Characterisation of mAb104 Antibody–Drug Conjugates Targeting a Tumour-Selective HER2 Epitope
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Antibody-Drug Conjugates
2.2. Cell Lines
2.3. Flow Cytometry (FACS)
2.4. Cell Proliferation Assay
2.5. In Vivo Studies
2.6. Dose-Finding Studies for mAb104 Conjugated with Different Payloads
2.7. Therapy Studies for mAb104 Conjugated with Different Payloads
2.8. Immunohistochemistry
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
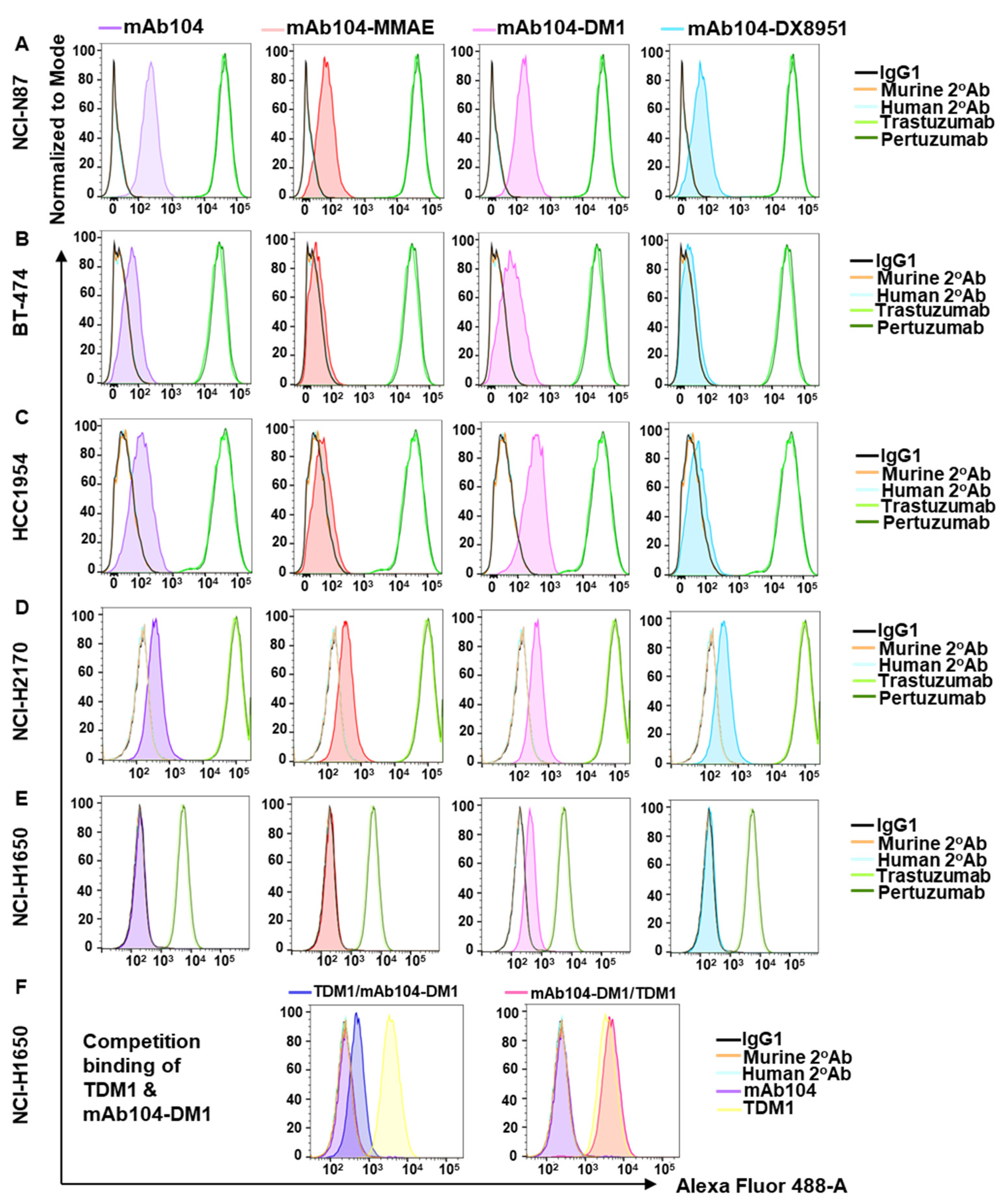
3.1. Generation of mAb104-ADCs
3.2. Mab104-ADC Binding
3.3. Effect of mAb104-ADCs on Proliferation in Vitro
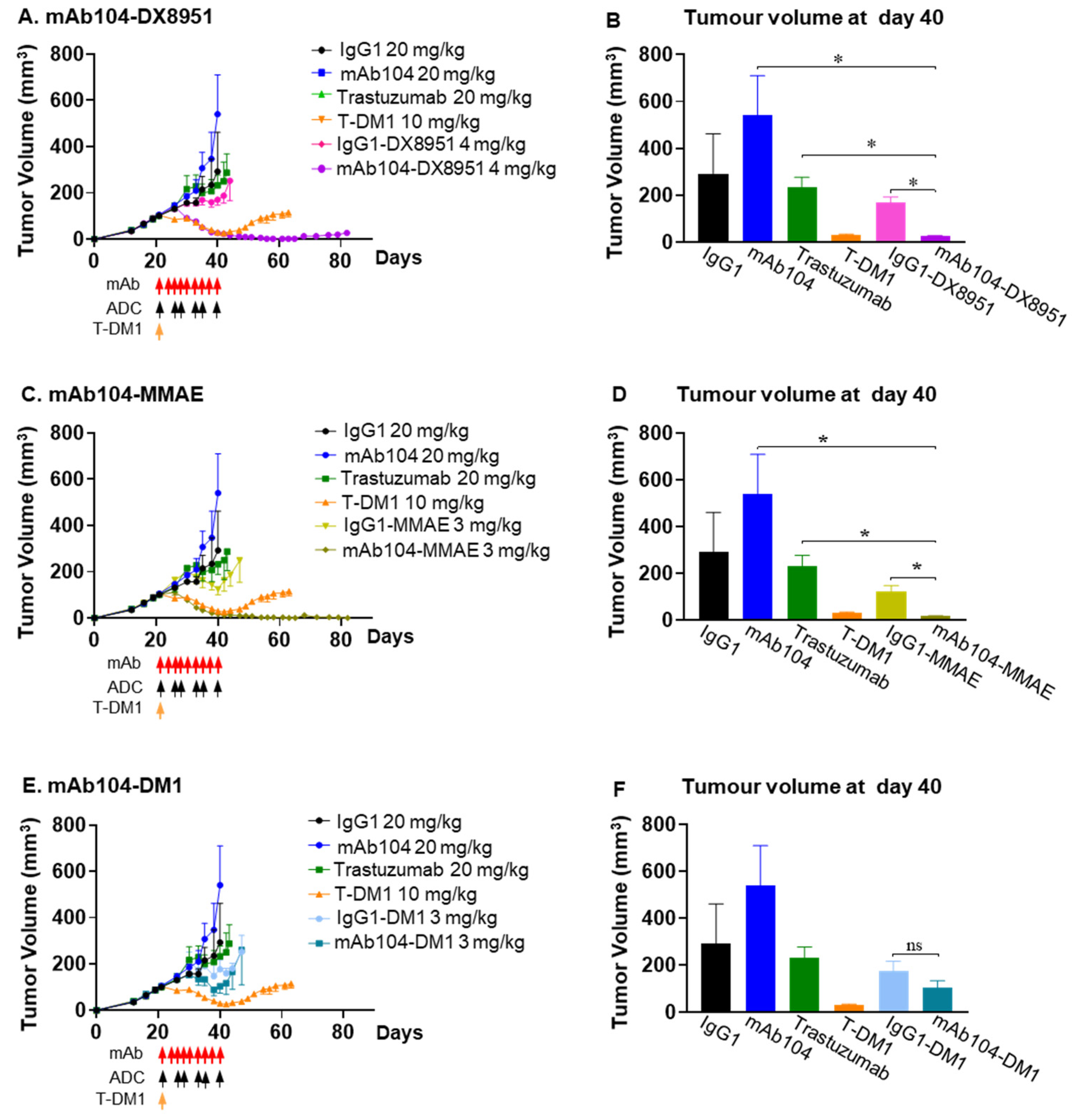
3.4. Effect of mAb104-ADCs In Vivo
3.4.1. Dose Finding Study
3.4.2. Efficacy of mAb104-ADCs in a HER2-Positive (IHC 3+) Trastuzumab Resistant Breast Cancer Xenograft Model
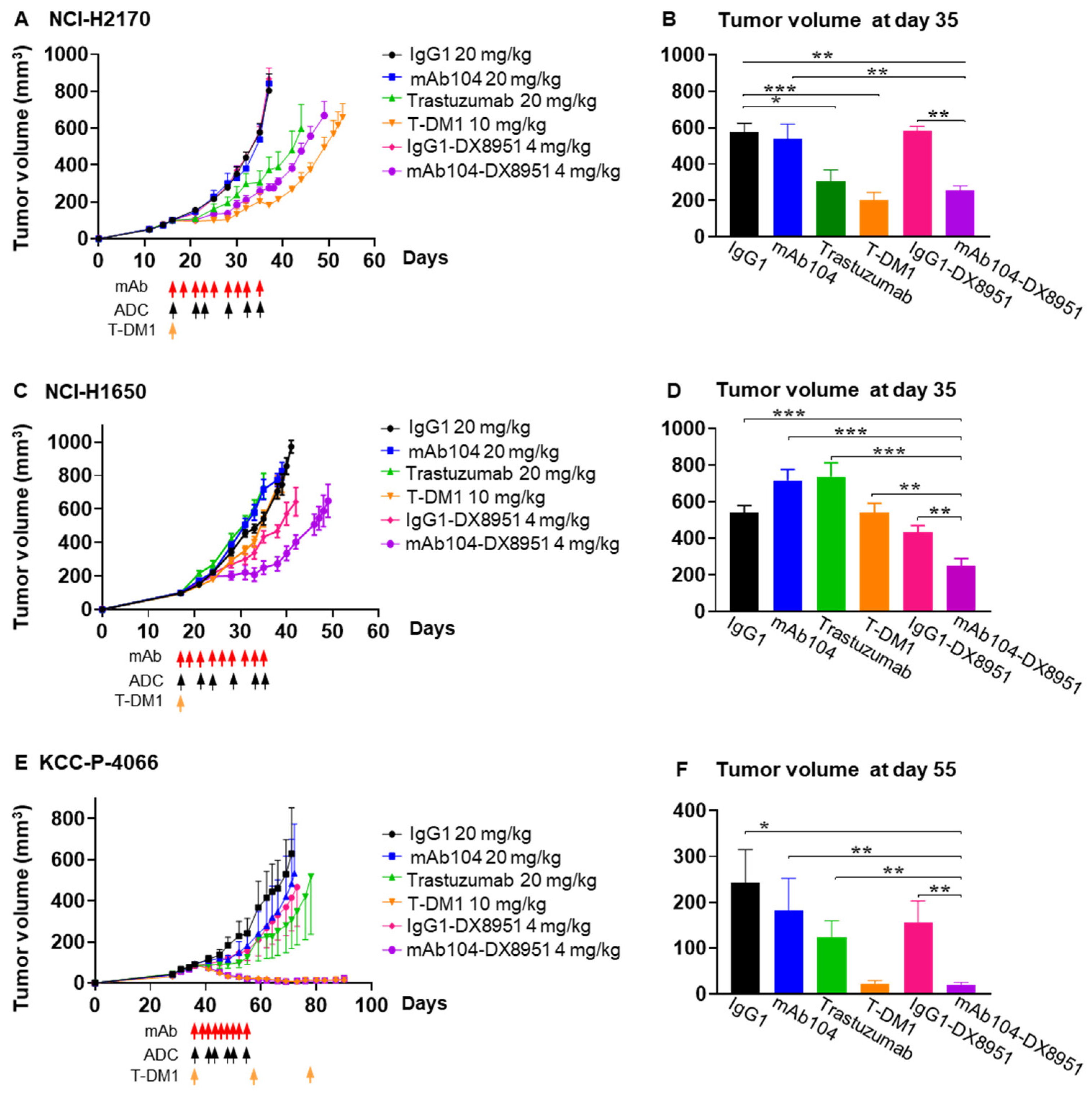
3.4.3. Efficacy of mAb104-DX8951 ADC Therapy in HER-Positive Squamous Cell Lung Carcinoma
3.4.4. Efficacy of mAb104-DX8951 ADC Therapy in EGFR Mutant, HER2 Low Adenocarcinoma Xenograft
3.4.5. Efficacy of mAb104-DX8951 ADC Therapy in HER2-Positive (IHC 2/3+), ER-Positive Breast Cancer PDX
4. Pharmacodynamic Studies
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-drug conjugates in lung cancer: Dawn of a new era? Npj Precis. Oncol. 2023, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Yang, X.; Tai, H.; Zhong, X.; Luo, T.; Zheng, H. HER2-targeted therapies in cancer: A systematic review. Biomark. Res. 2024, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: Primary results from the DESTINY-PanTumor02 phase II trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Galogre, M.; Rodin, D.; Pyatnitskiy, M.; Mackelprang, M.; Koman, I. A review of HER2 overexpression and somatic mutations in cancers. Crit. Rev. Oncol. Hematol. 2023, 186, 103997. [Google Scholar] [CrossRef]
- Parakh, S.; Huynh, N.; Cao, D.D.; Rigopoulos, A.; Gloria, B.; Burvenich, I.J.; Murone, C.; Wichmann, C.W.; Guo, N.Y.; Senko, C.; et al. Characterization of mAb104, a mAb Targeting a Conformationally Exposed, Tumor-Specific Epitope of HER2. Mol. Cancer Ther. 2025, 24, 1442–1452. [Google Scholar] [CrossRef]
- Arimori, T.; Mihara, E.; Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Takagi, J.; Kato, Y. Locally misfolded HER2 expressed on cancer cells is a promising target for development of cancer-specific antibodies. Structure 2024, 32, 536–549.e5. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Suzuki, H.; Ohishi, T.; Nakamura, T.; Tanaka, T.; Kato, Y. A cancer-specific monoclonal antibody against HER2 exerts antitumor activities in human breast cancer xenograft models. International. J. Mol. Sci. 2024, 25, 1941. [Google Scholar] [CrossRef]
- Liu, Z.; Panousis, C.; Smyth, F.E.; Murphy, R.; Wirth, V.; Cartwright, G.; Johns, T.G.; Scott, A.M. Generation of anti-idiotype antibodies for application in clinical immunotherapy laboratory analyses. Hybrid. Hybridomics 2003, 22, 219–228. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Barok, M.; Tanner, M.; Köninki, K.; Isola, J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011, 13, R46. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Ise, N.; Omi, K.; Nambara, D.; Higashiyama, S.; Goishi, K. Overexpressed HER2 in NSCLC is a possible therapeutic target of EGFR inhibitors. Anticancer Res. 2011, 31, 4155–4161. [Google Scholar] [PubMed]
- Paudyal, P.; Paudyal, B.; Hanaoka, H.; Oriuchi, N.; Iida, Y.; Yoshioka, H.; Tominaga, H.; Watanabe, S.; Watanabe, S.; Ishioka, N.S.; et al. Imaging and biodistribution of Her2/neu expression in non-small cell lung cancer xenografts with 64Cu-labeled trastuzumab PET. Cancer Sci. 2010, 101, 1045–1050. [Google Scholar] [CrossRef]
- Sos, M.L.; Koker, M.; Weir, B.A.; Heynck, S.; Rabinovsky, R.; Zander, T.; Seeger, J.M.; Weiss, J.; Fischer, F.; Frommolt, P.; et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009, 69, 3256–3261. [Google Scholar] [CrossRef]
- DeFazio-Eli, L.; Strommen, K.; Dao-Pick, T.; Parry, G.; Goodman, L.; Winslow, J. Quantitative assays for the measurement of HER1-HER2 heterodimerization and phosphorylation in cell lines and breast tumors: Applications for diagnostics and targeted drug mechanism of action. Breast Cancer Res. 2011, 13, R44. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, Y.; Zhou, L.; Du, J.; Wang, J.; Liu, S.H.; Cao, Y.; Li, Y.; Yang, W.; et al. Resistance mechanisms and prospects of trastuzumab. Front. Oncol. 2024, 14, 1389390. [Google Scholar] [CrossRef]
- Hotta, K.; Aoe, K.; Kozuki, T.; Ohashi, K.; Ninomiya, K.; Ichihara, E.; Kubo, T.; Ninomiya, T.; Chikamori, K.; Harada, D.; et al. A phase II study of trastuzumab emtansine in HER2-positive non–small cell lung cancer. J. Thorac. Oncol. 2018, 13, 273–279. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab deruxtecan in HER2-mutant non–small-cell lung cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Rodríguez, L.P.-A.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef]
- Italiano, A.; Besse, B.; Borghaei, H.; Popat, S.; Palacios, G.A.; Goncalves, A.; Meurer, M.; Mazieres, J.; Chouaid, C.; García, J.S.; et al. 118MO Trastuzumab deruxtecan (T-DXd) and pembrolizumab in immuno-oncology (IO)-naive HER2-expressing or HER2-mutant non-small cell lung cancer (NSCLC): Interim analysis of a phase Ib study. Immuno-Oncol. Technol. 2024, 24, 100747. [Google Scholar] [CrossRef]
- Ascione, L.; Guidi, L.; Prakash, A.; Trapani, D.; LoRusso, P.; Lou, E.; Curigliano, G. Unlocking the Potential: Biomarkers of Response to Antibody-Drug Conjugates. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e431766. [Google Scholar] [CrossRef] [PubMed]
- de Haas, S.L.; Slamon, D.J.; Martin, M.; Press, M.F.; Lewis, G.D.; Lambertini, C.; Prat, A.; Lopez-Valverde, V.; Boulet, T.; Hurvitz, S.A. Tumor biomarkers and efficacy in patients treated with trastuzumab emtansine+ pertuzumab versus standard of care in HER2-positive early breast cancer: An open-label, phase III study (KRISTINE). Breast Cancer Res. 2023, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Pandiella, A.; Vargas-Castrillón, E.; Diaz-Rodriguez, E.; Iglesias-Hernangomez, T.; Cano, C.M.; Fernández-Cuesta, I.; Winkow, E.; Perelló, M.F. Trastuzumab deruxtecan in breast cancer. Crit. Rev. Oncol. Hematol. 2024, 198, 104355. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Yoshino, T.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Wainberg, Z.; Elez, E.; et al. Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat. Commun. 2023, 14, 3332. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, Y.; et al. Trastuzumab deruxtecan in anti–human epidermal growth factor receptor 2 treatment–naive patients with human epidermal growth factor receptor 2–low gastric or gastroesophageal junction adenocarcinoma: Exploratory cohort results in a phase II trial. J. Clin. Oncol. 2023, 41, 816–825. [Google Scholar] [CrossRef]
- Quaquarini, E.; Grillo, F.; Gervaso, L.; Arpa, G.; Fazio, N.; Vanoli, A.; Parente, P. Prognostic and predictive roles of HER2 status in non-breast and non-gastroesophageal carcinomas. Cancers 2024, 16, 3145. [Google Scholar] [CrossRef]
- Gan, H.K.; Burgess, A.W.; Clayton, A.H.; Scott, A.M. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012, 72, 2924–2930. [Google Scholar] [CrossRef]


| Table | Median Survival (Days) | p Value |
|---|---|---|
| IgG1 | 41 | 0.0008 |
| Mab104 | 40 | 0.0008 |
| Trastuzumab | 39.5 | 0.0007 |
| T-DM1 | 41.5 | 0.0006 |
| IgG1-DX8951 | 48 | 0.0223 |
| Mab104-DX8951 | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parakh, S.; Huynh, N.; Osellame, L.D.; Cao, D.D.; Rigopoulos, A.; Gloria, B.; Guo, N.Y.; Scott, F.E.; Liu, Z.; Gan, H.K.; et al. Characterisation of mAb104 Antibody–Drug Conjugates Targeting a Tumour-Selective HER2 Epitope. Cancers 2025, 17, 2995. https://doi.org/10.3390/cancers17182995
Parakh S, Huynh N, Osellame LD, Cao DD, Rigopoulos A, Gloria B, Guo NY, Scott FE, Liu Z, Gan HK, et al. Characterisation of mAb104 Antibody–Drug Conjugates Targeting a Tumour-Selective HER2 Epitope. Cancers. 2025; 17(18):2995. https://doi.org/10.3390/cancers17182995
Chicago/Turabian StyleParakh, Sagun, Nhi Huynh, Laura D. Osellame, Diana D. Cao, Angela Rigopoulos, Benjamin Gloria, Nancy Yanan Guo, Fiona E. Scott, Zhanqi Liu, Hui K. Gan, and et al. 2025. "Characterisation of mAb104 Antibody–Drug Conjugates Targeting a Tumour-Selective HER2 Epitope" Cancers 17, no. 18: 2995. https://doi.org/10.3390/cancers17182995
APA StyleParakh, S., Huynh, N., Osellame, L. D., Cao, D. D., Rigopoulos, A., Gloria, B., Guo, N. Y., Scott, F. E., Liu, Z., Gan, H. K., & Scott, A. M. (2025). Characterisation of mAb104 Antibody–Drug Conjugates Targeting a Tumour-Selective HER2 Epitope. Cancers, 17(18), 2995. https://doi.org/10.3390/cancers17182995







