Mechanistic Insights into Proglumide’s Role in Immune Cell Efficacy and Response to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Liver Cancer Cells
2.2. Preliminary Dosing and Cell Number Pilot Experiments
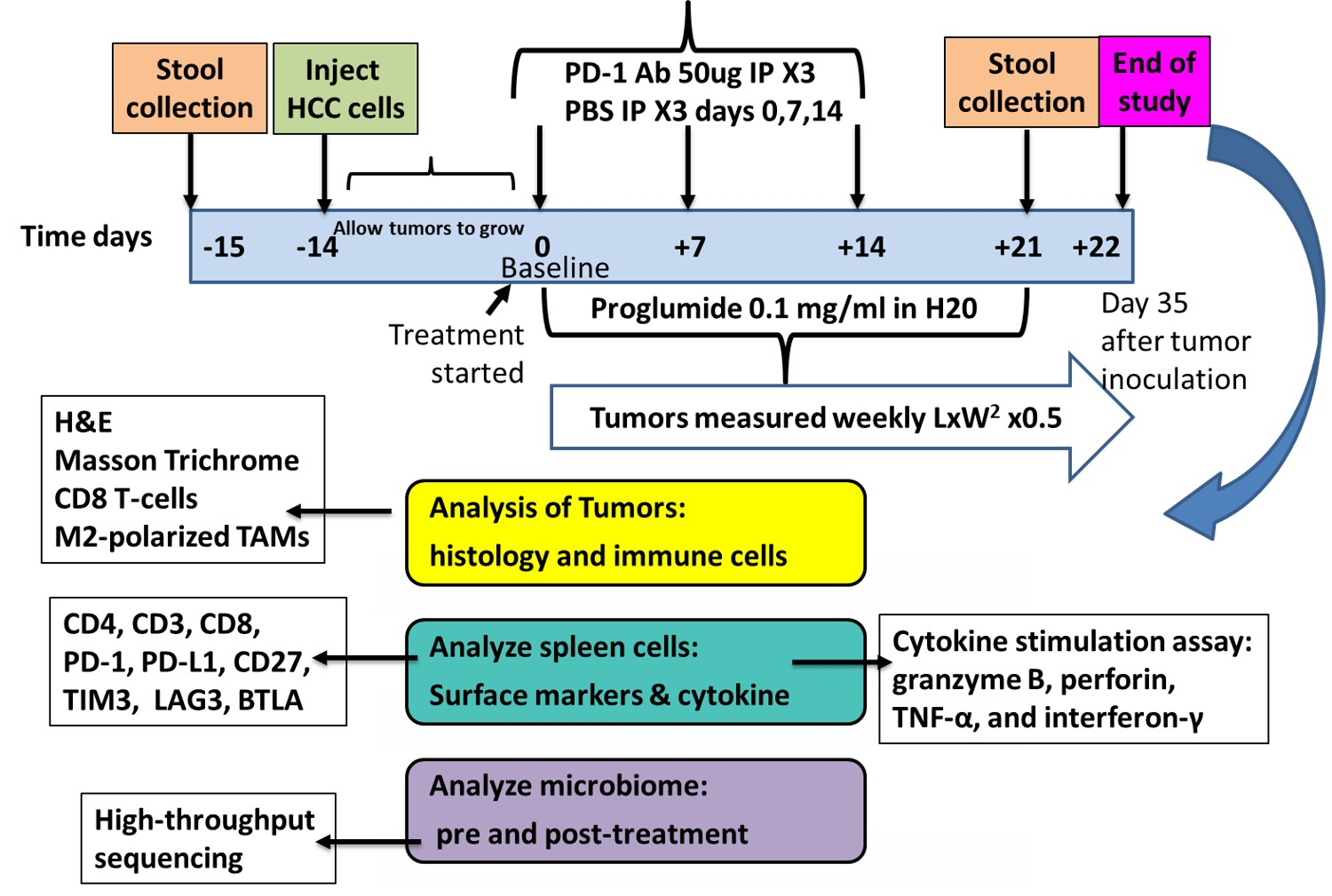
2.3. Study Design and Treatments
2.4. Evaluation of Treatments on Tumor Growth
2.5. Spleen T-Cell Isolation
2.6. Analysis of the Tumor Microenvironment by Histology and Immunohistochemistry
2.7. Evaluation of Spleen Lymphocytes for Exhaustion Markers
2.8. Measurement of T-Cell Activity by Cytokine Stimulation Assay
2.9. Microbiome Analysis
2.10. Statistical Analysis
3. Results
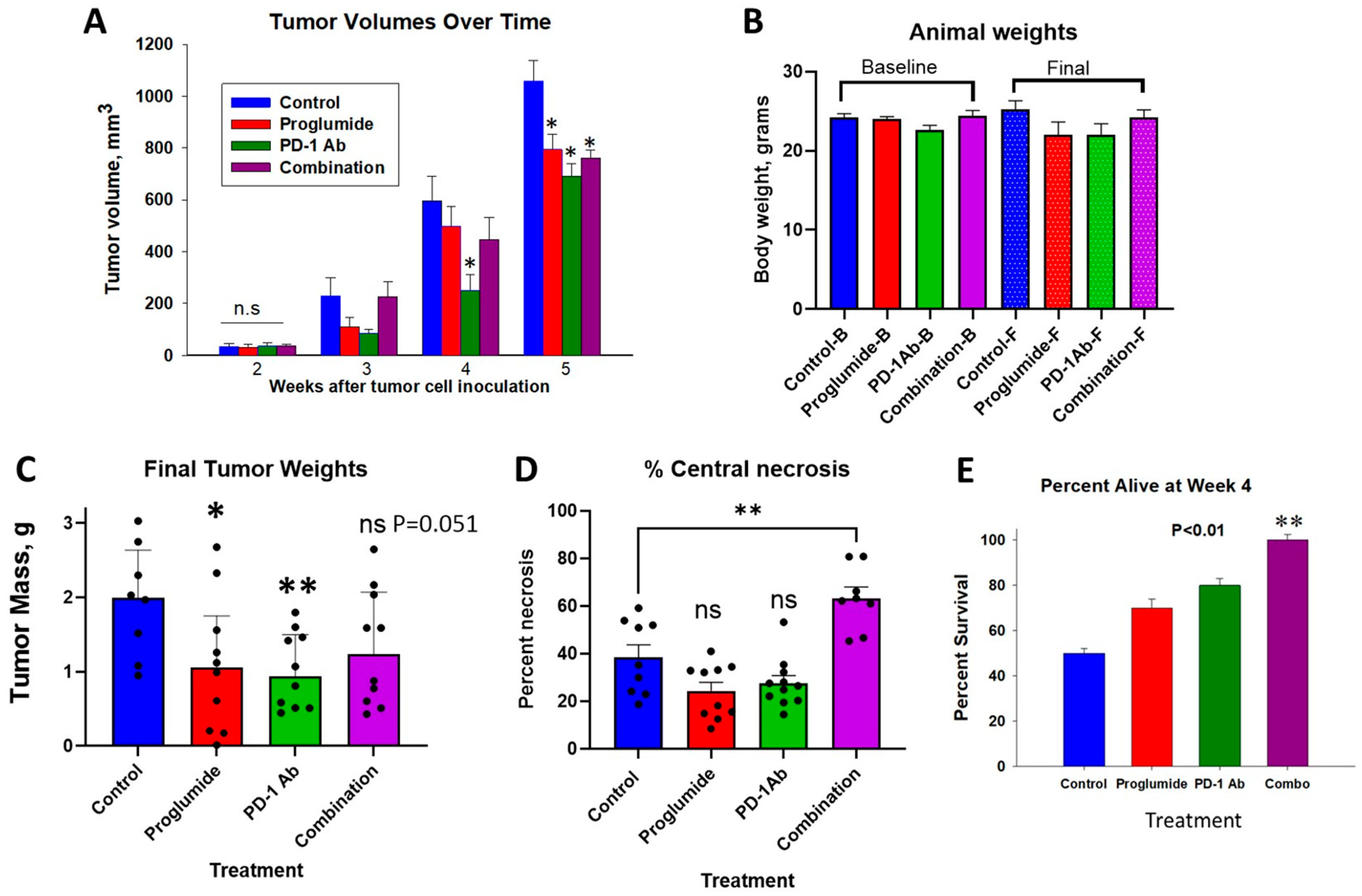
3.1. Effects of Proglumide and PD-1Ab Alone or in Combination on HCC Tumor Growth
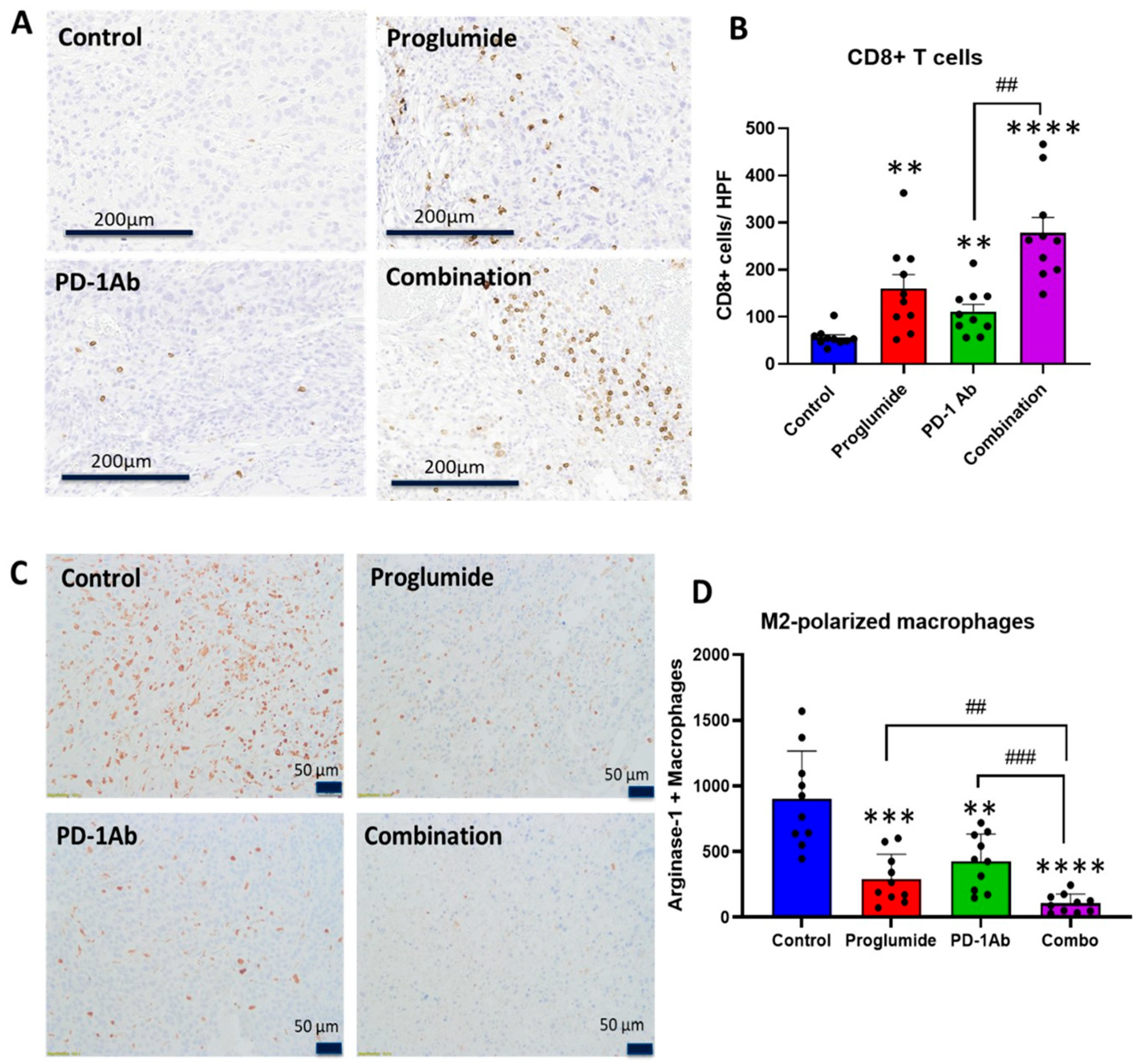
3.2. Proglumide Therapy Decreases Tumoral Fibrosis and Alters the Immune Cell Signature
3.3. Proglumide Alters the Immune Cell Signature of the HCC Tumor Microenvironment
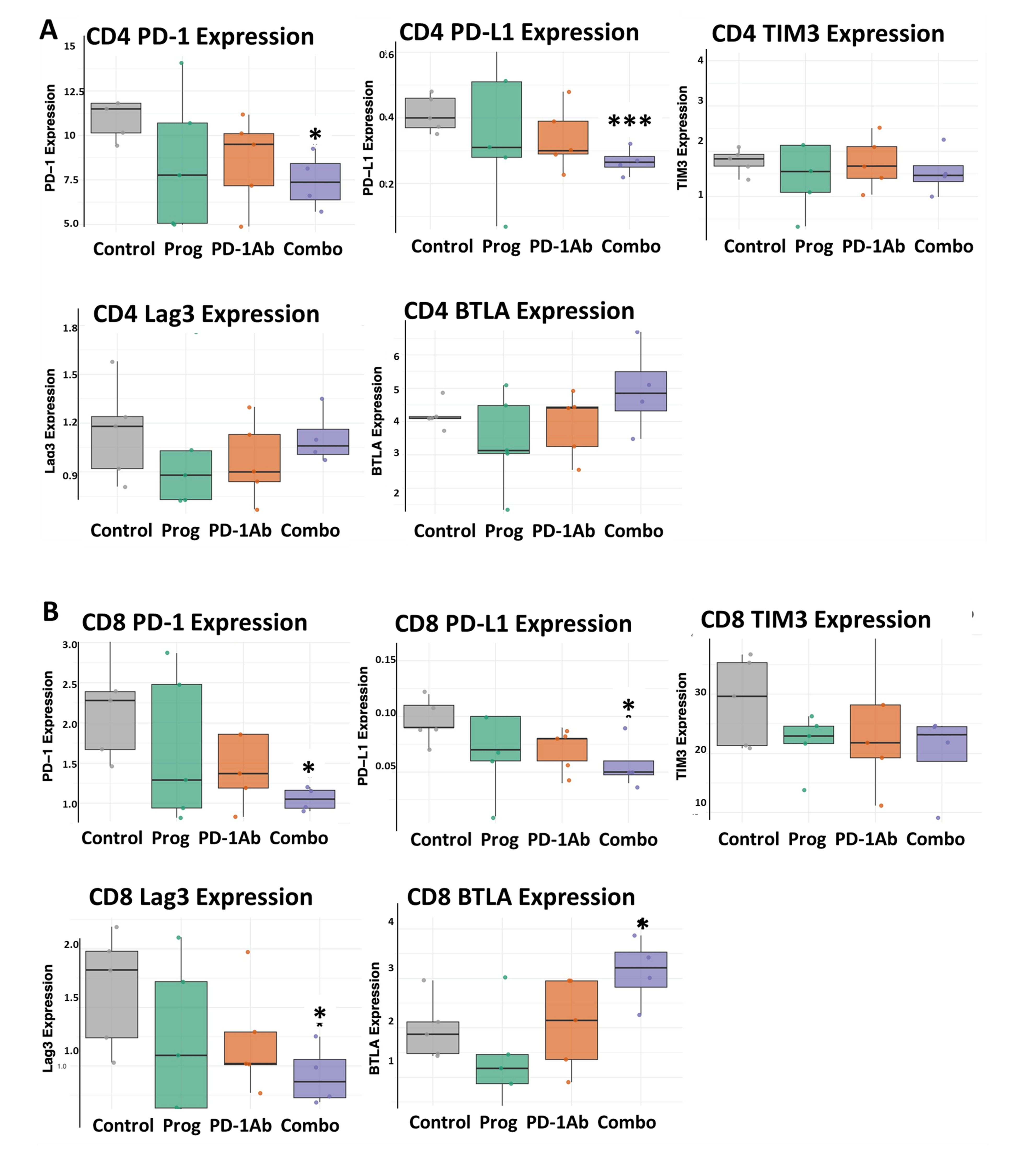
3.4. Combination Therapy Decreases T-Cell Exhaustion Markers
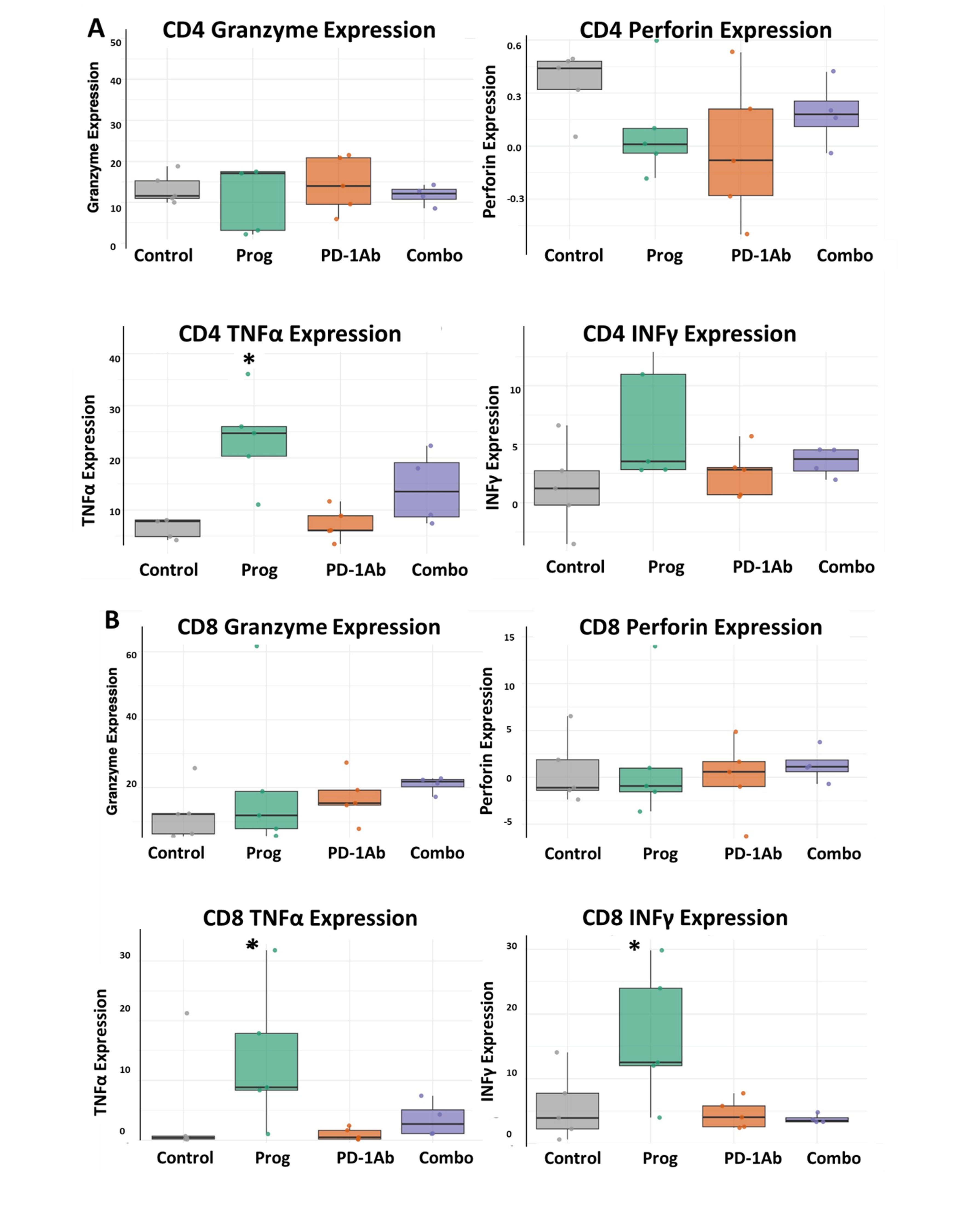
3.5. Improved Cytokine Release from T-Cells Treated with Proglumide
3.6. Combination Therapy with Proglumide and PD-1Ab Alters the Mouse Microbiome
4. Discussions
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCK | cholecystokinin |
| CCK-BR | cholecystokinin-B receptor |
| HCC | hepatocellular carcinoma |
| HPF | high-power field |
| ICI | immune checkpoint inhibitors |
| IFNγ | interferon-gamma |
| PD-1 Ab | programmed death-1 antibody |
| SCFAs | short-chain fatty acids |
| TAMs | tumor-associated macrophages |
| TME | tumor microenvironment |
| TNFα | tumor necrosis factor-alpha |
References
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Boland, P.; Wu, J. Systemic therapy for hepatocellular carcinoma: Beyond sorafenib. Chin. Clin. Oncol. 2018, 7, 50. [Google Scholar] [CrossRef]
- Kudo, M. Immuno-Oncology in Hepatocellular Carcinoma: 2017 Update. Oncology 2017, 93 (Suppl. 1), 147–159. [Google Scholar] [CrossRef]
- Pinter, M.; Scheiner, B.; Peck-Radosavljevic, M. Immunotherapy for advanced hepatocellular carcinoma: A focus on special subgroups. Gut 2021, 70, 204–214. [Google Scholar] [CrossRef]
- Abd El Aziz, M.A.; Facciorusso, A.; Nayfeh, T.; Saadi, S.; Elnaggar, M.; Cotsoglou, C.; Sacco, R. Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma. Vaccines 2020, 8, 616. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.R., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Alsudaney, M.; Ayoub, W.; Kosari, K.; Koltsova, E.; Abdulhaleem, M.N.; Adetyan, H.; Yalda, T.; Attia, A.M.; Liu, J.; Yang, J.D. Pathophysiology of liver cirrhosis and risk correlation between immune status and the pathogenesis of hepatocellular carcinoma. Hepatoma Res. 2025, 11, 7. [Google Scholar] [CrossRef]
- Basho, K.; Zoldan, K.; Schultheiss, M.; Bettinger, D.; Globig, A.M.; Bengsch, B.; Neumann-Haefelin, C.; Klocperk, A.; Warnatz, K.; Hofmann, M.; et al. IL-2 contributes to cirrhosis-associated immune dysfunction by impairing follicular T helper cells in advanced cirrhosis. J. Hepatol. 2021, 74, 649–660. [Google Scholar] [CrossRef]
- Gumber, D.; Wang, L.D. Improving CAR-T immunotherapy: Overcoming the challenges of T cell exhaustion. EBioMedicine 2022, 77, 103941. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Han, M.; Guo, Q.; Yan, F.; Wang, M.; Ning, Q.; Xi, D. Strategies to reinvigorate exhausted CD8(+) T cells in tumor microenvironment. Front. Immunol. 2023, 14, 1204363. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Ruff, S.M.; Pawlik, T.M. The effect of liver disease on hepatic microenvironment and implications for immune therapy. Front. Pharmacol. 2023, 14, 1225821. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, T.; Zeng, F.; Zhang, J. New insights into T cell metabolism in liver cancer: From mechanism to therapy. Cell Death. Discov. 2025, 11, 118. [Google Scholar] [CrossRef]
- Li, Q.; Han, J.; Yang, Y.; Chen, Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front. Immunol. 2022, 13, 1070961. [Google Scholar] [CrossRef]
- Mattos, A.Z.; Debes, J.D.; Boonstra, A.; Vogel, A.; Mattos, A.A. Immune aspects of hepatocellular carcinoma: From immune markers for early detection to immunotherapy. World J. Gastrointest. Oncol. 2021, 13, 1132–1143. [Google Scholar] [CrossRef]
- Singh, A.; Beechinor, R.J.; Huynh, J.C.; Li, D.; Dayyani, F.; Valerin, J.B.; Hendifar, A.; Gong, J.; Cho, M. Immunotherapy Updates in Advanced Hepatocellular Carcinoma. Cancers 2021, 13, 2164. [Google Scholar] [CrossRef]
- Gay, M.D.; Safronenka, A.; Cao, H.; Liu, F.H.; Malchiodi, Z.X.; Tucker, R.D.; Kroemer, A.; Shivapurkar, N.; Smith, J.P. Targeting the Cholecystokinin Receptor: A Novel Approach for Treatment and Prevention of Hepatocellular Cancer. Cancer Prev. Res. 2021, 14, 17–29. [Google Scholar] [CrossRef]
- Berna, M.J.; Seiz, O.; Nast, J.F.; Benten, D.; Blaker, M.; Koch, J.; Lohse, A.W.; Pace, A. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J. Biol. Chem. 2010, 285, 38905–38914. [Google Scholar] [CrossRef]
- Phillips, P.A.; Yang, L.; Shulkes, A.; Vonlaufen, A.; Poljak, A.; Bustamante, S.; Warren, A.; Xu, Z.; Guilhaus, M.; Pirola, R.; et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc. Natl. Acad. Sci. USA 2010, 107, 17397–17402. [Google Scholar] [CrossRef]
- Jolly, G.; Duka, T.; Shivapurkar, N.; Chen, W.; Bansal, S.; Cheema, A.; Smith, J.P. Cholecystokinin Receptor Antagonist Induces Pancreatic Stellate Cell Plasticity Rendering the Tumor Microenvironment Less Oncogenic. Cancers 2023, 15, 2811. [Google Scholar] [CrossRef] [PubMed]
- Gay, M.D.; Drda, J.C.; Chen, W.; Huang, Y.; Yassin, A.A.; Duka, T.; Fang, H.; Shivapurkar, N.; Smith, J.P. Implicating the cholecystokinin B receptor in liver stem cell oncogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G291–G309. [Google Scholar] [CrossRef]
- Tucker, R.D.; Ciofoaia, V.; Nadella, S.; Gay, M.D.; Cao, H.; Huber, M.; Safronenka, A.; Shivapurkar, N.; Kallakury, B.; Kruger, A.J.; et al. A Cholecystokinin Receptor Antagonist Halts Nonalcoholic Steatohepatitis and Prevents Hepatocellular Carcinoma. Dig. Dis. Sci. 2020, 65, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Shivapurkar, N.; Gay, M.D.; He, A.R.; Chen, W.; Golnazar, S.; Cao, H.; Duka, T.; Kallakury, B.; Vasudevan, S.; Smith, J.P. Treatment with a Cholecystokinin Receptor Antagonist, Proglumide, Improves Efficacy of Immune Checkpoint Antibodies in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2023, 24, 3625. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Gay, M.D.; Cao, H.; Shivapurkar, N.; Dakshanamurthy, S.; Kallakury, B.; Tucker, R.D.; Kwagyan, J.; Smith, J.P. Proglumide Reverses Nonalcoholic Steatohepatitis by Interaction with the Farnesoid X Receptor and Altering the Microbiome. Int. J. Mol. Sci. 2022, 23, 1899. [Google Scholar] [CrossRef]
- Frankel, A.E.; Deshmukh, S.; Reddy, A.; Lightcap, J.; Hayes, M.; McClellan, S.; Singh, S.; Rabideau, B.; Glover, T.G.; Roberts, B.; et al. Cancer Immune Checkpoint Inhibitor Therapy and the Gut Microbiota. Integr. Cancer Ther. 2019, 18, 1534735419846379. [Google Scholar] [CrossRef]
- Yi, M.; Yu, S.; Qin, S.; Liu, Q.; Xu, H.; Zhao, W.; Chu, Q.; Wu, K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 47. [Google Scholar] [CrossRef]
- Kapanadze, T.; Gamrekelashvili, J.; Ma, C.; Chan, C.; Zhao, F.; Hewitt, S.; Zender, L.; Kapoor, V.; Felsher, D.W.; Manns, M.P.; et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J. Hepatol. 2013, 59, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.; Sundseth, R.; Burks, J.; Cao, H.; Liu, X.; Kroemer, A.H.; Sutton, L.; Cato, A.; Smith, J.P. Gastrin vaccine improves response to immune checkpoint antibody in murine pancreatic cancer by altering the tumor microenvironment. Cancer Immunol. Immunother. 2019, 68, 1635–1648. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Diskin, B.; Adam, S.; Cassini, M.F.; Sanchez, G.; Liria, M.; Aykut, B.; Buttar, C.; Li, E.; Sundberg, B.; Salas, R.D.; et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 2020, 21, 442–454. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2021, 12, 796025. [Google Scholar] [CrossRef]
- Abdelsalam, N.A.; Hegazy, S.M.; Aziz, R.K. The curious case of Prevotella copri. Gut Microbes 2023, 15, 2249152. [Google Scholar] [CrossRef]
- Lee, P.C.; Wu, C.J.; Hung, Y.W.; Lee, C.J.; Chi, C.T.; Lee, I.C.; Yu-Lun, K.; Chou, S.H.; Luo, J.C.; Hou, M.C.; et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004779. [Google Scholar] [CrossRef]
- Kang, X.; Lau, H.C.; Yu, J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep. Med. 2024, 5, 101478. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Liu, C.; Ding, Y.; Ni, Y.; Ji, F.; Lau, H.C.H.; Jiang, L.; Sung, J.J.; Wong, S.H.; Yu, J. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8(+) T cells. Gut 2023, 72, 2112–2122. [Google Scholar] [CrossRef]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open. Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Renukadevi, J.; Helinto, J.S.; Prena, D. Immunotherapeutic Potential of Lactobacillus Species as Immune Checkpoint Inhibitors in Cancer Immunotherapy. J. Bio-X Res. 2025, 8, 0028. [Google Scholar] [CrossRef]
- Yan, S.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Chen, H.; Zhai, Q. Ligilactobacillus salivarius CCFM 1266 modulates gut microbiota and GPR109a-mediated immune suppression to attenuate immune checkpoint blockade-induced colitis. Food Funct. 2023, 14, 10549–10563. [Google Scholar] [CrossRef]
- Failing, J.J.; Dudek, O.A.; Marin Acevedo, J.A.; Chirila, R.M.; Dong, H.; Markovic, S.N.; Dronca, R.S. Biomarkers of hyperprogression and pseudoprogression with immune checkpoint inhibitor therapy. Future. Oncol. 2019, 15, 2645–2656. [Google Scholar] [CrossRef]
- Jiang, X.; Dudzinski, S.; Beckermann, K.E.; Young, K.; McKinley, E.; McIntyre, J.; Rathmell, J.C.; Xu, J.; Gore, J.C. MRI of tumor T cell infiltration in response to checkpoint inhibitor therapy. J. Immunother. Cancer 2020, 8, e000328. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, J.; Wu, X. Pseudoprogression and hyperprogression after checkpoint blockade. Int. Immunopharmacol. 2018, 58, 125–135. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Wang, M.M.; Coupland, S.E.; Aittokallio, T.; Figueiredo, C.R. Resistance to immune checkpoint therapies by tumour-induced T-cell desertification and exclusion: Key mechanisms, prognostication and new therapeutic opportunities. Br. J. Cancer 2023, 129, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, A.; Yada, M.; Miyazaki, Y.; Tanaka, K.; Kurosaka, K.; Ohishi, Y.; Masumoto, A.; Motomura, K. Tumor-infiltrating CD8(+) T cells as a biomarker for chemotherapy efficacy in unresectable hepatocellular carcinoma. Oncol. Lett. 2023, 25, 259. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell. 2018, 33, 547–562. [Google Scholar]
- Aoyama, S.; Nakagawa, R.; Nemoto, S.; Perez-Villarroel, P.; Mule, J.J.; Mailloux, A.W. Checkpoint blockade accelerates a novel switch from an NKT-driven TNFalpha response toward a T cell driven IFN-gamma response within the tumor microenvironment. J. Immunother. Cancer 2021, 9, e002269. [Google Scholar] [CrossRef]
- Ding, G.; Shen, T.; Yan, C.; Zhang, M.; Wu, Z.; Cao, L. IFN-gamma down-regulates the PD-1 expression and assist nivolumab in PD-1-blockade effect on CD8+ T-lymphocytes in pancreatic cancer. BMC. Cancer 2019, 19, 1053. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Sztefko, K.; Li, P.; Ballatori, N.; Chey, W.Y. CCK-receptor antagonists proglumide and loxiglumide stimulate bile flow and biliary glutathione excretion. Dig. Dis. Sci. 1994, 39, 1974–1980. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Cholecystokinin-From Local Gut Hormone to Ubiquitous Messenger. Front. Endocrinol. 2017, 8, 47. [Google Scholar] [CrossRef]
- Thor, P.; Laskiewicz, J.; Konturek, P.; Konturek, S.J. Cholecystokinin in the regulation of intestinal motility and pancreatic secretion in dogs. Am. J. Physiol. 1988, 255, G498–G504. [Google Scholar] [CrossRef] [PubMed]
- Miederer, S.E.; Lindstaedt, H.; Kutz, K.; Mayershofer, R. Efficient treatment of gastric ulcer with proglumide (Milid) in outpatients (double blind trial). Acta Hepatogastroenterol. 1979, 26, 314–318. [Google Scholar]
- Sullivan, R.J.; Weber, J.S. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 2022, 21, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Spiers, L.; Coupe, N.; Payne, M. Toxicities associated with checkpoint inhibitors-an overview. Rheumatology 2019, 58, vii7–vii16. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.P.; Postow, M.A.; Faleck, D.M. Efficacy of Infliximab Dose Escalation in Patients with Refractory Immunotherapy-Related Colitis: A Case Series. Oncologist 2022, 27, e350–e352. [Google Scholar] [CrossRef]
- Sanjeevaiah, A.; Kerr, T.; Beg, M.S. Approach and management of checkpoint inhibitor-related immune hepatitis. J. Gastrointest. Oncol. 2018, 9, 220–224. [Google Scholar] [CrossRef]
- Kramer, S.; van Hee, K.; Blokzijl, H.; van der Heide, F.; Visschedijk, M.C. Immune Checkpoint Inhibitor-related Pancreatitis: A Case Series, Review of the Literature and an Expert Opinion. J. Immunother. 2023, 46, 271–275. [Google Scholar] [CrossRef]
- Rabiee, A.; Gay, M.D.; Shivapurkar, N.; Cao, H.; Nadella, S.; Smith, C.I.; Lewis, J.H.; Bansal, S.; Cheema, A.; Kwagyan, J.; et al. Safety and Dosing Study of a Cholecystokinin Receptor Antagonist in Non-alcoholic Steatohepatitis. Clin. Pharmacol. Ther. 2022, 112, 1271–1279. [Google Scholar] [CrossRef]
- Ciofoaia, V.; Chen, W.; Tarek, B.; Gay, M.; Shivapurkar, N.; Smith, J. The Role of a Cholecystokinin Receptor Antagonist in the Management of Chronic Pancreatitis: A Phase 1 Trial. Pharmaceutics 2024, 16, 611. [Google Scholar] [CrossRef]
- Hsu, C.C.; Bansal, S.; Cao, H.; Smith, C.I.; He, A.R.; Gay, M.D.; Li, Y.; Cheema, A.; Smith, J.P. Safety and Pharmacokinetic Assessment of Oral Proglumide in Those with Hepatic Impairment. Pharmaceutics. 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Azzariti, A.; Galetta, D.; Nardulli, P.; Leonetti, F.; Silvestris, N. Strategies to Improve Cancer Immune Checkpoint Inhibitors Efficacy, Other Than Abscopal Effect: A Systematic Review. Cancers 2019, 11, 539. [Google Scholar] [CrossRef] [PubMed]


| Clinical Trial | Registration Number | Outcome | Publication |
|---|---|---|---|
| Metabolic dysfunction-associated steatohepatitis, MASH | NCT04152473 | Decreased transaminases, less fibrosis by FibroScan | [65] |
| Cirrhosis pharmacokinetic and safety study | NCT04814602 | Renal excretion, no changes in liver function | [67] |
| Chronic pancreatitis | NCT05551858 | Decreased pain, C-RP, lipase | [66] |
| Pancreatic cancer | NCT05827055 | Safe with chemotherapy, decreased fibrosis and altered immune cell signature in tumor | (AACR abstract, Boston September 2025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doneparthi, P.S.; Cao, H.; Chen, W.; Dou, W.; Fang, H.-B.; Smith, J.P. Mechanistic Insights into Proglumide’s Role in Immune Cell Efficacy and Response to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma. Cancers 2025, 17, 2998. https://doi.org/10.3390/cancers17182998
Doneparthi PS, Cao H, Chen W, Dou W, Fang H-B, Smith JP. Mechanistic Insights into Proglumide’s Role in Immune Cell Efficacy and Response to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma. Cancers. 2025; 17(18):2998. https://doi.org/10.3390/cancers17182998
Chicago/Turabian StyleDoneparthi, Priyanka S., Hong Cao, Wenqiang Chen, Wenyu Dou, Hong-Bin Fang, and Jill. P. Smith. 2025. "Mechanistic Insights into Proglumide’s Role in Immune Cell Efficacy and Response to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma" Cancers 17, no. 18: 2998. https://doi.org/10.3390/cancers17182998
APA StyleDoneparthi, P. S., Cao, H., Chen, W., Dou, W., Fang, H.-B., & Smith, J. P. (2025). Mechanistic Insights into Proglumide’s Role in Immune Cell Efficacy and Response to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma. Cancers, 17(18), 2998. https://doi.org/10.3390/cancers17182998








