Exposure to Occupational Carcinogens and Non-Oncogene Addicted Phenotype in Lung Cancer: Results from a Real-Life Observational Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Anatomopathological Data: Classification of Non-Oncogene-Addicted (nOA) Cases
2.3. Occupational Exposure Evaluation
2.4. Smoking Exposure Assessment
2.5. Statistical Analysis
3. Results
3.1. Smoking Exposure Assessment
3.2. Age at Diagnosis and Age at First Employment
3.3. Tumor Histological Types and Gene Mutations
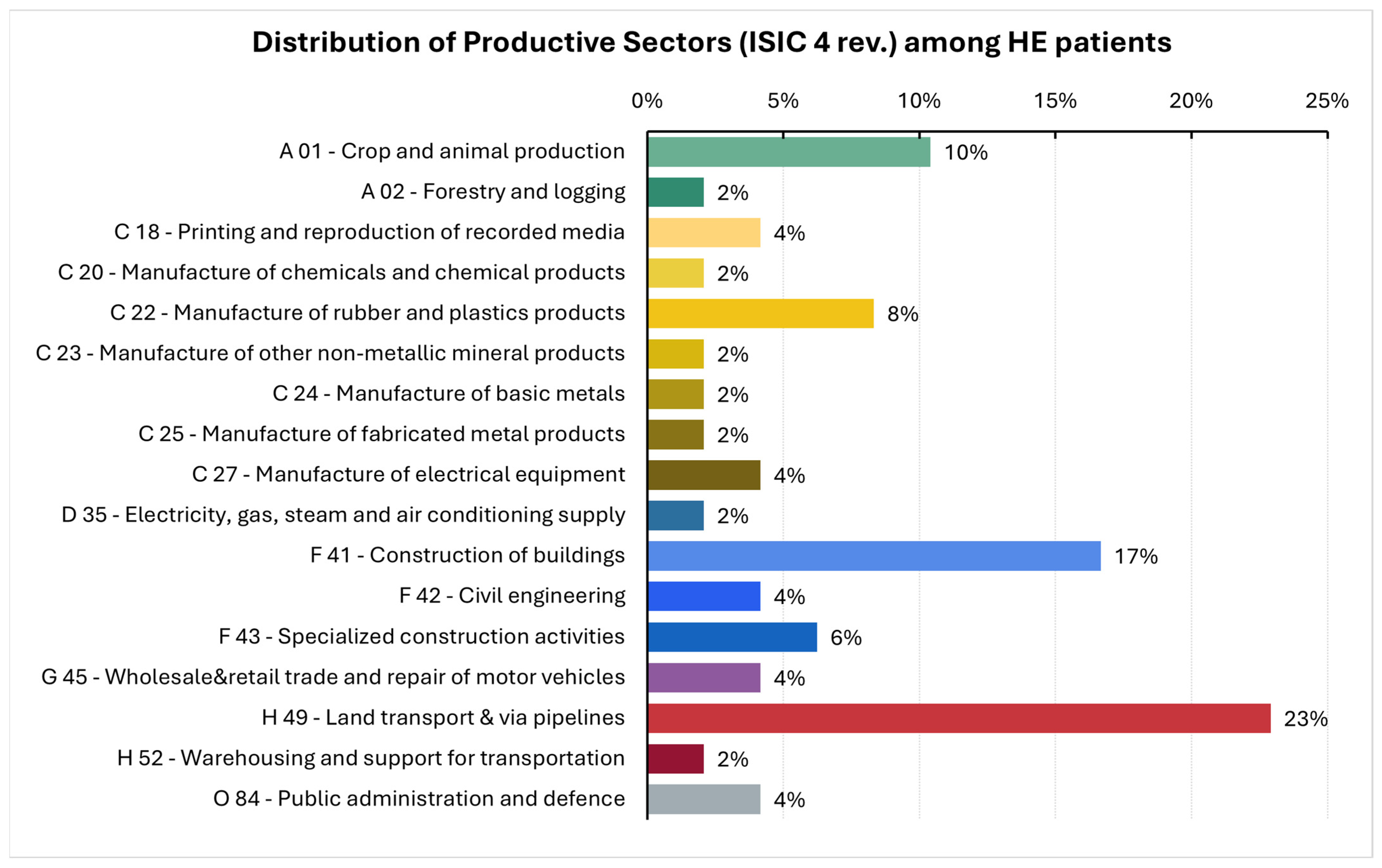
3.4. Occupational Exposures
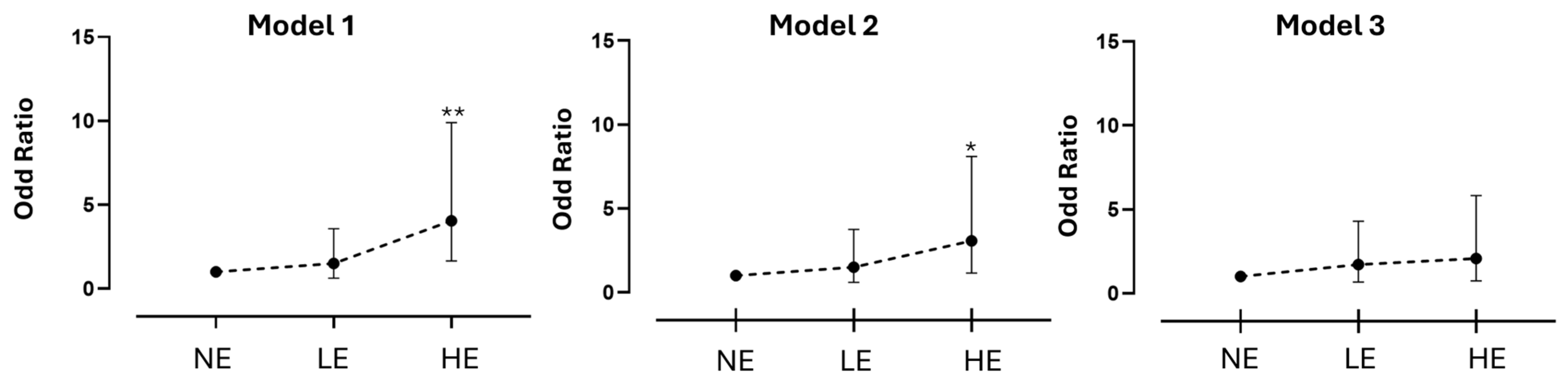
3.5. Occupational Exposure and Oncogene-Addiction State
4. Discussion
4.1. Actionable Gene Mutations, Histotypes, and Occupational Exposure
4.2. Occupational Exposures
4.3. Smoking Habits
4.4. Timing of Exposure
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC | Lung cancer |
| SCLC | Small-cell lung cancer |
| NSCLC | Non-small-cell lung cancer |
| NGS | Next-generation sequencing |
| ISIC | International Standard Industrial Classification of All Economic Activities |
| INAIL | Istituto Nazionale per l’Assicurazione per gli Infortuni sul Lavoro |
| IARC | International Agency for Research on Cancer |
| EGFR | Epidermal Growth Factor Receptor |
| ALK | Anaplastic Lymphoma Kinase |
| ROS1 | ROS proto-oncogene 1, receptor tyrosine kinase |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| KRAS | Kirsten rat sarcoma viral oncogene |
| OA | Oncogene-addicted |
| nOA | Non-oncogene-addicted |
References
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and Heritable Factors in the Causation of Cancer--Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Veglia, F.; Vineis, P.; Overvad, K.; Boeing, H.; Bergmann, M.M.; Trichopoulou, A.; Trichopoulos, D.; Palli, D.; Krogh, V.; Tumino, R.; et al. Occupational Exposures, Environmental Tobacco Smoke, and Lung Cancer. Epidemiology 2007, 18, 769–775. [Google Scholar] [CrossRef]
- Colditz, G.; DeJong, D.; Hunter, D.; Trichopoulos, D.; Willett, W. Harvard Report on Cancer Prevention: Volume 1: Causes of Human Cancer Harvard Center for Cancer Prevention Harvard School of Public Health. Cancer Causes Control 1996, 7, S3–S4. [Google Scholar] [CrossRef]
- GBD 2016 Occupational Carcinogens Collaborators. Global and Regional Burden of Cancer in 2016 Arising from Occupational Exposure to Selected Carcinogens: A Systematic Analysis for the Global Burden of Disease Study 2016. Occup. Environ. Med. 2020, 77, 151–159. [Google Scholar] [CrossRef]
- Curti, S.; Sauni, R.; Spreeuwers, D.; De Schryver, A.; Valenty, M.; Rivière, S.; Mattioli, S. Interventions to Increase the Reporting of Occupational Diseases by Physicians. Cochrane Database Syst. Rev. 2015, 2015, CD010305. [Google Scholar] [CrossRef]
- Morelle, I.; Berghmans, T.; CsToth, I.; Sculier, J.-P.; Meert, A.-P. Apport du repérage des expositions professionnelles en oncologie thoracique: Une expérience belge. Rev. Mal. Respir. 2014, 31, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Orriols, R.; Isidro, I.; Abu-Shams, K.; Costa, R.; Boldu, J.; Rego, G.; Zock, J.; other Members of the Enfermedades Respiratorias Ocupacionales y Medioambientales (EROM) Group. Reported Occupational Respiratory Diseases in Three Spanish Regions. Am. J. Ind. Med. 2010, 53, 922–930. [Google Scholar] [CrossRef]
- Pérol, O.; Lepage, N.; Noelle, H.; Lebailly, P.; De Labrusse, B.; Clin, B.; Boulanger, M.; Praud, D.; Fournié, F.; Galvaing, G.; et al. A Multicenter Study to Assess a Systematic Screening of Occupational Exposures in Lung Cancer Patients. Int. J. Environ. Res. Public Health 2023, 20, 5068. [Google Scholar] [CrossRef]
- Assennato, G.; De Giampaulis, C. When Occupational Cancer Recognition Falters. Med. Lav. 2025, 116, 16997. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Friedlaender, A.; Perol, M.; Banna, G.L.; Parikh, K.; Addeo, A. Oncogenic Alterations in Advanced NSCLC: A Molecular Super-Highway. Biomark. Res. 2024, 12, 24. [Google Scholar] [CrossRef]
- O’Leary, C.; Gasper, H.; Sahin, K.B.; Tang, M.; Kulasinghe, A.; Adams, M.N.; Richard, D.J.; O’Byrne, K.J. Epidermal Growth Factor Receptor (EGFR)-Mutated Non-Small-Cell Lung Cancer (NSCLC). Pharmaceuticals 2020, 13, 273. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the Transforming EML4–ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, A. Trk Receptor Tyrosine Kinases: A Bridge between Cancer and Neural Development. Cancer Lett. 2001, 169, 107–114. [Google Scholar] [CrossRef]
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ostrem, J.; Pellini, B.; Imbody, D.; Stern, Y.; Solanki, H.S.; Haura, E.B.; Villaruz, L.C. Overcoming KRAS-Mutant Lung Cancer. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology (ASCO): Alexandria, VA, USA, 2022; pp. 700–710. [Google Scholar] [CrossRef]
- Nadler, D.L.; Zurbenko, I.G. Developing a Weibull Model Extension to Estimate Cancer Latency. ISRN Epidemiol. 2013, 2013, 750857. [Google Scholar] [CrossRef]
- Nadler, D.L.; Zurbenko, I.G. Estimating Cancer Latency Times Using a Weibull Model. Adv. Epidemiol. 2014, 2014, 746769. [Google Scholar] [CrossRef]
- United Nations (UN). International Standard Industrial Classification of All Economic Activities (ISIC), Rev.4; Statistical Papers (Ser. M); United Nations: New York, NY, USA, 2008; ISBN 978-92-1-161518-0. [Google Scholar]
- Massari, S. Renaloccam: Il Sistema di Monitoraggio delle Neoplasie a Bassa Frazione Eziologica: Manuale Operativo; Inail: Roma, Italy, 2021; ISBN 978-88-7484-700-6. [Google Scholar]
- Samet, J.M.; Chiu, W.A.; Cogliano, V.; Jinot, J.; Kriebel, D.; Lunn, R.M.; Beland, F.A.; Bero, L.; Browne, P.; Fritschi, L.; et al. The IARC Monographs: Updated Procedures for Modern and Transparent Evidence Synthesis in Cancer Hazard Identification. JNCI J. Natl. Cancer Inst. 2020, 112, 30–37. [Google Scholar] [CrossRef]
- Italian Republic Law No. 257 of 27 March 1992: Provisions on the Cessation of the Use of Asbestos 1992. Available online: https://uk.practicallaw.thomsonreuters.com/Glossary/UKPracticalLaw/If6efc3cf42e311ec9f24ec7b211d8087?transitionType=Default&contextData=(sc.Default)&firstPage=true&comp=pluk (accessed on 25 June 2025).
- Olsson, A.; Bouaoun, L.; Schüz, J.; Vermeulen, R.; Behrens, T.; Ge, C.; Kromhout, H.; Siemiatycki, J.; Gustavsson, P.; Boffetta, P.; et al. Lung Cancer Risks Associated with Occupational Exposure to Pairs of Five Lung Carcinogens: Results from a Pooled Analysis of Case-Control Studies (SYNERGY). Environ. Health Perspect. 2024, 132, 017005. [Google Scholar] [CrossRef]
- Saw, S.P.L.; Le, X.; Hendriks, L.E.L.; Remon, J. New Treatment Options for Patients With Oncogene-Addicted Non–Small Cell Lung Cancer Focusing on EGFR-Mutant Tumors. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432516. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597. [Google Scholar] [CrossRef]
- De Matteis, S.; Consonni, D.; Lubin, J.H.; Tucker, M.; Peters, S.; Vermeulen, R.C.; Kromhout, H.; Bertazzi, P.A.; Caporaso, N.E.; Pesatori, A.C.; et al. Impact of Occupational Carcinogens on Lung Cancer Risk in a General Population. Int. J. Epidemiol. 2012, 41, 711–721. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, C. Lung Cancer in Never Smokers\u2014the East Asian Experience. Transl. Lung Cancer Res. 2018, 7, 450–463. [Google Scholar] [CrossRef]
- Ha, S.Y.; Choi, S.-J.; Cho, J.H.; Choi, H.J.; Lee, J.; Jung, K.; Irwin, D.; Liu, X.; Lira, M.E.; Mao, M.; et al. Lung Cancer in Never-Smoker Asian Females Is Driven by Oncogenic Mutations, Most Often Involving EGFR. Oncotarget 2015, 6, 5465–5474. [Google Scholar] [CrossRef] [PubMed]
- Bogni, M.; Cervino, D.; Rossi, M.R.; Galli, P. A 7-Year Active Surveillance Experience for Occupational Lung Cancer in Bologna, Italy (2017–2023). Med. Lav. 2025, 116, 16173. [Google Scholar] [CrossRef]
- Thumerel, M.; Carles, C.; Begueret, H.; Thomas, Q.; Jougon, J.; Audoin, C.; Bernaudin, J.-F.; Brochard, P.; Belaroussi, Y. Does Occupational Exposure Affect the Surgical Management of Patients with Non-Small Cell Lung Cancer? A Single-Center Retrospective Experience. Respir. Med. Res. 2025, 88, 101183. [Google Scholar] [CrossRef] [PubMed]
- Oddone, E.; Bollon, J.; Nava, C.R.; Consonni, D.; Marinaccio, A.; Magnani, C.; Gasparrini, A.; Barone-Adesi, F. Effect of Asbestos Consumption on Malignant Pleural Mesothelioma in Italy: Forecasts of Mortality up to 2040. Cancers 2021, 13, 3338. [Google Scholar] [CrossRef]
- International Ban Asbestos Secretariat (IBAS) Current Asbestos Bans. Available online: https://www.ibasecretariat.org/alpha_ban_list.php?utm_source=chatgpt.com (accessed on 25 August 2025).
- Vineis, P.; Airoldi, L.; Veglia, F.; Olgiati, L.; Pastorelli, R.; Autrup, H.; Dunning, A.; Garte, S.; Gormally, E.; Hainaut, P.; et al. Environmental Tobacco Smoke and Risk of Respiratory Cancer and Chronic Obstructive Pulmonary Disease in Former Smokers and Never Smokers in the EPIC Prospective Study. BMJ 2005, 330, 277. [Google Scholar] [CrossRef] [PubMed]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A Review of Human Carcinogens—Part E: Tobacco, Areca Nut, Alcohol, Coal Smoke, and Salted Fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Bassig, B.A.; Lubin, J.; Graubard, B.; Blair, A.; Vermeulen, R.; Attfield, M.; Appel, N.; Rothman, N.; Stewart, P.; et al. The Diesel Exhaust in Miners Study (DEMS) II: Temporal Factors Related to Diesel Exhaust Exposure and Lung Cancer Mortality in the Nested Case–Control Study. Environ. Health Perspect. 2023, 131, 087002. [Google Scholar] [CrossRef]
- Hauptmann, M.; Lubin, J.H.; Rosenberg, P.; Wellmann, J.; Kreienbrock, L. The Use of Sliding Time Windows for the Exploratory Analysis of Temporal Effects of Smoking Histories on Lung Cancer Risk. Stat. Med. 2000, 19, 2185–2194. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Gibson, C.J. Turning Gray: The Natural History of Lung Cancer Over Time. J. Thorac. Oncol. 2008, 3, 781–792. [Google Scholar] [CrossRef]
- Schabel, F.M. Concepts for Systemic Treatment of Micrometastases. Cancer 1975, 35, 15–24. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Wu, Y.; Wei, C.; Fang, Y. Global, Regional and National Burden of Lung Cancer Attributable to Occupational Carcinogens, 1990–2019: A Study of Trends, Inequalities and Predictions Based on GBD 2019. Cancer Epidemiol. 2025, 94, 102737. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Budtz-Jørgensen, E.; Keiding, N.; Weihe, P. Underestimation of Risk Due to Exposure Misclassification. Int. J. Occup. Med. Environ. Health 2004, 17, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Blair, A.; Stewart, P.; Lubin, J.H.; Forastiere, F. Methodological Issues Regarding Confounding and Exposure Misclassification in Epidemiological Studies of Occupational Exposures. Am. J. Ind. Med. 2007, 50, 199–207. [Google Scholar] [CrossRef]
| ICS Maugeri | CC-HRH | All | p | |
|---|---|---|---|---|
| Sex | ||||
| Male | 58 (58.0%) | 52 (52.5%) | 110 (55.3%) | |
| Female | 42 (42.0%) | 47 (47.5%) | 89 (44.7%) | 0.437 |
| Age at enrollment (years) | ||||
| 40–49 | 3 (3.0%) | 4 (4.0%) | 7 (3.5%) | |
| 50–59 | 15 (15.0%) | 19 (19.2%) | 34 (17.1%) | |
| 60–69 | 29 (29.0%) | 37 (37.4%) | 66 (33.2%) | |
| 70–79 | 40 (40.0%) | 30 (30.3%) | 70 (35.2%) | |
| 80+ | 13 (13.0%) | 9 (9.1%) | 22 (11.1%) | 0.443 |
| Smoking habits | ||||
| Never | 12 (12.0%) | 7 (7.1%) | 19 (9.5%) | |
| Former at diagnosis | ||||
| 0–9 years before diagnosis | 19 (19.0%) | 11 (11.1%) | 30 (15.1%) | |
| 10–19 years before diagnosis | 11 (11.0%) | 13 (13.1%) | 24 (12.1%) | |
| 20+ years before diagnosis | 24 (24.0%) | 17 (17.2%) | 41 (20.6%) | |
| Current at diagnosis | 34 (34.0%) | 51 (51.5%) | 85 (42.7%) | 0.084 |
| Pack-years (median, IQR) | 40.0 (20.8–60.8) | 46.0 (27.0–58.0) | 42.8 (22.0–60.0) | 0.508 |
| Occupational exposure a | ||||
| Not exposed | 49 (49.0%) | 69 (69.7%) | 118 (59.3%) | |
| Low exposure | 20 (20.0%) | 14 (14.1%) | 34 (17.1%) | |
| High exposure | 31 (31.0%) | 16 (16.2%) | 47 (23.6%) | 0.010 |
| ICS Maugeri | CC-HRH | All | p | |
|---|---|---|---|---|
| Age at diagnosis (years) | ||||
| 40–49 | 1 (1.0%) | 11 (11.1%) | 12 (6.0%) | |
| 50–59 | 22 (22.0%) | 16 (16.2%) | 38 (19.1%) | |
| 60–69 | 31 (31.0%) | 44 (44.4%) | 75 (37.7%) | |
| 70–79 | 38 (38.0%) | 23 (23.2%) | 61 (30.7%) | |
| 80+ | 8 (8.0%) | 5 (5.1%) | 13 (6.5%) | 0.003 |
| Histological type | ||||
| Adenocarcinoma | 78 (78.0%) | 72 (73.4%) | 150 (75.8%) | |
| Squamous | 13 (13.0%) | 13 (13.3%) | 26 (13.1%) | |
| Small cell | 9 (9.0%) | 10 (10.2%) | 19 (9.6%) | |
| Others | 0 - | 3 (3.1%) | 3 (1.5%) | 0.351 |
| Gene mutations a | ||||
| EGFR | 5 (6.4%) | 9 (12.5%) | 14 (9.3%) | 0.200 |
| ALK | 5 (5.4%) | 1 (1.4%) | 6 (4.0%) | 0.117 |
| ROS1 | 2 (2.6%) | 1 (1.4%) | 3 (2.0%) | 0.608 |
| BRAF | 4 (5.1%) | 5 (6.9%) | 9 (6.0%) | 0.640 |
| KRAS | 8 (10.3%) | 27 (37.5%) | 35 (23.3%) | <0.001 |
| RET | 1 (1.3%) | 0 - | 1 (0.7%) | 0.335 |
| MET | 0 - | 3 (4.2%) | 3 (2.0%) | 0.069 |
| HER2 | 0 - | 3 (4.2%) | 3 (2.0%) | 0.359 |
| Center | n | Model 1 | p | Model 2 | p | Model 3 | p |
|---|---|---|---|---|---|---|---|
| OR (95%IC) | OR (95%IC) | OR (95%IC) | |||||
| ICS Maugeri | |||||||
| Never Exposed | 37 | 1 (ref.) | - | 1 (ref.) | - | 1 (ref.) | - |
| Low Exposure | 17 | 1.46 (0.42–5.03) | 0.548 | 1.25 (0.31–5.08) | 0.751 | 1.79 (0.48–6.67) | 0.383 |
| High Exposure | 24 | 6.70 (1.36–32.92) | 0.019 | 5.94 (1.05–33.51) | 0.044 | 2.97 (0.45–19.29) | 0.255 |
| t: 0.02 | t: 0.04 | t: 0.26 | |||||
| CC-HRH | |||||||
| Never Exposed | 51 | 1 (ref.) | - | 1 (ref.) | - | 1 (ref.) | - |
| Low Exposed | 10 | 0.85 (0.20–3.73) | 0.873 | 0.69 (0.15–3.19) | 0.630 | 0.80 (0.17–3.77) | 0.781 |
| High Exposed | 11 | 1.67 (0.44–6.25) | 0.449 | 1.08 (0.26–4.51) | 0.914 | 1.08 (0.25–4.63) | 0.914 |
| t: 0.45 | t: 0.91 | t: 0.91 | |||||
| Total | |||||||
| Never Exposed | 88 | 1 (ref.) | - | 1 (ref.) | - | 1 (ref.) | - |
| Low Exposed | 27 | 1.50 (0.63–3.57) | 0.360 | 1.50 (0.60–3.75) | 0.383 | 1.71 (0.68–4.30) | 0.251 |
| High Exposed | 35 | 4.05 (1.66–9.90) | 0.002 | 3.07 (1.16–8.11) | 0.023 | 2.08 (0.74–5.82) | 0.162 |
| t: <0.01 | t: 0.02 | t: 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oddone, E.; D’Amato, L.; Pernetti, R.; Madeo, D.; Toschi, L.; Farinatti, S.; Riva, G.; Spina, L.; Ferrante, L.; Conde, C.; et al. Exposure to Occupational Carcinogens and Non-Oncogene Addicted Phenotype in Lung Cancer: Results from a Real-Life Observational Study. Cancers 2025, 17, 2997. https://doi.org/10.3390/cancers17182997
Oddone E, D’Amato L, Pernetti R, Madeo D, Toschi L, Farinatti S, Riva G, Spina L, Ferrante L, Conde C, et al. Exposure to Occupational Carcinogens and Non-Oncogene Addicted Phenotype in Lung Cancer: Results from a Real-Life Observational Study. Cancers. 2025; 17(18):2997. https://doi.org/10.3390/cancers17182997
Chicago/Turabian StyleOddone, Enrico, Luca D’Amato, Roberta Pernetti, Domenico Madeo, Luca Toschi, Sara Farinatti, Giulia Riva, Lucrezia Spina, Luigia Ferrante, Catharina Conde, and et al. 2025. "Exposure to Occupational Carcinogens and Non-Oncogene Addicted Phenotype in Lung Cancer: Results from a Real-Life Observational Study" Cancers 17, no. 18: 2997. https://doi.org/10.3390/cancers17182997
APA StyleOddone, E., D’Amato, L., Pernetti, R., Madeo, D., Toschi, L., Farinatti, S., Riva, G., Spina, L., Ferrante, L., Conde, C., Locati, L. D., Sottotetti, F., & Barbic, F. (2025). Exposure to Occupational Carcinogens and Non-Oncogene Addicted Phenotype in Lung Cancer: Results from a Real-Life Observational Study. Cancers, 17(18), 2997. https://doi.org/10.3390/cancers17182997









