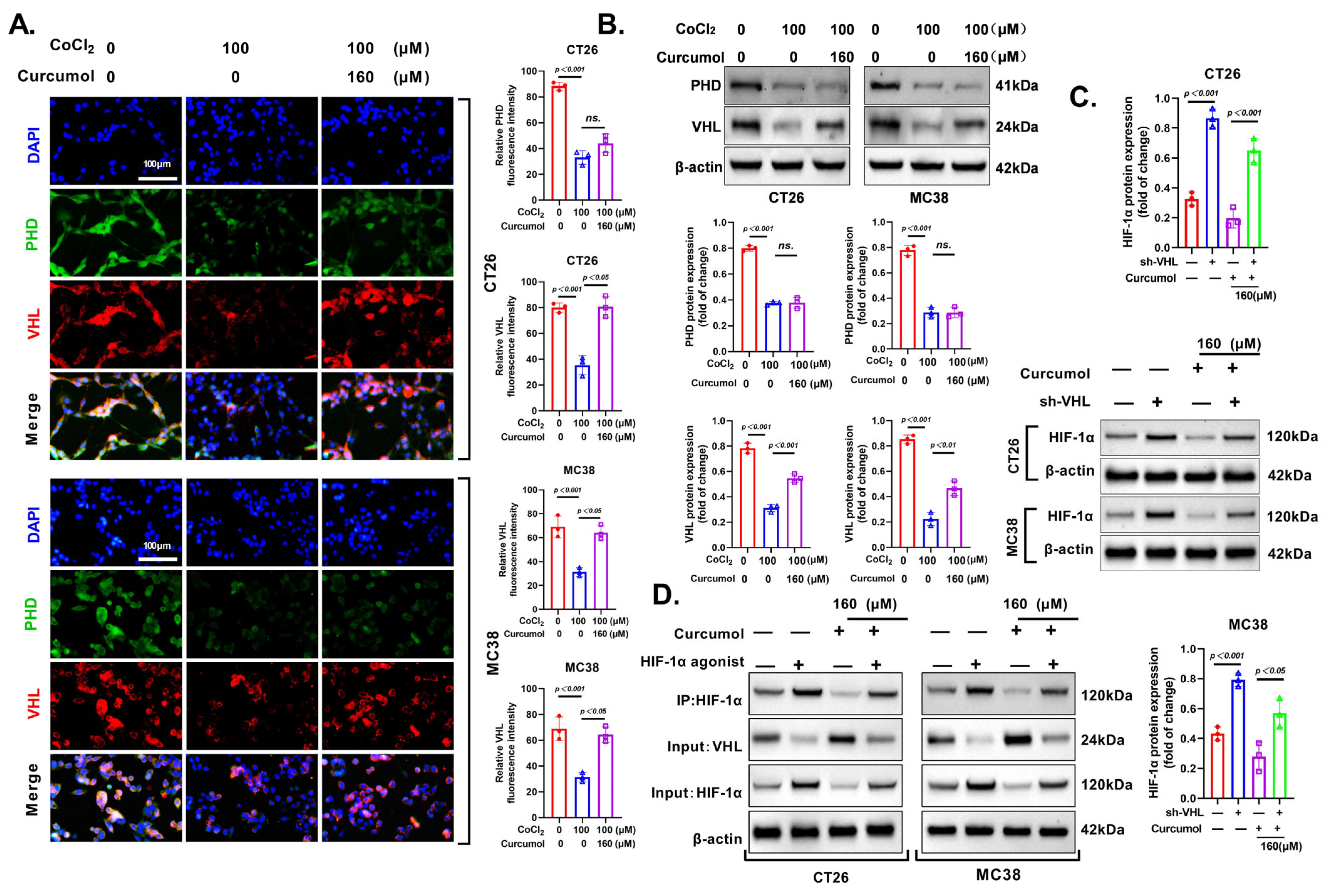
3.7. Curcumol Enhances HIF-1α Degradation via VHL Pathway
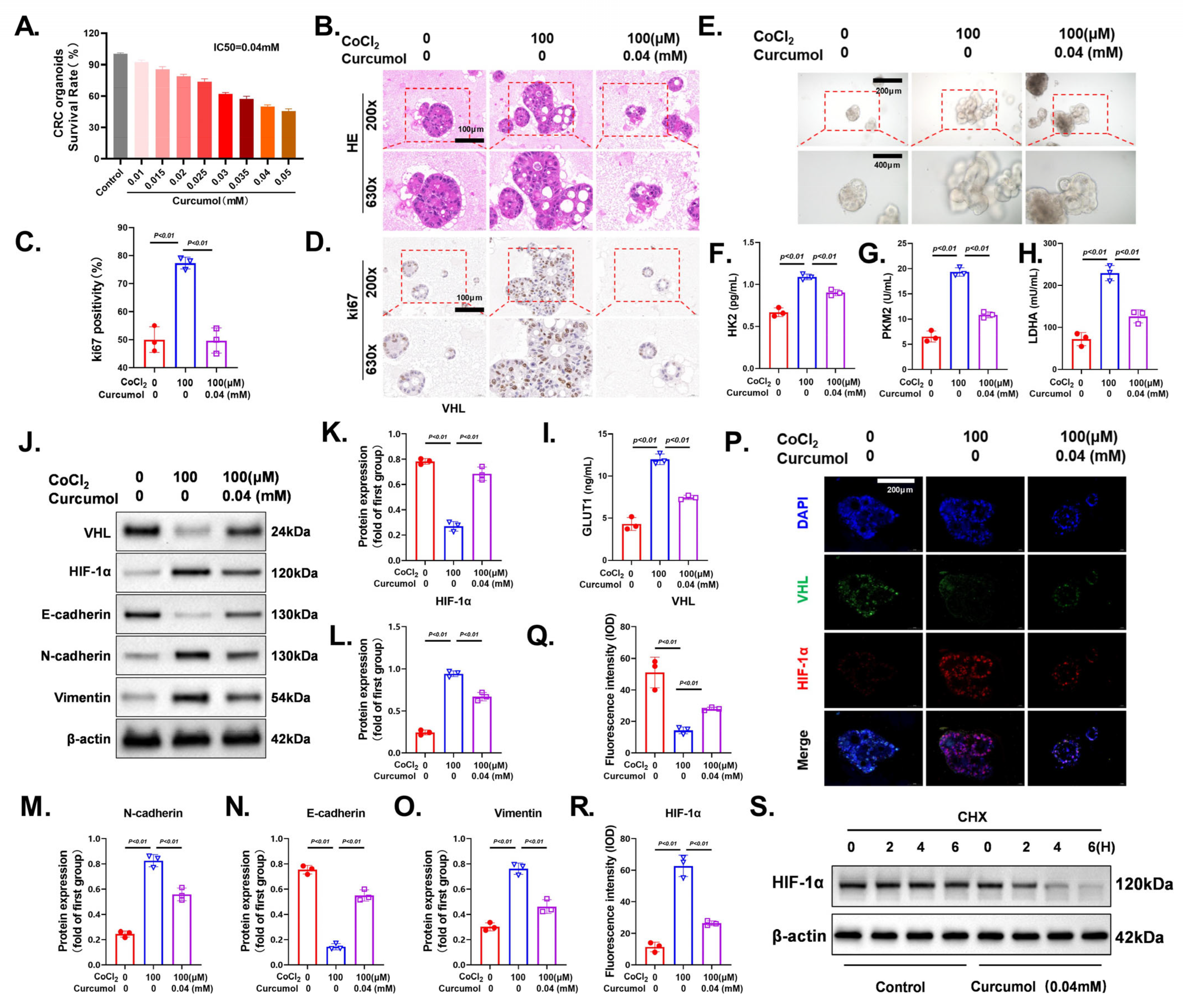
3.8. Curcumol Reverses CoCl2-Induced Glycolytic Activation and EMT by Restoring the VHL/HIF-1α Axis
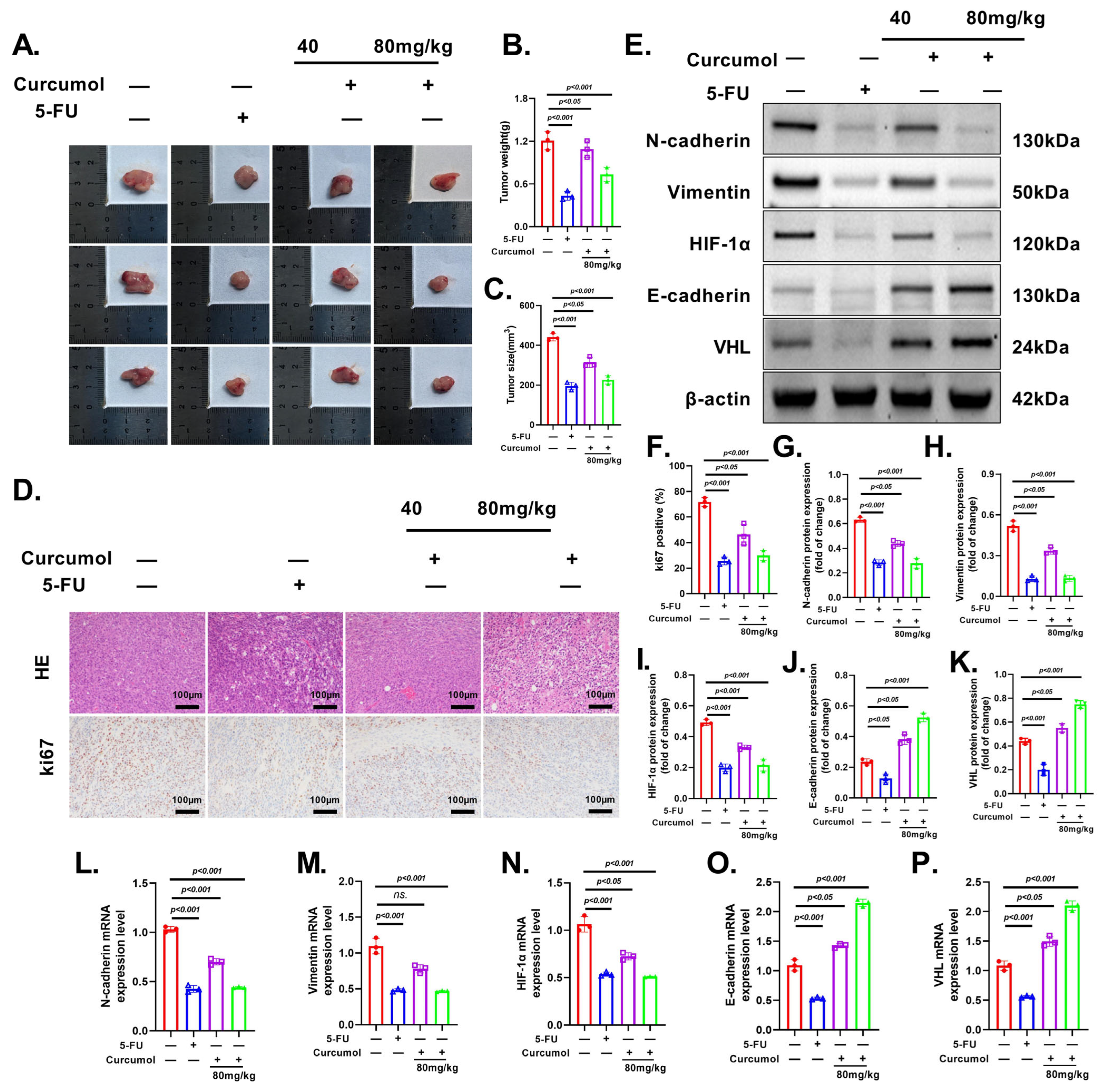
3.9. Curcumol Exhibits Potent and Dose-Dependent Anti-Tumor Effects In Vivo
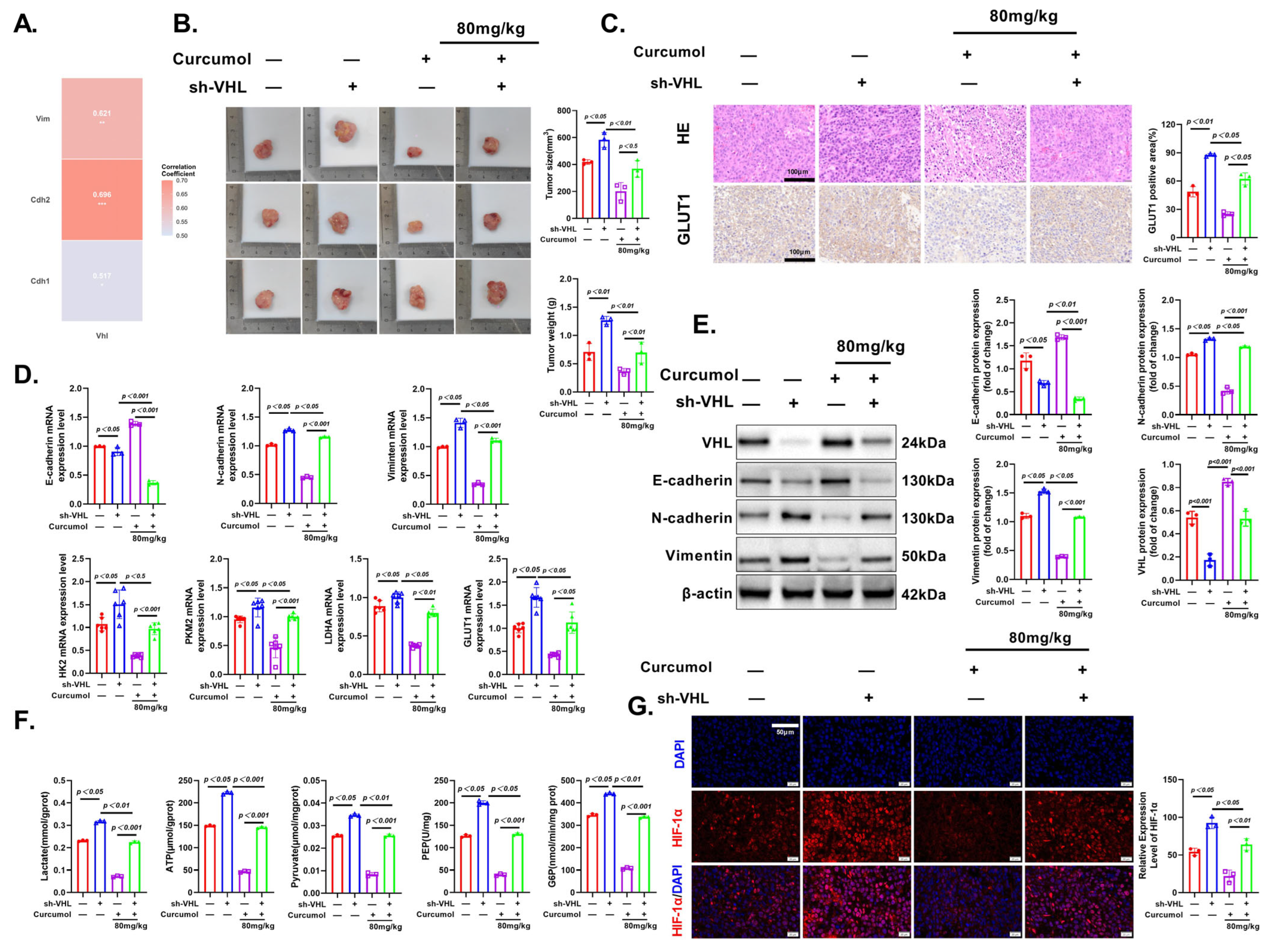
3.10. Curcumol Exerts Anti-Tumor Effects via VHL/HIF-1α Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| Co-IP | co-immunoprecipitation |
| CRC | colorectal cancer |
| ECAR | extracellular acidification rate |
| EMT | epithelial–mesenchymal transition |
| G6P | glucose-6-phosphate |
| GLUT1 | glucose transporter 1 |
| HIF-1α | hypoxia-inducible factor 1 alpha |
| HK2 | hexokinase 2 |
| IHC | immunohistochemistry |
| IF | immunofluorescence |
| LDHA | lactate dehydrogenase A |
| MAPK | mitogen-activated protein kinase |
| OCR | oxygen consumption rate |
| PEP | phosphoenolpyruvate |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| VHL | von Hippel–Lindau protein |
| WB | Western blot |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sun, L.T.; Zhang, L.Y.; Shan, F.Y.; Shen, M.H.; Ruan, S.M. Jiedu Sangen decoction inhibits chemoresistance to 5-fluorouracil of colorectal cancer cells by suppressing glycolysis via PI3K/AKT/HIF-1α signaling pathway. Chin. J. Nat. Med. 2021, 19, 143–152. [Google Scholar] [CrossRef]
- Haynes, J.; Manogaran, P. Mechanisms and Strategies to Overcome Drug Resistance in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 1988. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Grassi, A.; Perilli, L.; Albertoni, L.; Tessarollo, S.; Mescoli, C.; Urso, E.D.L.; Fassan, M.; Rugge, M.; Zanovello, P. A coordinate deregulation of microRNAs expressed in mucosa adjacent to tumor predicts relapse after resection in localized colon cancer. Mol. Cancer 2018, 17, 17. [Google Scholar] [CrossRef]
- Gill, K.S.; Fernandes, P.; O’Donovan, T.R.; McKenna, S.L.; Doddakula, K.K.; Power, D.G.; Soden, D.M.; Forde, P.F. Glycolysis inhibition as a cancer treatment and its role in an anti-tumour immune response. Biochim. Biophys. Acta 2016, 1866, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Su, F.; Li, Q.; Wen, Y.; Wei, F.; He, Z.; Chen, X.; Yin, X.; Wang, J.; Cai, Y.; et al. Phytochemicals targeting glycolysis in colorectal cancer therapy: Effects and mechanisms of action. Front. Pharmacol. 2023, 14, 1257450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Zhao, K.; Lin, Q.; Li, H.; Xue, X.; Ge, W.; He, H.; Liu, D.; Xie, H.; et al. A novel LncRNA HITT forms a regulatory loop with HIF-1α to modulate angiogenesis and tumor growth. Cell Death Differ. 2020, 27, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Hsieh, P.C.; Chiu, V.; Lan, C.C.; Lu, K.C. The von Hippel-Lindau Tumor Suppressor Gene Mutations Modulate Lipocalin-2 Expression in Ferroptotic-Inflammatory Pathways. Oxid. Med. Cell Longev. 2023, 2023, 7736638. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Targeting intratumoral hypoxia to enhance anti-tumor immunity. Semin. Cancer Biol. 2023, 96, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Ding, Y.; Huang, J.; Yao, J.; Xie, Z.; Lv, Y.; Zuo, J. Regulating the Expression of HIF-1α or lncRNA: Potential Directions for Cancer Therapy. Cells 2022, 11, 2811. [Google Scholar] [CrossRef]
- Shen, Z.; Yu, N.; Zhang, Y.; Jia, M.; Sun, Y.; Li, Y.; Zhao, L. The potential roles of HIF-1α in epithelial-mesenchymal transition and ferroptosis in tumor cells. Cell. Signal. 2024, 122, 111345. [Google Scholar] [CrossRef]
- Bian, Y.; Yin, G.; Wang, G.; Liu, T.; Liang, L.; Yang, X.; Zhang, W.; Tang, D. Degradation of HIF-1α induced by curcumol blocks glutaminolysis and inhibits epithelial-mesenchymal transition and invasion in colorectal cancer cells. Cell Biol. Toxicol. 2023, 39, 1957–1978. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Kai, J.; Wang, F.; Jia, Y.; Wang, S.; Tan, S.; Shen, X.; Chen, A.; Shao, J.; et al. Curcumol attenuates liver sinusoidal endothelial cell angiogenesis via regulating Glis-PROX1-HIF-1α in liver fibrosis. Cell Prolif. 2020, 53, e12762. [Google Scholar] [CrossRef]
- Zuo, H.X.; Jin, Y.; Wang, Z.; Li, M.Y.; Zhang, Z.H.; Wang, J.Y.; Xing, Y.; Ri, M.H.; Jin, C.H.; Xu, G.H.; et al. Curcumol inhibits the expression of programmed cell death-ligand 1 through crosstalk between hypoxia-inducible factor-1α and STAT3 (T705) signaling pathways in hepatic cancer. J. Ethnopharmacol. 2020, 257, 112835. [Google Scholar] [CrossRef]
- Wang, J.; Huang, F.; Bai, Z.; Chi, B.; Wu, J.; Chen, X. Curcumol Inhibits Growth and Induces Apoptosis of Colorectal Cancer LoVo Cell Line via IGF-1R and p38 MAPK Pathway. Int. J. Mol. Sci. 2015, 16, 19851–19867. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, Y.; Yang, G.; Liu, Y.; Zou, J.; Zhao, H.; Yin, G.; Wu, Y.; Li, X.; Wei, Z.; et al. Kaempferol impairs aerobic glycolysis against melanoma metastasis via inhibiting the mitochondrial binding of HK2 and VDAC1. Eur. J. Pharmacol. 2022, 931, 175226. [Google Scholar] [CrossRef]
- Zahedi, M.; Salmani Izadi, H.; Arghidash, F.; Gumpricht, E.; Banach, M.; Sahebkar, A. The effect of curcumin on hypoxia in the tumour microenvironment as a regulatory factor in cancer. Arch. Med. Sci. 2023, 19, 1616–1629. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: Progress and prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef]
- Paul, S.; Ghosh, S.; Kumar, S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin. Cancer Biol. 2022, 86 Pt 3, 1216–1230. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, P.; Chen, A.; Deng, Y.; Zhang, Z.; Lu, H.; Zhu, A.; Zhou, C.; Wu, Y.; Li, S. Aerobic glycolysis in colon cancer is repressed by naringin via the HIF1A pathway. J. Zhejiang Univ. Sci. B 2023, 24, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Yang, R.; Wei, Y.; Wang, D.; Zhu, Y.; Wang, X.; Lu, Y.; Wang, Y.; Zen, K.; Li, L. HIF-1α-induced miR-23a-27a-24 cluster promotes colorectal cancer progression via reprogramming metabolism. Cancer Lett. 2019, 440–441, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, X.; Wu, S.; Shi, W.; Zhang, G.; Cao, Y.; Wang, Z.; Wu, J.; Jiang, C. UBE2S promotes malignant properties via VHL/HIF-1α and VHL/JAK2/STAT3 signaling pathways and decreases sensitivity to sorafenib in hepatocellular carcinoma. Cancer Med. 2023, 12, 18078–18097. [Google Scholar] [CrossRef]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updat. 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Fontana, R.; Mestre-Farrera, A.; Yang, J. Update on Epithelial-Mesenchymal Plasticity in Cancer Progression. Annu. Rev. Pathol. 2024, 19, 133–156. [Google Scholar] [CrossRef]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Sabouni, E.; Nejad, M.M.; Mojtabavi, S.; Khoshduz, S.; Mojtabavi, M.; Nadafzadeh, N.; Nikpanjeh, N.; Mirzaei, S.; Hashemi, M.; Aref, A.R.; et al. Unraveling the function of epithelial-mesenchymal transition (EMT) in colorectal cancer: Metastasis, therapy response, and revisiting molecular pathways. Biomed. Pharmacother. 2023, 160, 114395. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Dai, J.; Torabian, P.; Nabavi, N.; Aref, A.R.; Aljabali, A.A.A.; Tambuwala, M.; Zhu, M. Circular RNAs in EMT-driven metastasis regulation: Modulation of cancer cell plasticity, tumorigenesis and therapy resistance. Cell Mol. Life Sci. 2024, 81, 214. [Google Scholar] [CrossRef]
- Musleh Ud Din, S.; Streit, S.G.; Huynh, B.T.; Hana, C.; Abraham, A.N.; Hussein, A. Therapeutic Targeting of Hypoxia-Inducible Factors in Cancer. Int. J. Mol. Sci. 2024, 25, 2060. [Google Scholar] [CrossRef]
- Shen, S.; Xu, Y.; Gong, Z.; Yao, T.; Qiao, D.; Huang, Y.; Zhang, Z.; Gao, J.; Ni, H.; Jin, Z.; et al. Positive Feedback Regulation of Circular RNA Hsa_circ_0000566 and HIF-1α promotes Osteosarcoma Progression and Glycolysis Metabolism. Aging Dis. 2023, 14, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Qiu, S.; Wang, P.; Liang, X.; Huang, F.; Wu, H.; Zhang, B.; Zhang, W.; Tian, X.; Xu, R.; et al. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. 2019, 38, 377. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ding, J.; Kuang, S.; Li, B.; Sun, T.; Zhu, C.; Liu, J.; Zhu, L.; Li, Y.; Sheng, W. Icariin-Curcumol promotes docetaxel sensitivity in prostate cancer through modulation of the PI3K-Akt signaling pathway and the Warburg effect. Cancer Cell Int. 2023, 23, 190. [Google Scholar] [CrossRef]
- Hashemi, M.; Mirdamadi, M.S.A.; Talebi, Y.; Khaniabad, N.; Banaei, G.; Daneii, P.; Gholami, S.; Ghorbani, A.; Tavakolpournegari, A.; Farsani, Z.M.; et al. Pre-clinical and clinical importance of miR-21 in human cancers: Tumorigenesis, therapy response, delivery approaches and targeting agents. Pharmacol. Res. 2023, 187, 106568. [Google Scholar] [CrossRef]
- Yang, Z.; Su, W.; Wei, X.; Qu, S.; Zhao, D.; Zhou, J.; Wang, Y.; Guan, Q.; Qin, C.; Xiang, J.; et al. HIF-1α drives resistance to ferroptosis in solid tumors by promoting lactate production and activating SLC1A1. Cell Rep. 2023, 42, 112945. [Google Scholar] [CrossRef]
- Singh, L.; Nair, L.; Kumar, D.; Arora, M.K.; Bajaj, S.; Gadewar, M.; Mishra, S.S.; Rath, S.K.; Dubey, A.K.; Kaithwas, G.; et al. Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival. Front. Oncol. 2023, 13, 1034205. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Zhang, J.; Muri, J.; Fitzgerald, G.; Gorski, T.; Gianni-Barrera, R.; Masschelein, E.; D’Hulst, G.; Gilardoni, P.; Turiel, G.; Fan, Z.; et al. Endothelial Lactate Controls Muscle Regeneration from Ischemia by Inducing M2-like Macrophage Polarization. Cell Metab. 2020, 31, 1136–1153.e7. [Google Scholar] [CrossRef]
- Alberghina, L. The Warburg Effect Explained: Integration of Enhanced Glycolysis with Heterogeneous Mitochondria to Promote Cancer Cell Proliferation. Int. J. Mol. Sci. 2023, 24, 15787. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.; Ferrari, P. Involvement of tumor immune microenvironment metabolic reprogramming in colorectal cancer progression, immune escape, and response to immunotherapy. Front. Immunol. 2024, 15, 1353787. [Google Scholar] [CrossRef] [PubMed]
- Elzakra, N.; Kim, Y. HIF-1α Metabolic Pathways in Human Cancer. Adv. Exp. Med. Biol. 2021, 1280, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, J. Curcumol Enhances the Antitumor Effect of Lenvatinib on Hepatocellular Carcinoma Cells. Discov. Med. 2024, 36, 199–208. [Google Scholar] [CrossRef]

| Primer Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| VHL-CRISPR | CACC GCGTTCCAATAATGCCCCGGA | AAAC TCCGGGGCATTATTGGAACGC |
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | ACTCTTCCAGCCTTCCTTCC | CGTCATACTCCTGCTTGCTG |
| HIF-1α | CTGCCACTGCCACCACAACTG | TGCCACTGTATGCTGATGCCTTAG |
| HK2 | GACGAGAGCATCCTCCTCAAGTG | TCACCACAGCAACCACATCCAG |
| PKM2 | TTGCCTGCTGTGTCGGAGAAG | CAGATGCCTTGCGGATGAATGAC |
| LDHA | TCAGCCCGATTCCGTTACCTAATG | CACCAGCAACATTCATTCCACTCC |
| GLUT1 | GATGAAAGAAGAGGGTCGGCAGATG | CAGCACCACAGCGATGAGGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Yue, Z.; Yin, G.; Zhu, L.; Zhou, W.; Sun, R.; Bi, T.; Zhao, L.; Bian, Y.; Tang, D. Curcumol Targets the VHL/HIF-1α Axis to Suppress Glycolysis-Driven Progression in Colorectal Cancer. Cancers 2025, 17, 3000. https://doi.org/10.3390/cancers17183000
Wang G, Yue Z, Yin G, Zhu L, Zhou W, Sun R, Bi T, Zhao L, Bian Y, Tang D. Curcumol Targets the VHL/HIF-1α Axis to Suppress Glycolysis-Driven Progression in Colorectal Cancer. Cancers. 2025; 17(18):3000. https://doi.org/10.3390/cancers17183000
Chicago/Turabian StyleWang, Gang, Zengyaran Yue, Gang Yin, Lifeng Zhu, Wen Zhou, Ruiqian Sun, Tingting Bi, Lin Zhao, Yong Bian, and Decai Tang. 2025. "Curcumol Targets the VHL/HIF-1α Axis to Suppress Glycolysis-Driven Progression in Colorectal Cancer" Cancers 17, no. 18: 3000. https://doi.org/10.3390/cancers17183000
APA StyleWang, G., Yue, Z., Yin, G., Zhu, L., Zhou, W., Sun, R., Bi, T., Zhao, L., Bian, Y., & Tang, D. (2025). Curcumol Targets the VHL/HIF-1α Axis to Suppress Glycolysis-Driven Progression in Colorectal Cancer. Cancers, 17(18), 3000. https://doi.org/10.3390/cancers17183000





