Simple Summary
Cognitive difficulties are a common and often persistent side effect experienced by many breast cancer patients following chemotherapy, negatively affecting daily functioning and overall quality of life. These cognitive impairments, frequently referred to as “chemo brain”, may include problems with memory, attention, and processing speed. This study systematically reviewed and analysed recent clinical trials to evaluate the effectiveness of cognitive stimulation interventions in managing these symptoms. The review aimed to identify not only the efficacy of such interventions but also key characteristics such as optimal timing, techniques, and duration. Although the findings suggest a moderate beneficial effect on cognitive functioning, methodological variability among studies limits the strength of the conclusions. These results emphasize the need for further research to develop standardized, evidence-based approaches for early cognitive support in breast cancer care.
Abstract
Background: A considerable proportion of breast cancer (BC) patients experience chemotherapy-related cognitive impairment (CRCI) and other symptoms even after the completion of treatment. The persistence of CRCI throughout the oncological process highlights the need for routine assessment of its severity, impact on quality of life, and the effectiveness of interventions aimed at addressing it. Objectives: To analyse the effectiveness of cognitive stimulation interventions on CRCI in BC patients and to identify the characteristics of such interventions, including the most appropriate timing for their implementation, the most suitable techniques, and their duration. Methodology: A systematic review and meta-analysis were conducted in accordance with PRISMA guidelines. Randomized controlled trials published between 1 January 2020 and 31 December 2024 were searched across three electronic databases. Studies involving cognitive stimulation interventions for the management of CRCI in BC patients were included. Results: A total of 12 eligible studies were identified for the systematic review and 10 for the meta-analysis. The review revealed a wide range of cognitive stimulation interventions, differing in techniques, duration, format, and timing of implementation. Group-based therapies lasting between 6 and 12 weeks predominated, with cognitive outcomes primarily assessed using the FACT-Cog scale. The meta-analysis demonstrated a moderate positive effect of cognitive stimulation interventions on cognitive functioning in BC patients (d = 0.59), although not statistically significant (p = 0.07), and showed high heterogeneity across studies (I2 = 93%). Conclusions: Cognitive stimulation interventions show potential benefits in improving cognitive functioning in BC patients following chemotherapy. However, the high methodological heterogeneity limits the strength of the evidence. Further research is needed to develop standardized and personalized early intervention protocols.
1. Introduction
Although breast cancer (BC) is one of the most prevalent cancers among women worldwide, early detection and current treatment strategies have significantly improved survival rates [1].
It has been previously demonstrated that BC patients often experience a psychoneurological symptom cluster, which includes sleep disturbances, fatigue, anxiety, depressive symptoms, and cognitive impairment [2,3,4]. These symptoms, which tend to occur concurrently during the active phase of oncological treatment, form an inseparable unit with disruptive consequences for emotional, physical, cognitive, and social functioning. Identifying symptom clusters and their relationship with clinical and therapeutic management variables is enabling a better understanding of the clinical picture of these patients and facilitating the planning of interventions to improve their quality of life (QoL) [5].
One of the primary symptoms negatively affecting the QoL in BC patients is chemotherapy-related cognitive impairment (CRCI). CRCI is characterized by cognitive deficits in areas such as memory, attention, and executive function, which can interfere with daily functioning and reduce occupational performance [6]. The prevalence of CRCI has been estimated to be as high as 75% [7], making it a growing focus of clinical and research interest. CRCI has been linked to various treatments, including radiotherapy, hormonal therapy, immunotherapy, and most notably chemotherapy [8]. Chemotherapy, in particular, has been identified as a key predictor of CRCI severity. Lower perceived cognitive functioning scores have been associated with reduced cerebral oxygenation and poorer performance on phonemic and semantic verbal fluency tasks [9].
A substantial proportion of BC patients experience CRCI even after the completion of treatment, with prevalence rates remaining elevated for more than one year, and in some cases persisting five years or more post-chemotherapy [10]. The persistence of CRCI and other symptoms throughout the disease trajectory underscores the importance of routinely assessing their severity, impact, and influence on QoL [11]. Within this context, it is equally essential to assess the effectiveness of interventions aimed at symptom improvement. Most intervention studies focus on a primary symptom and monitor whether related symptoms improve as a result of the intervention. Numerous non-pharmacological interventions have been explored for the management and prevention of CRCI. These can be broadly categorized into four main types: cognitive training or rehabilitation, cognitive behavioural therapy, physical exercise, and interventions including yoga, tai chi, or mindfulness [12].
Several meta-analyses have evaluated the effectiveness of non-pharmacological interventions in improving cognitive function in BC survivors, suggesting that such approaches should be promoted as viable strategies for preventing and treating CRCI [13,14,15].
Cognitive stimulation serves as an overarching concept that includes both cognitive training and cognitive rehabilitation, each with distinct approaches. According to Woods et al. (2012), cognitive stimulation involves group-based activities aimed at enhancing general cognitive and social functioning [16]. Cognitive training refers to repetitive, standardized exercises targeting specific domains such as memory, attention, or executive function. In contrast, cognitive rehabilitation is a personalized, goal-oriented intervention that combines cognitive training with compensatory strategies to address everyday functional challenges. This classification helps differentiate the varied approaches used in interventions for CRCI.
Although some authors have conducted systematic reviews on cognitive stimulation to address CRCI [17,18,19], to date, no review has specifically focused on the efficacy and characteristics of these therapies in BC patients. Therefore, the aim of the present study was to analyse the effectiveness of cognitive stimulation interventions for CRCI in BC patients through a systematic review and meta-analysis, and to identify key characteristics of these interventions, such as the most appropriate timing, optimal techniques, and duration.
2. Materials and Methods
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [20]. The review was not registered.
2.1. Inclusion Criteria
- Studies involving women aged 18 years or older, diagnosed with stage 0 to IV BC, at any point in the treatment continuum.
- Studies investigating cognitive stimulation interventions (cognitive rehabilitation or cognitive stimulation), either as stand-alone approaches or in combination with other interventions.
- Comparators were not restricted; studies comparing the intervention to usual care, waitlist control, or other non-pharmacological interventions were eligible.
- Outcomes included at least one measure of cognitive function assessed both at baseline and at follow-up using any validated neuropsychological test, whether reported as a primary or secondary outcome.
- Among study designs, only randomized controlled trials were included.
- Pilot studies and studies published in English between 2020 and 2025 were considered.
2.2. Exclusion Criteria
- Studies involving patients with cancers other than BC.
- Studies that did not assess and/or report cognitive functioning as a dependent variable.
- Non-interventional studies.
- Review articles.
2.3. Search Strategy
A comprehensive literature search was performed on 12 January 2025, across three electronic databases: PubMed/MEDLINE, Web of Science, and Scopus.
Medical Subject Headings (MeSH) and keywords included terms related to:
- Breast cancer (e.g., “breast neoplasm” OR “breast cancer”),
- Cognitive impairment (e.g., “cognitive impairment” OR “chemotherapy-related cognitive impairment” OR “chemo brain”),
- Cognitive interventions (e.g., “cognitive stimulation” OR “cognitive rehabilitation” OR “cognitive training”).
These terms were combined using the Boolean operator AND. Relevant systematic reviews and meta-analyses were also screened, and reference lists from these reviews were examined iteratively until no further eligible studies were found.
2.4. Study Selection Process
All references retrieved from the electronic databases were imported into Zotero (version 7.0), a reference management software, and duplicates were removed. Following de-duplication, two independent reviewers (L.R.-S. and C.F.-M.) screened the titles and abstracts of the remaining studies for relevance. Full-text reviews were conducted for studies that met the initial inclusion criteria.
Any disagreements between reviewers during the selection process were resolved through discussion and consensus, guided by the predefined eligibility criteria. Additionally, reference lists of identified systematic reviews were manually screened to identify any further relevant studies.
A PRISMA flow diagram was used to illustrate the study selection process (Figure 1).
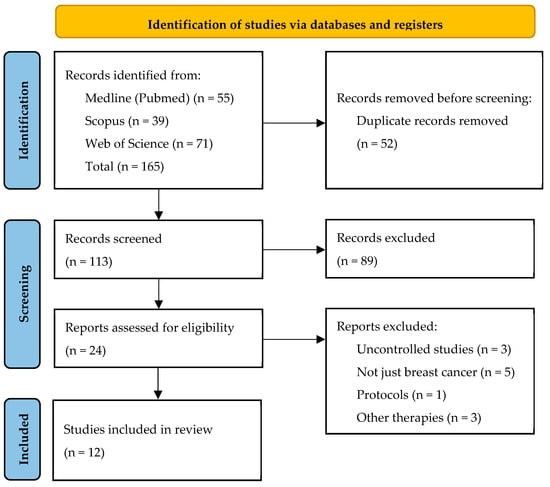
Figure 1.
PRISMA Flow Diagram of the Study Selection Process.
2.5. Data Extraction Process
Following the initial review of studies, a second researcher independently coded and entered information from each included study into standardized data extraction forms. Zotero software was used to manage the studies and support the data extraction process.
The extracted data included: study characteristics (first author, year of publication, regions of origin), sample size, intervention characteristics (type of intervention, intervention duration, total hours, number of sessions, setting [individual vs. group]), outcome measures used to evaluate cognitive function. Outcome data included the cognitive domains assessed and the total scores from self-reported cognitive measures, whose reliability and validity had been previously established. Additionally, data regarding the eligibility criteria, the time of CRCI measurement, and the characteristics of the control group were also extracted.
2.6. Risk of Bias Assessment
Two reviewers independently assessed the risk of bias for each study using the Cochrane Risk of Bias Tool version 2.0 [21]. Disagreements were resolved through discussion with the full review team.
The assessment focused on five domains:
- Bias arising from the randomization process,
- Bias due to deviations from intended interventions,
- Bias due to missing outcome data,
- Bias in the measurement of outcomes, and
- Bias in the selection of the reported results.
2.7. Meta-Analysis
Data analysis was performed using IBM SPSS Statistics (version 29). A random-effects meta-analysis model for continuous outcomes was conducted. In each study, a single intervention group was compared to a control group, with cognitive outcomes assessed after the intervention.
The analysis was based on standardized mean differences (Cohen’s d) rather than raw scores, due to the heterogeneity of cognitive measures across studies. Heterogeneity was assessed using τ2, Higgins and Thompson’s I2, and Cochran’s Q test. As per Ioannidis et al. (2007), an I2 value above 60% was considered indicative of substantial heterogeneity [22].
Forest plots and funnel plots were used to assess heterogeneity and publication bias.
When cognitive function was assessed multiple times after the intervention, only the immediate post-intervention result was included. In studies with multiple intervention groups, these were combined into a single group using pooled means and variances.
Baseline cognitive scores were not used as the primary comparison metric due to the randomized design of all studies. Although pre-post differences would have been more informative, this approach was not feasible in all cases, as some studies did not report sufficient data to calculate the variance of the change. In two studies where baseline differences between experimental and control groups were statistically significant, post-intervention means were adjusted accordingly.
When cognitive assessment tools used inverse scoring (i.e., higher scores indicated worse performance), the direction of Cohen’s d was reversed to ensure consistency across studies.
3. Results
3.1. Intervention Characteristics
The general characteristics of the 12 studies included in this systematic review are summarized in Table 1.

Table 1.
General Characteristics of the 12 Studies Included in the Systematic Review.
A detailed description of the included studies is provided in Table 2. Multiple cognitive stimulation techniques were identified, each employing different approaches. These include: Cancer and Living Meaningfully (CALM) therapy [23,24], Brief Acceptance and Commitment Therapy (ACT) [25], Psychoeducation-based Cognitive Stimulation [26], Neurosensory Stimulation [27], Attention Process Training (APT) and Compensatory Strategy Training (CST) [28], Cognitive Behavioral Therapy for Insomnia (CBT-I) [29], Dual N-Back training [30], video game-based therapies [31,32], and cognitive stimulation combined with physical exercise or music [33,34].

Table 2.
Detailed description of the studies included in the systematic review.
Of the interventions, 58.3% (n = 7) were delivered in group settings [23,24,25,26,28,29,33], while the remainder were individual-based interventions [27,30,31,32,34].
A high degree of variability was observed in the number of sessions and overall therapy duration. Intervention lengths ranged from 2 to 24 weeks. Most studies (58.3%) were conducted over a period of 6 to 12 weeks [24,25,26,28,29,32,33]. Two studies involved shorter interventions: 2 weeks (Dual N-Back) [30] and 4 weeks (Computer-Assisted Cognitive Training [CACT]) [34]. In contrast, two studies employed longer interventions: Multisensory stimulation [27], lasting approximately 15 weeks, and video game-based cognitive stimulation [31], which extended over 6 months.
The average total duration of sessions was 15.57 h (±21.90). In eight studies (67%), the total duration was 10 h or less [23,24,25,27,29,30,33,34]. APT [28] and psychoeducation-based training [26] lasted between 12 and 15 h, while the most intensive interventions reached up to 77 h (BrainHQ and video game-based therapies) [31,32].
3.2. Cognitive Assessment Methods
Different eligibility criteria and cognitive assessment methods were used across studies. Some relied solely on self-reported cognitive complaints (41.7%) [26,28,30,31,32], while others used standardized cognitive scales, such as the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) or Mini Mental State Examination (MMSE) [23,24,25,27,29,33,34].
The FACT-Cog was the most commonly used tool to assess CRCI, appearing in 75% of the studies [23,24,25,26,28,29,30,33,34]. This scale evaluates the impact of cognitive function on QoL, including domains such as memory, attention, concentration, thought organization, and ability to carry out daily activities.
Other instruments used included: Cognitive Failures Questionnaire (CFQ) [31], Patient-Reported Outcomes Measurement Information System (PROMIS) Cognitive Function Scale [32], Rivermead Behavioural Memory Test-Second Edition (RBMT-II) [27], and MMSE [23,24]. In general, these instruments assess domains such as memory, attention, executive function, and overall cognitive performance, either through self-report or objective testing.
3.3. Timing of the Intervention and Follow-Up
A subset of the selected studies (n = 5) implemented cognitive interventions during active chemotherapy treatment (defined as ≥2 chemotherapy cycles) [23,24,25,27,33]. In contrast, the remaining studies initiated cognitive training following the completion of cancer treatment, with post-treatment intervals ranging from 2 months to 5 years [26,28,29,30,32]. Notably, Smith et al. (2021) and Bellens et al. (2020) applied interventions during both treatment and the post-treatment period, thereby offering a broader scope of cognitive rehabilitation timing [31,34].
Regarding the assessment timeline, five studies conducted evaluations of CRCI both at baseline and immediately after the intervention [23,24,27,32,34]. In addition, seven studies included longitudinal follow-up evaluations ranging from 3 to 12 months post-intervention [25,26,28,29,30,31,33], allowing for a more comprehensive understanding of sustained intervention effects over time.
All included studies employed a comparative design to evaluate the efficacy of cognitive stimulation interventions. These were contrasted against control groups that received various types of alternative or minimal interventions. Importantly, five of the studies (41.6%) utilized waitlist control groups [23,24,25,26,29], providing a basis for assessing the specific effect of the active intervention in the absence of competing treatments.
Beyond the primary outcome of CRCI, several studies examined additional psychological and functional variables known to influence cognitive outcomes. These included anxiety and depression [25,28,30,31], fatigue [25], and insomnia [29]—all of which are frequently reported among cancer survivors and may exacerbate cognitive impairment. Furthermore, six studies evaluated QoL as a secondary outcome, underscoring the broader psychosocial impact of cognitive rehabilitation [23,24,30,32,33,34].
3.4. Risk of Bias in Studies
A detailed risk of bias assessment is presented in Figure 2.
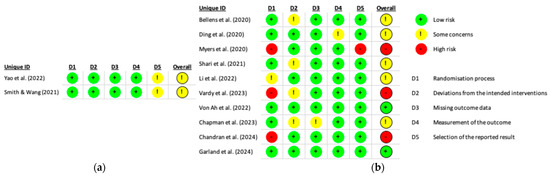
Figure 2.
Summary of risk of bias for studies included in (a) the Systematic Review [24,34] and (b) the Systematic Review and Meta-analysis [23,25,26,27,28,29,30,31,32,33].
The randomization process showed the greatest variability, with approximately 70% of studies rated as having low risk of bias, while the remainder were assessed as having either some concerns or a high risk of bias. The domain of deviations from intended interventions was predominantly rated as low risk, although around 30% of studies raised some concerns. In contrast, the domains related to missing outcome data and measurement of the outcome were associated with a high proportion, approximately 90%, of low-risk ratings. The first domain concerning the selection of the reported result revealed some concerns in 20% of studies and a high risk of bias in 10%.
Regarding the overall risk of bias, fewer than 20% of studies were judged to be at low risk. The majority were classified as raising some concerns, and approximately 25% were considered to be at high risk of bias. These findings indicate potential methodological limitations that may compromise the internal validity of the included studies.
3.5. Meta-Analysis
Two studies [24,34] were excluded from the meta-analysis due to insufficient data required to compute effect sizes.
3.5.1. Overall Effect of Cognitive Training Interventions
The outcomes of the cognitive assessment studies are summarised in Figure 3 and Figure 4. The overall standardised mean difference between experimental and control groups was d = 0.59, with a 95% confidence interval of [−0.05, 1.23] and a p-value of 0.07. While this reflects a moderate positive effect of cognitive training, the difference did not reach statistical significance.
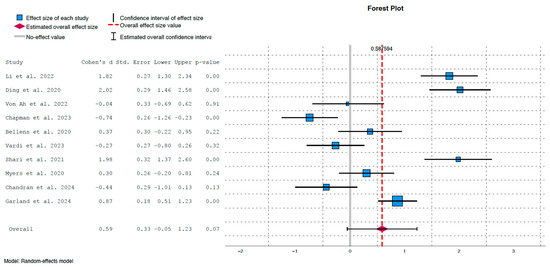
Figure 3.
Forest plot illustrating effect sizes and confidence intervals for each individual study [23,25,26,27,28,29,30,31,32,33].
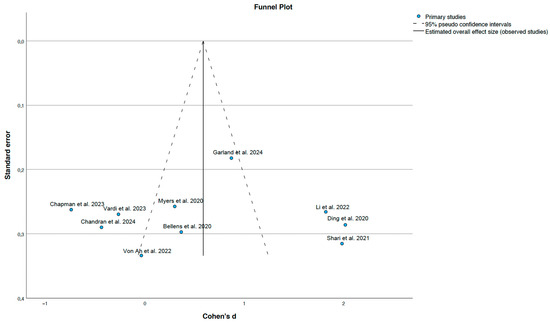
Figure 4.
Funnel plot of standard errors and effect sizes [23,25,26,27,28,29,30,31,32,33].
3.5.2. Heterogeneity Analysis
The lack of statistical significance may be attributed to substantial heterogeneity, indicated by τ2 = 0.99, p < 0.01, and an I2 value of 93%, which implies that a large proportion of the variance is due to heterogeneity rather than chance. This heterogeneity is clearly depicted in Figure 3, where some studies showed strong and statistically significant effects in favour of the intervention group:
- Ding et al. (2020) [23]: d = 2.02, p < 0.01
- Shari et al. (2021) [25]: d = 1.98, p < 0.01
- Li et al. (2022) [27]: d = 1.82, p < 0.01
- Garland et al. (2024) [29]: d = 0.87, p < 0.01
- Conversely, weaker and non-significant positive effects were observed in:
- Bellens et al. (2020) [31]: d = 0.37, p = 0.22
- Myers et al. (2020) [26]: d = 0.30, p = 0.24
Moreover, several studies showed non-significant effects favouring the control group:
- Von Ah et al. (2022) [32]: d = −0.04, p = 0.91
- Vardy et al. (2023) [28]: d = −0.27, p = 0.32
- Chandran et al. (2024) [33]: d = −0.44, p = 0.13
Interestingly, the study by Chapman et al. (2023) showed a moderate and significant effect in favour of the control group immediately post-intervention (d = −0.74, p < 0.01) [30]. However, if we apply a Bonferroni correction due to the multiplicity of contrasts, the confidence intervals in Figure 3 are distributed as follows: (1.09, 2.54) for Li et al. (2020) [27], (1.24, 2.80) for Ding et al. (2020) [23], (−0.95, 0.87) for Von Ah et al. (2022) [32], (−1,47, 0.00) for Chapman et al. (2023) [30], (−0.438, 1.182) for Bellens et al. (2020) [31], (−1.01, 0.47) for Vardy et al. (2023) [28], (1.11, 2.85) for Shari et al. (2021) [25], (−0.42, 1.02) for Myers et al. (2020) [26], (−1.24, 0.36) for Chandran et al. (2024) [33] and (0.37, 1.37) for Garland et al. (2024) [29]. Therefore, the conclusions remain the same in all studies except Chapman et al. (2023), in which the effect in favour of control group becomes borderline [30].
3.5.3. Publication Bias
The funnel plot presented in Figure 4 does not reveal any substantial asymmetry, suggesting the absence of publication bias. However, the ability to detect such bias may be limited due to the relatively uniform standard errors across the included studies. Overall, no clear evidence of publication bias was identified.
4. Discussion
4.1. Study Characteristics
This study investigated the effects of cognitive stimulation for managing CRCI in BC patients through a systematic review and meta-analysis. Out of the 165 studies initially identified, a total of 12 met the inclusion and exclusion criteria and were selected for systematic review. Nearly 80% of the included studies were conducted in North America or Asia, with minimal representation from Europe.
Cognitive stimulation therapies have long been used to support patients with dementia at various stages and are now being explored as targeted interventions for CRCI across different types of cancer. Fernandes et al. (2019) reviewed 19 studies—mostly randomized clinical trials—implementing cognitive rehabilitation programs, including structured cognitive training and compensatory strategies, in patients with non-central nervous system (non-CNS) cancers [18]. They reported significant improvements in memory, processing speed, and executive functioning, though they also noted considerable methodological heterogeneity across studies. Binarelli et al. (2021) focused on 20 computer-based interventions, primarily digital cognitive stimulation, and found positive outcomes in most studies, particularly in attention and memory domains, although the overall quality of evidence was moderate to low [19]. Iulio et al. (2019) conducted a broader review on neuropsychological impairments in non-CNS cancers, documenting frequent objective cognitive decline even in the absence of direct brain involvement [17]. They highlighted the clinical potential of cognitive rehabilitation interventions, which remain insufficiently standardized in routine care. Taken together, these reviews support the use of cognitive stimulation strategies across cancer populations while underscoring the need for more rigorous and cancer-specific studies—particularly in BC.
In addition to reviews specifically focused on cognitive stimulation, others have addressed a wider range of non-pharmacological interventions for CRCI, including cognitive stimulation, rehabilitation, and training. These reviews differ in population focus, intervention types, and the cognitive domains assessed. Four notable reviews [14,15,35,36] concentrated specifically on women with BC following chemotherapy. Floyd et al. identified limited evidence across ten randomized trials and highlighted the need for high-quality, multidomain studies [35]. Liu et al., through a network meta-analysis of 12 studies, found that psychotherapy was the most effective intervention for subjective cognition, whereas exercise and qigong yielded better outcomes on objective measures [14]. Park et al. included 23 studies (17 in quantitative synthesis) and reported positive effects of cognitive rehabilitation, cognitive-behavioral therapy (CBT), and physical activity on attention, immediate memory, and executive function. Pan Yang et al., in the most comprehensive review to date (42 studies), concluded that psychological interventions were most effective for subjective symptoms, while cognitive training and cognitive rehabilitation were more effective for working memory, learning, and executive functions [36]. Other reviews focusing on various cancer types, such as those by Mackenzie et al. and Zeng et al., found similar benefits from CBT, exercise, meditation, and cognitive training, although with greater heterogeneity and reduced applicability to BC specifically. Collectively, these reviews affirm the potential value of non-pharmacological interventions in mitigating CRCI but also emphasize the need for methodologically robust studies targeting well-defined clinical populations [12,37].
Our review observed substantial diversity in cognitive stimulation and training interventions, with durations ranging from less than 6 to more than 12 weeks, total hours from fewer than 3 to over 75, and implementation at various points in the treatment timeline (during chemotherapy or 3 months to 1 year post-treatment). The timing of intervention is a particularly important factor. The literature remains divided on the optimal period for addressing CRCI. Some authors advocate intervening during chemotherapy to mitigate its impact on QoL and support emotional and occupational recovery. However, most studies are conducted at least 6 months after chemotherapy, largely due to recruitment challenges during active treatment, when patients experience significant physical and emotional side effects and may be less willing to participate. Additionally, maladaptive coping strategies often develop during this phase, potentially compromising study outcomes [9].
When interpreting these findings, it is also important to consider patient-related factors. Root et al. (2023) have shown that advanced age, particularly in patients over 75 years, may contribute to cognitive decline through both cancer treatment and age-related processes [38]. Emerging evidence further indicates that biologic subtype, such as HER2 status, may also influence cognitive outcomes in BC patients [39]. In addition, while our review focused primarily on mean-based measures of cognitive performance, growing attention has been directed toward cognitive intra-individual variability (IIV) as a complementary and potentially more sensitive indicator of cognitive functioning in breast cancer survivors. IIV, which reflects fluctuations in performance across tasks or within repeated trials, has been proposed as an early marker of cognitive inefficiency and neurological vulnerability. A recent systematic review by Vance et al. (2025) synthesized evidence on IIV in this population, concluding that higher IIV may be associated with CRCI and could provide insights beyond traditional mean-based scores [40]. Future studies should therefore incorporate both demographic and tumor-related factors, as well as IIV measures, to provide a more comprehensive understanding of cognitive health in breast cancer survivors.
The quality assessment of the selected studies revealed considerable methodological variability. Fewer than 20% of the studies were judged to be at low risk of bias, while the majority raised some concerns, and approximately 25% were categorized as having a high risk of bias. Overall, the evidence base of this review presents a moderate risk of bias, with notable weaknesses particularly in the domains of randomization and selective reporting. These limitations may affect the internal validity of the findings and should be taken into account when interpreting the results of the systematic review and meta-analysis.
4.2. Intervention Effects
The results of the meta-analysis examining the effects of cognitive stimulation interventions indicate a moderate overall effect size (Cohen’s d = 0.59), suggesting potential benefits for cognitive functioning in BC patients experiencing CRCI. However, this effect did not reach statistical significance (p = 0.07), and the wide confidence interval (95% CI: −0.05 to 1.23) warrants caution in interpreting the magnitude of the benefit. Although the observed effect trends positively, the high degree of between-study variability may partly explain the lack of significance.
The heterogeneity analysis revealed substantial inconsistency across studies (τ2 = 0.99, p < 0.01; I2 = 93%), indicating that most of the variance in effect sizes is attributable to differences in study characteristics rather than random error. This is clearly illustrated in the forest plot (Figure 3), where some interventions [23,25,27,29] demonstrated strong and statistically significant improvements in cognitive outcomes. In contrast, other studies reported weak effects favouring the control group [28,30], with one study showing statistical significance; however, this significance was borderline when adjusted using the Bonferroni correction [30]. These discrepancies may stem from methodological differences, including the type, timing, duration, and delivery format of cognitive stimulation, as well as variations in outcome measures (e.g., FACT-Cog vs. alternative cognitive scales).
Despite the substantial heterogeneity, the direction of the effect generally favoured cognitive stimulation interventions. Moreover, the symmetrical distribution of studies in the funnel plot (Figure 4) suggests a low risk of publication bias. Nonetheless, the relatively small number of studies and the homogeneity in standard errors may limit the sensitivity of funnel plot-based asymmetry detection.
4.3. Limitations
This review provides a meaningful contribution to the current understanding of cognitive stimulation interventions for CRCI in BC patients; however, several limitations should be acknowledged. First, the relatively small number of included studies, combined with the restriction of the inclusion period to 2020–2025, may reduce the representativeness and generalizability of the findings. This temporal restriction was intentional, as our aim was to synthesize the most methodologically up-to-date evidence. Second, there was substantial inconsistency in how cognitive stimulation was defined and implemented, reflecting both heterogeneity in the literature and the evolving nature of these interventions. Outcome measures also varied, with some studies employing the FACT-Cog scale and others using alternative instruments, thereby complicating cross-study comparisons. Nonetheless, this variation reflects a growing methodological interest in the field. Additionally, two studies were excluded from the meta-analysis due to insufficient statistical reporting, which may have influenced the overall pooled estimate. Despite these limitations, the review offers a timely synthesis of recent evidence and identifies key directions for future research and clinical practice.
5. Conclusions
Cognitive stimulation and training interventions have the potential to improve cognitive functioning in BC patients who have undergone chemotherapy. However, substantial heterogeneity exists across studies in terms of both intervention characteristics and the assessment criteria used for CRCI. Consequently, further research is needed to comprehensively investigate the pathophysiology of CRCI, as well as effective methods for its prevention and treatment. Such efforts would support the development of unified strategies or early, personalised intervention protocols tailored to the specific needs of each patient.
Author Contributions
Conceptualization, M.C.C., M.Á.M.-P., J.M.-F., A.S.-F., C.F.L.-J. and N.D.-G.; Data curation, J.M.-F.; Formal analysis, M.Á.M.-P. and J.M.-F.; Funding acquisition, N.D.-G.; Investigation, M.C.C., M.Á.M.-P. and A.S.-F.; Methodology, M.C.C., M.Á.M.-P., J.M.-F. and N.D.-G.; Project administration, N.D.-G.; Resources, M.C.C. and M.Á.M.-P.; Supervision, N.D.-G.; Validation, M.C.C., M.Á.M.-P. and N.D.-G.; Visualization, M.C.C., M.Á.M.-P., J.M.-F. and C.F.L.-J.; Writing—original draft, M.C.C., M.Á.M.-P., J.M.-F. and A.S.-F.; Writing—review & editing, M.C.C., M.Á.M.-P. and N.D.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This project has been co-financed at 85% by the European Union (European Regional Development Fund) and the Regional Government of Extremadura. Managing Authority: Ministry of Finance. Reference number: GR24011.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACT | Acceptance and Commitment Therapy |
| ADLs | Activities of Daily Living |
| APT | Attention Process Training |
| BC | Breast Cancer |
| BDNF | Brain-Derived Neurotrophic Factor |
| BADS | Behavioural Assessment of the Dysexecutive Syndrome |
| CACT | Computer-Assisted Cognitive Training |
| CALM | Cancer and Living Meaningfully |
| CBT-I | Cognitive Behavioral Therapy for Insomnia |
| CDT | Cognitive Dysfunction Test |
| CFQ | Cognitive Failures Questionnaire |
| CRCI | Chemotherapy-Related Cognitive Impairment |
| CST | Compensatory Strategy Training |
| EEG | Electroencephalogram |
| FACT-B | Functional Assessment of Cancer Therapy-Breast |
| FACT-Cog | Functional Assessment of Cancer Therapy-Cognitive Function |
| IIV | Intra-individual Variability |
| ISI | Insomnia Severity Index |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MDR | Multidimensional Rehabilitation Program |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| QoL | Quality of Life |
| RBMT-II | Rivermead Behavioural Memory Test-Second Edition |
| RRS | Ruminative Response Scale |
| WAI | Working Alliance Inventory |
References
- Pimentel-Parra, G.A.; García-Vivar, C.; Escalada-Hernández, P.; San Martín-Rodríguez, L.; Soto-Ruiz, N. Systematic Review of Clinical Practice Guidelines for Long-Term Breast Cancer Survivorship: Assessment of Quality and Evidence-Based Recommendations. Br. J. Cancer 2025, 2025, 178–193. [Google Scholar] [CrossRef]
- Cáceres, M.C.; Nadal-Delgado, M.; López-Jurado, C.; Pérez-Civantos, D.; Guerrero-Martín, J.; Durán-Gómez, N. Factors Related to Anxiety, Depressive Symptoms and Quality of Life in Breast Cancer. Int. J. Environ. Res. Public Health 2022, 19, 3547. [Google Scholar] [CrossRef]
- Cáceres, M.C.; Pérez-Civantos, D.; Guerrero-Martín, J.; Delgado, M.N.; Jurado, C.L.; Durán-Gómez, N. Depressive Symptoms and Quality of Life Associated with the Use of Monoclonal Antibodies in Breast Cancer Treatment. Oncol. Nurs. Forum 2021, 48, 535–545. [Google Scholar] [CrossRef]
- Durán-Gómez, N.; Martín-Parrilla, M.Á.; López-Jurado, C.F.; Montanero-Fernández, J.; Nadal-Delgado, M.; Cáceres, M.C. The Need for Comprehensive Sleep Disturbances Assessment and Management in Breast Cancer Care. Sci. Rep. 2025, 15, 21235. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, H.; Guo, Y.; Cao, Y.; Wong, C.L. Symptom Clusters in Breast Cancer Patients Receiving Adjuvant Chemotherapy: A Systematic Review. J. Clin. Nurs. 2024, 33, 4554–4567. [Google Scholar] [CrossRef]
- Yang, Y.; Von Ah, D. Cancer-Related Cognitive Impairment: Updates to Treatment, the Need for More Evidence, and Impact on Quality of Life—A Narrative Review. Ann. Palliat. Med. 2024, 13, 1261280–1265280. [Google Scholar] [CrossRef]
- Kohler, C.; Chang, M.; Allemann-Su, Y.-Y.; Vetter, M.; Jung, M.; Jung, M.; Conley, Y.; Paul, S.; Kober, K.M.; Cooper, B.A.; et al. Changes in Attentional Function in Patients from before through 12 Months after Breast Cancer Surgery. J. Pain. Symptom Manag. 2020, 59, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.; Schaffrath, N.; Schmidt, K.; Bloch, W.; Jäger, E.; Steindorf, K.; Hartig, P.; Joisten, N.; Zimmer, P. Protocol for the “Chemobrain in Motion--Study”(CIM--Study): A Randomized Placebo-Controlled Trial of the Impact of a High-Intensity Interval Endurance Training on Cancer Related Cognitive Impairments in Women with Breast Cancer Receiving First-Line Chemot. BMC Cancer 2018, 18, 1071. [Google Scholar] [CrossRef] [PubMed]
- Durán-Gómez, N.; López-Jurado, C.F.; Nadal-Delgado, M.; Pérez-Civantos, D.; Guerrero-Martín, J.; Cáceres, M.C. Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Based on Functional Assessment and NIRS Analysis. J. Clin. Med. 2022, 11, 2363. [Google Scholar] [CrossRef]
- Whittaker, A.L.; George, R.P.; O’Malley, L. Prevalence of Cognitive Impairment Following Chemotherapy Treatment for Breast Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 2135. [Google Scholar] [CrossRef]
- Vrancken Peeters, N.J.M.C.; Koppert, L.B.; Jager, A.; Hendriks, M.P.; Siesling, S.; van den Hurk, C.J.G. Symptom Monitoring and Health-Related Quality of Life in Non-Metastatic Breast Cancer Patients: A Systematic Review. Curr. Breast Cancer Rep. 2024, 16, 417–428. [Google Scholar] [CrossRef]
- Mackenzie, L.; Marshall, K. Effective Non-Pharmacological Interventions for Cancer Related Cognitive Impairment in Adults (Excluding Central Nervous System or Head and Neck Cancer): Systematic Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2021, 58, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Jung, Y.S.; Kim, K.S.; Bae, S.H. Effects of Compensatory Cognitive Training Intervention for Breast Cancer Patients Undergoing Chemotherapy: A Pilot Study. Support. Care Cancer 2017, 25, 1887–1896. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.-E.; Chen, S.; Zhao, F.; Chen, L.; Li, R. Effectiveness of Nonpharmacologic Interventions for Chemotherapy-Related Cognitive Impairment in Breast Cancer Patients: A Systematic Review and Network Meta-Analysis. Cancer Nurs. 2023, 46, E305–E319. [Google Scholar] [CrossRef]
- Yang, P.; Hu, Q.; Zhang, L.; Shen, A.; Zhang, Z.; Wang, Q.; Lu, Q. Effects of Non-Pharmacological Interventions on Cancer-Related Cognitive Impairment in Patients with Breast Cancer: A Systematic Review and Network Meta-Analysis. Eur. J. Oncol. Nurs. 2025, 75, 102804. [Google Scholar] [CrossRef]
- Woods, B.; Aguirre, E.; Spector, A.E.; Orrell, M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012, CD005562. [Google Scholar] [CrossRef] [PubMed]
- Di Iulio, F.; Cravello, L.; Shofany, J.; Paolucci, S.; Caltagirone, C.; Morone, G. Neuropsychological Disorders in Non-Central Nervous System Cancer: A Review of Objective Cognitive Impairment, Depression, and Related Rehabilitation Options. Neurol. Sci. 2019, 40, 1759–1774. [Google Scholar] [CrossRef]
- Fernandes, H.A.; Richard, N.M.; Edelstein, K. Cognitive Rehabilitation for Cancer-Related Cognitive Dysfunction: A Systematic Review. Support. Care Cancer 2019, 27, 3253–3279. [Google Scholar] [CrossRef]
- Binarelli, G.; Joly, F.; Tron, L.; Lefevre Arbogast, S.; Lange, M. Management of Cancer-Related Cognitive Impairment: A Systematic Review of Computerized Cognitive Stimulation and Computerized Physical Activity. Cancers 2021, 13, 5161. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Patsopoulos, N.A.; Evangelou, E. Uncertainty in Heterogeneity Estimates in Meta-Analyses. BMJ 2007, 335, 914–916. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, X.; Zhao, J.; Zuo, H.; Bi, Z.; Cheng, H. Managing Cancer and Living Meaningfully (CALM) Intervention on Chemotherapy-Related Cognitive Impairment in Breast Cancer Survivors. Integr. Cancer Ther. 2020, 19, 1534735420938450. [Google Scholar] [CrossRef]
- Yao, S.; Ding, K.; Liu, S.; Zhang, Q.; Li, W.; Tang, L.; Yu, S.; Pang, L.; Yin, X.; Cheng, H. The Managing Cancer and Living Meaningfully (CALM) Intervention Alleviates Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer by Modulating Pan-Immune-Inflammation Values. Integr. Cancer Ther. 2022, 21, 15347354221140498. [Google Scholar] [CrossRef]
- Shari, N.I.; Zainal, N.Z.; Ng, C.G. Effects of Brief Acceptance and Commitment Therapy (ACT) on Subjective Cognitive Impairment in Breast Cancer Patients Undergoing Chemotherapy. J. Psychosoc. Oncol. 2021, 39, 695–714. [Google Scholar] [CrossRef]
- Myers, J.S.; Cook-Wiens, G.; Baynes, R.; Jo, M.Y.; Bailey, C.; Krigel, S.; Klemp, J.; Asher, A. Emerging From the Haze: A Multicenter, Controlled Pilot Study of a Multidimensional, Psychoeducation-Based Cognitive Rehabilitation Intervention for Breast Cancer Survivors Delivered With Telehealth Conferencing. Arch. Phys. Med. Rehabil. 2020, 101, 948–959. [Google Scholar] [CrossRef]
- Li, Z.; Hao, X.; Lei, P.; Zhou, L.; Chen, C.; Tan, T.; Yue, L. Patients With Breast Cancer Receiving Chemotherapy: Effects of Multisensory Stimulation Training on Cognitive Impairment. Clin. J. Oncol. Nurs. 2022, 26, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.L.; Pond, G.R.; Bell, M.L.; Renton, C.; Dixon, A.; Dhillon, H.M. A Randomised Controlled Trial Evaluating Two Cognitive Rehabilitation Approaches for Cancer Survivors with Perceived Cognitive Impairment. J. Cancer Surviv. 2023, 17, 1583–1595. [Google Scholar] [CrossRef]
- Garland, S.N.; Tulk, J.; Savard, J.; Rash, J.A.; Browne, S.; Urquhart, R.; Seal, M.; Thoms, J.; Laing, K. Randomized Controlled Trial of Virtually Delivered Cognitive Behavioral Therapy for Insomnia to Address Perceived Cancer-Related Cognitive Impairment in Cancer Survivors. J. Clin. Oncol. 2024, 42, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Louis, C.C.; Moser, J.; Grunfeld, E.A.; Derakshan, N. Benefits of Adaptive Cognitive Training on Cognitive Abilities in Women Treated for Primary Breast Cancer: Findings from a 1-Year Randomised Control Trial Intervention. Psychooncology 2023, 32, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Bellens, A.; Roelant, E.; Sabbe, B.; Peeters, M.; van Dam, P.A. A Video-Game Based Cognitive Training for Breast Cancer Survivors with Cognitive Impairment: A Prospective Randomized Pilot Trial. Breast 2020, 53, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, D.; McDonald, B.C.; Crouch, A.D.; Ofner, S.; Perkins, S.; Storey, S.; Considine, R.; Unverzagt, F. Randomized Double-Masked Controlled Trial of Cognitive Training in Breast Cancer Survivors: A Preliminary Study. Support. Care Cancer 2022, 30, 7457–7467. [Google Scholar] [CrossRef] [PubMed]
- Chandran, G.; Tang, N.; Ngo, E.L.P.; Huang, S.; Tong, S.I.; Ong, J.X.; Chew, E. Comparing the Efficacy of a Multi-Dimensional Breast Cancer Rehabilitation Programme versus a Home-Based Exercise Programme during Adjuvant Cancer Treatment. BMC Cancer 2024, 24, 361. [Google Scholar] [CrossRef]
- Smith, T.M.; Wang, W. Comparison of a Standard Computer-Assisted Cognitive Training Program to a Music Enhanced Program: A Mixed Methods Study. Cancer Rep. 2021, 4, e1325. [Google Scholar] [CrossRef]
- Floyd, R.; Dyer, A.H.; Kennelly, S.P. Non-Pharmacological Interventions for Cognitive Impairment in Women with Breast Cancer Post-Chemotherapy: A Systematic Review. J. Geriatr. Oncol. 2021, 12, 173–181. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, S.J.; Lee, L.J.; Rhu, J.; Bae, S.H. Impact of Nonpharmacological Interventions on Cognitive Impairment in Women with Breast Cancer: A Systematic Review and Meta-Analysis. Asia Pac. J. Oncol. Nurs. 2023, 10, 100212. [Google Scholar] [CrossRef]
- Zeng, Y.; Dong, J.; Huang, M.; Zhang, J.; Zhang, X.; Xie, M.; Wefel, J.S. Nonpharmacological Interventions for Cancer-Related Cognitive Impairment in Adult Cancer Patients: A Network Meta-Analysis. Int. J. Nurs. Stud. 2020, 104, 103514. [Google Scholar] [CrossRef]
- Root, J.C.; Li, Y.; Schofield, E.; Orlow, I.; Ryan, E.; Traina, T.; Patel, S.K.; Ahles, T.A. Cognitive Aging in Older Breast Cancer Survivors. Cancers 2023, 15, 3208. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, H.J.; Wu, S.I.; Tzang, B.S.; Hsieh, C.C.; Weng, Y.P.; Hsu, Y.T.; Hsiao, H.P.; Chen, V.C.H. Cognitive Function and Breast Cancer Molecular Subtype before and after Chemotherapy. Appl. Neuropsychol. Adult 2025, 32, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Collette, C.; Frank, J.S.; Billings, B.; Deaver, J.; del Bene, V.A.; Fazeli, P.L.; Bail, J.R.; Li, W.; Triebel, K.L. Cognitive Intra-Individual Variability in Breast Cancer Survivors: A Systematic Review. Appl. Neuropsychol. Adult 2025, 32, 1346–1360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).