The Imaging of Primary Fallopian Tube Carcinoma: A Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Clinical Features
4. Pathological Diagnosis and Characteristics of Primary Fallopian Tube Carcinoma
5. From Benign to Malignant: Imaging Strategies for Accurate Fallopian Tube Pathology
5.1. Transvaginal Ultrasonography
5.2. Magnetic Resonance Imaging
The O-RADS MRI Score and Its Implications for PFTC Diagnosis
5.3. PET/CT
6. The Role of Imaging in the Early Diagnosis of Primary Fallopian Tube Carcinoma
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PFTCs | Primary Fallopian Tube Carcinomas |
| EOC | Epithelial Ovarian Carcinoma |
| PPSC | Primary Peritoneal Serous Carcinoma |
| WHO | World Health Organization |
| FIGO | International Federation of Gynaecology and Obstetrics |
| HGSCs | high-grade serous carcinomas |
| FT | Fallopian Tube |
| STIC | Serous Tubal Intraepithelial Carcinoma |
| US | Ultrasonography |
| MRI | Magnetic Resonance Imaging |
| HSG | Hysterosalpingography |
| CT | Computed Tomography |
| IOTA | International Ovarian Tumor Analysis |
| O-RADS | Ovarian-Adnexal Reporting and Data System |
| DWI | Diffusion-weighted imaging |
| DCE | Dynamic contrast-enhanced |
| ESGO | European Society of Gynaecological Oncology |
| PET-CT | Positron emission tomography–computed tomography |
| FDG | Fluorodeoxyglucose |
| NCCN | National Comprehensive Cancer Network |
References
- Maeda, D.; Takazawa, Y.; Ota, S.; Takeuchi, Y.; Seta, A.; Nakagawa, S.; Yano, T.; Taketani, Y.; Fukayama, M. Bilateral microscopic adenocarcinoma of the Fallopian tubes detected by an endometrial cytologic smear. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 2010, 29, 273–277. [Google Scholar] [CrossRef]
- Ural, U.M.; Balik, G.; Tekin, Y.B.; Sehitoglu, I.; Bedir, R.; Sahin, F.K. Primary Fallopian tube carcinoma diagnosed preoperatively by cervical smear. Ann. Saudi Med. 2014, 34, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Kalampokas, E.; Kalampokas, T.; Tourountous, I. Primary Fallopian tube carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Seidman, J.D.; Zhao, P.; Yemelyanova, A. “Primary peritoneal” high-grade serous carcinoma is very likely metastatic from serous tubal intraepithelial carcinoma: Assessing the new paradigm of ovarian and pelvic serous carcinogenesis and its implications for screening for ovarian cancer. Gynecol. Oncol. 2011, 120, 470–473. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, Fallopian tube, and peritoneum: 2021 update. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstetrics 2021, 155 (Suppl. S1), 61–85. [Google Scholar] [CrossRef]
- Duska, L.R.; Kohn, E.C. The new classifications of ovarian, Fallopian tube, and primary peritoneal cancer and their clinical implications. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28 (Suppl. S8), viii8–viii12. [Google Scholar] [CrossRef]
- Crum, C.P.; Drapkin, R.; Miron, A.; Ince, T.A.; Muto, M.; Kindelberger, D.W.; Lee, Y. The distal Fallopian tube: A new model for pelvic serous carcinogenesis. Curr. Opin. Obstet. Gynecol. 2007, 19, 3–9. [Google Scholar] [CrossRef]
- Deffieux, X.; Morice, P.; Thoury, A.; Camatte, S.; Duvillard, P.; Castaigne, D. Anatomy of pelvic and para-aortic nodal spread in patients with primary Fallopian tube carcinoma. J. Am. Coll. Surg. 2005, 200, 45–48. [Google Scholar] [CrossRef]
- Kalampokas, E.; Sofoudis, C.; Boutas, I.; Kalampokas, T.; Tourountous, I. Primary Fallopian tube carcinoma: A case report and mini review of the literature. Eur. J. Gynaecol. Oncol. 2014, 35, 595–596. [Google Scholar]
- Veloso Gomes, F.; Dias, J.L.; Lucas, R.; Cunha, T.M. Primary Fallopian tube carcinoma: Review of MR imaging findings. Insights Imaging 2015, 6, 431–439. [Google Scholar] [CrossRef]
- Chaudhry, S.; Hussain, R.; Zuberi, M.M.; Zaidi, Z. Rare primary Fallopian tube carcinoma; a gynaecologist’s dilemma. JPMA J. Pak. Med. Assoc. 2016, 66, 107–110. [Google Scholar] [PubMed]
- Stoler, M.; Bergeron, C.; Colgan, T.J. WHO Classification of Tumours of Female Reproductive Organs; International Agency for Research on Cancer: Lyon, France, 2014; pp. 169–189. [Google Scholar]
- Revzin, M.V.; Moshiri, M.; Katz, D.S.; Pellerito, J.S.; Mankowski Gettle, L.; Menias, C.O. Imaging Evaluation of Fallopian Tubes and Related Disease: A Primer for Radiologists. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2020, 40, 1473–1501. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gilks, C.B.; Wilkinson, N.; McCluggage, W.G. Assignment of primary site in high-grade serous tubal, ovarian and peritoneal carcinoma: A proposal. Histopathology 2014, 65, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Aich, R.K.; Dasgupta, S.; Chakraborty, B.; Karim, R.; Bhattacharya, J.; Sen, P. Primary Fallopian tube carcinoma with metastasis in the contralateral ovary. J. Indian Med. Assoc. 2012, 110, 494–498. [Google Scholar]
- Yang, Y.; Xiao, Z.; Liu, Z.; Lv, F. MRI can be used to differentiate between primary Fallopian tube carcinoma and epithelial ovarian cancer. Clin. Radiol. 2020, 75, 457–465. [Google Scholar] [CrossRef]
- Kessler, M.; Fotopoulou, C.; Meyer, T. The molecular fingerprint of high grade serous ovarian cancer reflects its Fallopian tube origin. Int. J. Mol. Sci. 2013, 14, 6571–6596. [Google Scholar] [CrossRef]
- Kaundal, A.; Kaur, G.; Renjhen, P.; Parsad, S.; Sharma, S. Fallopian Tube Papilloma: A Systematic Review of Case Reports. Niger. Med. J. 2025, 65, 811–823. [Google Scholar]
- Nogales, F.F.; Goyenaga, P.; Preda, O.; Nicolae, A.; Vieites, B.; Ruiz-Marcellan, M.C.; Pedrosa, A.; Merino, M.J. An analysis of five clear cell papillary cystadenomas of mesosalpinx and broad ligament: Four associated with von Hippel-Lindau disease and one aggressive sporadic type. Histopathology 2012, 60, 748–757. [Google Scholar] [CrossRef]
- Tavares, M.A.; Silva, R.C.; Lourenço, M.; Ambrósio, A. Giant serous adenofibroma of the Fallopian tube. BMJ Case Rep. 2020, 13, e234267. [Google Scholar] [CrossRef]
- Hodzic, E.; Pusina, S.; Bajramagic, S.; Salibasic, M.; Holjan, S. Papillary Cystadenofibroma of Fallopian Tube: Case Report with a Literature Review. Med. Arch. 2020, 74, 73–76. [Google Scholar] [CrossRef]
- Sunitsch, S.; Reisinger, J.; Abete, L.; Kashofer, K.; Regitnig, P. Metaplastic papillary tumour of the Fallopian tube, a rare entity, analysed by next-generation sequencing. Histopathology 2020, 76, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Ludovisi, M.; De Blasis, I.; Virgilio, B.; Fischerova, D.; Franchi, D.; Pascual, M.A.; Savelli, L.; Epstein, E.; Van Holsbeke, C.; Guerriero, S.; et al. Imaging in gynecological disease (9): Clinical and ultrasound characteristics of tubal cancer. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2014, 43, 328–335. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, R.; Fu, Y.; Fu, X. Leiomyoma of the Fallopian tube found during laparoscopic myomectomy: A case report and review of the literature. Front. Surg. 2023, 10, 997338. [Google Scholar] [CrossRef]
- Jozwik, M.; Bednarczuk, K.; Osierda, Z.; Wojtkiewicz, J.; Kocik, J.; Jozwik, M. A Case Report of an Adenomatoid Tumor of the Fallopian Tube: The Histopathologic Challenges and a Review of Literature. J. Clin. Med. 2025, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Kayastha, S.; Bharati, A.; Shrestha, A.; Shrestha, S.; Pandey, A.; Panday, P. Mature Cystic Teratoma at Fallopian Tubes: A Case Series. JNMA J. Nepal Med. Assoc. 2023, 61, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.; Lanjewar, S.; Gupta, R. Placental Site Nodule. Available online: https://www.pathologyoutlines.com/topic/placentaplacentalsitenodule.html (accessed on 24 April 2025).
- Beena, D.; Teerthanath, S.; Jose, V.; Shetty, J. Molar Pregnancy Presents as Tubal Ectopic Pregnancy: A Rare Case Report. J. Clin. Diagn. Res. JCDR 2016, 10, ED10–ED11. [Google Scholar] [CrossRef]
- Malak, M.; Klam, S. Primary Fallopian Tube Clear Cell Adenocarcinoma in Pregnancy: Case Presentation and Review of Literature. Case Rep. Obstet. Gynecol. 2015, 183243. [Google Scholar] [CrossRef]
- National Cancer Institute. NCI Thesaurus Version 18.11d. 2018. Available online: https://ncit.nci.nih.gov/ncitbrowser/ (accessed on 8 May 2025).
- Wegscheider, A.S.; Tauber, N.; Graubner, K.; Ziegeler, G.; Behr, M.; Lindner, C.; Niendorf, A. Synchronous High-Grade Squamous Intraepithelial Lesion of the Fimbria of the Fallopian Tube in a 51-Year-Old Woman with Invasive Squamous Cell Carcinoma of the Uterine Cervix. Diagnostics 2023, 13, 2836. [Google Scholar] [CrossRef]
- Bean, G.R.; Anderson, J.; Sangoi, A.R.; Krings, G.; Garg, K. DICER1 mutations are frequent in müllerian adenosarcomas and are independent of rhabdomyosarcomatous differentiation. Mod. Pathol. Off. J. Can. Acad. Pathol. Inc. 2019, 32, 280–289. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, A.; Wu, J.J.; Niu, M.; Zhao, Y.; Tian, S.F.; Chen, A.; Zhong, L. Primary malignant mixed Müllerian tumors of the Fallopian tube with cervix metastasis: A rare case report and literature review. Medicine 2018, 97, e11311. [Google Scholar] [CrossRef]
- Baginski, L.; Yazigi, R.; Sandstad, J. Immature (malignant) teratoma of the Fallopian tube. Am. J. Obstet. Gynecol. 1989, 160, 671–672. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Wang, Q.; Jiang, W.; Lin, L.; Yi, T.; Zhao, L.; Li, M.; Wang, P. Primary leiomyosarcoma of the Fallopian tube: A case report and literature review. Medicine 2018, 97, e0536. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Song, X.; Jin, C.; Li, Y. Tubal choriocarcinoma presented as ruptured ectopic pregnancy: A case report and review of the literature. World J. Surg. Oncol. 2020, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.; Lanjewar, S.; Gupta, R. Placental Site Trophoblastic Tumor. Available online: https://www.pathologyoutlines.com/topic/placentaPSTT.html (accessed on 24 April 2025).
- Zhang, J.; Hou, Z.; Huang, J.; Xu, W.; Wang, C.; Ma, X.; Lu, N.; Liu, J.; Mao, Y.; Qian, Y. Successful pregnancy via in vitro fertilization in a primary infertile woman with primary lymphoma of the Fallopian tube after surgery: A case report and literature review. Medicine 2022, 101, e29353. [Google Scholar] [CrossRef]
- Stein, R.G.; Diessner, J.; Hönig, A.; Wischhusen, J.; Dietl, J. Fallopian Tube Tumors: An Overview. Atlas of Genetics and Cytogenetics in Oncology and Haematology. 2013. Available online: http://atlasgeneticsoncology.org/solid-tumor/5279/Fallopian-tube-tumors-an-overview (accessed on 26 May 2025).
- Riska, A.; Leminen, A. Updating on primary Fallopian tube carcinoma. Acta Obstet. Gynecol. Scand. 2007, 86, 1419–1426. [Google Scholar] [CrossRef]
- Nebgen, D.R.; Lu, K.H.; Bast, R.C., Jr. Novel Approaches to Ovarian Cancer Screening. Curr. Oncol. Rep. 2019, 21, 75. [Google Scholar] [CrossRef]
- Zafarani, F.; Ghaffari, F.; Ahmadi, F.; Soleimani Mehranjani, M.; Shahrzad, G. Hysterosalpingography in the assessment of proximal tubal pathology: A review of congenital and acquired abnormalities. Br. J. Radiol. 2021, 94, 20201386. [Google Scholar] [CrossRef]
- Olinger, K.; Liu, X.; Khoshpouri, P.; Khoshpouri, P.; Scoutt, L.M.; Khurana, A.; Chaubal, R.N.; Moshiri, M. Added Value of Contrast-enhanced US for Evaluation of Female Pelvic Disease. RadioGraphics 2024, 44, e230092. [Google Scholar] [CrossRef]
- Tian, T.; Ding, R.; Xue, T.; Sun, J.; Ling, J. The Typical Computed Tomography Findings of Primary Fallopian Tube Carcinoma. Curr. Med. Imaging 2025, 21, e15734056274106. [Google Scholar] [CrossRef]
- Zhan, D.; Saavedra, H.; Torne, A.; Saco, A.; Cabedo, L.; Nicolau, C.; Sebastia, C. Primarian Fallopian tube carcinoma: Clinical and radiological keys for diagnosis. Radiol. Case Rep. 2024, 19, 4380–4384. [Google Scholar] [CrossRef]
- Klein, M.; Rosen, A.; Graf, A.; Lahousen, M.; Kucera, H.; Pakisch, B.; Vavra, N.; Beck, A. Primary Fallopian tube carcinoma--a retrospective survey of 51 cases. Austrian Cooperative Study Group for Fallopian Tube Carcinoma. Arch. Gynecol. Obstet. 1994, 255, 141–146. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Mogensen, H.; Amtrup, F.; Honore, E. Primary carcinoma of the Fallopian tube. A retrospective study of patients reported to the Danish Cancer Registry in a five-year period. Acta Oncol. 1989, 28, 7–11. [Google Scholar] [CrossRef]
- Tongsong, T.; Wanapirak, C.; Tantipalakorn, C.; Tinnangwattana, D. Sonographic Diagnosis of Tubal Cancer with IOTA Simple Rules Plus Pattern Recognition. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3011–3015. [Google Scholar]
- Horng, H.C.; Teng, S.W.; Huang, B.S.; Sun, H.D.; Yen, M.S.; Wang, P.H.; Tsui, K.H.; Wen, K.C.; Chen, Y.J.; Chuang, C.M.; et al. Primary Fallopian tube cancer: Domestic data and up-to-date review. Taiwan. J. Obstet. Gynecol. 2014, 53, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Yazbek, J.; Ambler, G.; Hoo, W.; Naftalin, J.; Jurkovic, D. Prospective evaluation of the IOTA logistic regression model LR2 for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2012, 40, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Sayasneh, A.; Kaijser, J.; Preisler, J.; Johnson, S.; Stalder, C.; Husicka, R.; Guha, S.; Naji, O.; Abdallah, Y.; Raslan, F.; et al. A multicenter prospective external validation of the diagnostic performance of IOTA simple descriptors and rules to characterize ovarian masses. Gynecol. Oncol. 2013, 130, 140–146. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Pascual, M.Á.; Olartecoechea, B.; Graupera, B.; Aubá, M.; Ajossa, S.; Hereter, L.; Julve, R.; Gastón, B.; Peddes, C.; et al. IOTA simple rules for discriminating between benign and malignant adnexal masses: Prospective external validation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2013, 42, 467–471. [Google Scholar] [CrossRef]
- Ma, F.H.; Cai, S.Q.; Qiang, J.W.; Zhao, S.H.; Zhang, G.F.; Rao, Y.M. MRI for differentiating primary Fallopian tube carcinoma from epithelial ovarian cancer. J. Magn. Reson. Imaging JMRI 2015, 42, 42–47. [Google Scholar] [CrossRef]
- La Parra Casado, C.; Molina Fàbrega, R.; Forment Navarro, M.; Cano Gimeno, J. Estudio de las enfermedades de las trompas de Falopio mediante resonancia magnética [Fallopian tube disease on magnetic resonance imaging]. Radiologia 2013, 55, 385–397. [Google Scholar] [CrossRef]
- Radswiki, T. Primary Fallopian Tube Carcinoma. Available online: https://radiopaedia.org/cases/primary-fallopian-tube-carcinoma (accessed on 4 May 2025).
- Rockall, A.G.; Jalaguier-Coudray, A.; Thomassin-Naggara, I. MR imaging of the Adnexa: Technique and Imaging Acquisition. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 149–161. [Google Scholar] [CrossRef]
- Dabi, Y.; Rockall, A.; Sadowski, E.; Touboul, C.; Razakamanantsoa, L.; Thomassin-Naggara, I.; EURAD study group. O-RADS MRI to classify adnexal tumors: From clinical problem to daily use. Insights Imaging 2024, 15, 29. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Dabi, Y.; Florin, M.; Saltel-Fulero, A.; Manganaro, L.; Bazot, M.; Razakamanantsoa, L. O-RADS MRI SCORE: An Essential First-Step Tool for the Characterization of Adnexal Masses. J. Magn. Reson. Imaging JMRI 2024, 59, 720–736. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.E.; Fine, G.C.; Covington, M.F.; Koppula, B.R.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology-IV. Gynecologic and Genitourinary Malignancies. Cancers 2022, 14, 3000. [Google Scholar] [CrossRef] [PubMed]
- Borley, J.; Wilhelm-Benartzi, C.; Yazbek, J.; Williamson, R.; Bharwani, N.; Stewart, V.; Carson, I.; Hird, E.; McIndoe, A.; Farthing, A.; et al. Radiological predictors of cytoreductive outcomes in patients with advanced ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 843–849. [Google Scholar] [CrossRef]
- Roze, J.F.; Hoogendam, J.P.; van de Wetering, F.T.; Spijker, R.; Verleye, L.; Vlayen, J.; Veldhuis, W.B.; Scholten, R.J.; Zweemer, R.P. Positron emission tomography (PET) and magnetic resonance imaging (MRI) for assessing tumour resectability in advanced epithelial ovarian/Fallopian tube/primary peritoneal cancer. Cochrane Database Syst. Rev. 2018, 10, CD012567. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Grigsby, P.W.; Dehdashti, F.; Delbeke, D. The role of 18F-FDG PET in assessing therapy response in cancer of the cervix and ovaries. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2009, 50 (Suppl. S1), 64S–73S. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Z. Radiomics Analysis of PET and CT Components of 18F-FDG PET/CT Imaging for Prediction of Progression-Free Survival in Advanced High-Grade Serous Ovarian Cancer. Front. Oncol. 2021, 11, 638124. [Google Scholar] [CrossRef]
- NCCN. [Guideline] NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1.2021—9 September 2021. Available online: https://www.nccn.org/professionals/physician_gls/PDF/ovarian.pdf (accessed on 8 May 2025).
- Dendl, K.; Koerber, S.A.; Finck, R.; Mokoala, K.M.G.; Staudinger, F.; Schillings, L.; Heger, U.; Röhrich, M.; Kratochwil, C.; Sathekge, M.; et al. 68Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4089–4100. [Google Scholar] [CrossRef]
- Sponholtz, S.E.; Mogensen, O.; Hildebrandt, M.G.; Jensen, P.T. Clinical impact of pre-treatment FDG-PET/CT staging of primary ovarian, Fallopian tube, and peritoneal cancers in women. Acta Obstet. Gynecol. Scand. 2020, 99, 186–195. [Google Scholar] [CrossRef]
- Dai, N.; Deng, S.; Yang, Y.; Sang, S. 18-F fluorodeoxyglucose positron emission tomography/computed tomography findings of bilateral primary Fallopian tube carcinoma and metastasis to the uterus: A case report and literature review. J. Int. Med. Res. 2022, 50, 3000605221118678. [Google Scholar] [CrossRef]
- Alvarado-Cabrero, I.; Stolnicu, S.; Kiyokawa, T.; Yamada, K.; Nikaido, T.; Santiago-Payán, H. Carcinoma of the Fallopian tube: Results of a multi-institutional retrospective analysis of 127 patients with evaluation of staging and prognostic factors. Ann. Diagn. Pathol. 2013, 17, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sumtsov, D.H.; Gladchuk, I.Z.; Kashtalian, N.M.; Sumtsov, G.O. PRACTICAL MEANS OF PREOPERATIVE DIAGNOSTICS OF PRIMARY FALLOPIAN TUBE CANCER. Wiad. Lek. 2021, 74, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Jeung, I.C.; Lee, Y.S.; Lee, H.N.; Park, E.K. Primary carcinoma of the Fallopian tube: Report of two cases with literature review. Cancer Res. Treat. 2009, 41, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Saksouk, F.A.; Johnson, S.C. Recognition of the ovaries and ovarian origin of pelvic masses with CT. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2004, 24 (Suppl. S1), S133–S146. [Google Scholar] [CrossRef]
- Nougaret, S.; Nikolovski, I.; Paroder, V.; Vargas, H.A.; Sala, E.; Carrere, S.; Tetreau, R.; Hoeffel, C.; Forstner, R.; Lakhman, Y. MRI of Tumors and Tumor Mimics in the Female Pelvis: Anatomic Pelvic Space-based Approach. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2019, 39, 1205–1229. [Google Scholar] [CrossRef]
- Thawait, S.K.; Batra, K.; Johnson, S.I.; Torigian, D.A.; Chhabra, A.; Zaheer, A. Magnetic resonance imaging evaluation of non ovarian adnexal lesions. Clin. Imaging 2016, 40, 33–45. [Google Scholar] [CrossRef]
- Elsherif, S.B.; Agely, A.; Gopireddy, D.R.; Ganeshan, D.; Hew, K.E.; Sharma, S.; Lall, C. Mimics and Pitfalls of Primary Ovarian Malignancy Imaging. Tomography 2022, 8, 100–119. [Google Scholar] [CrossRef]
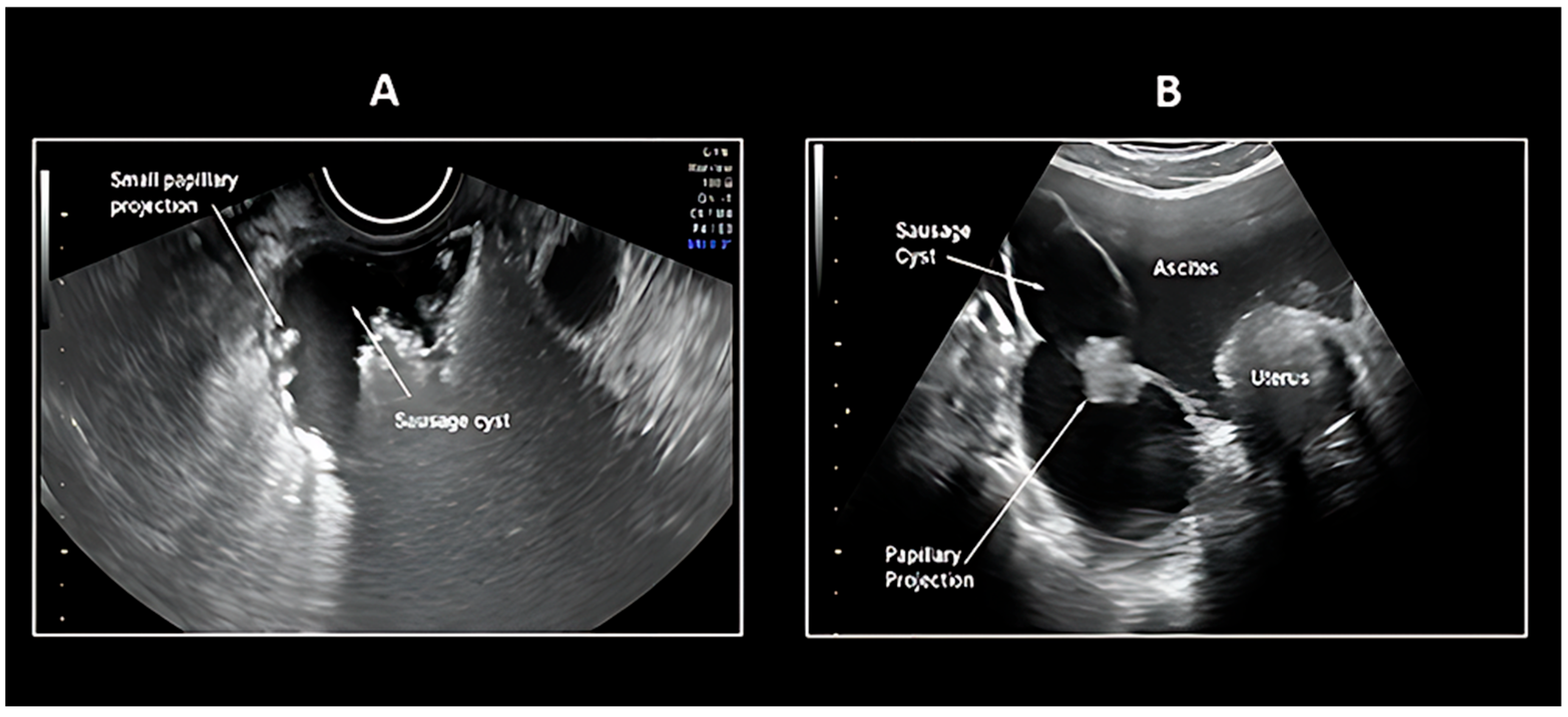
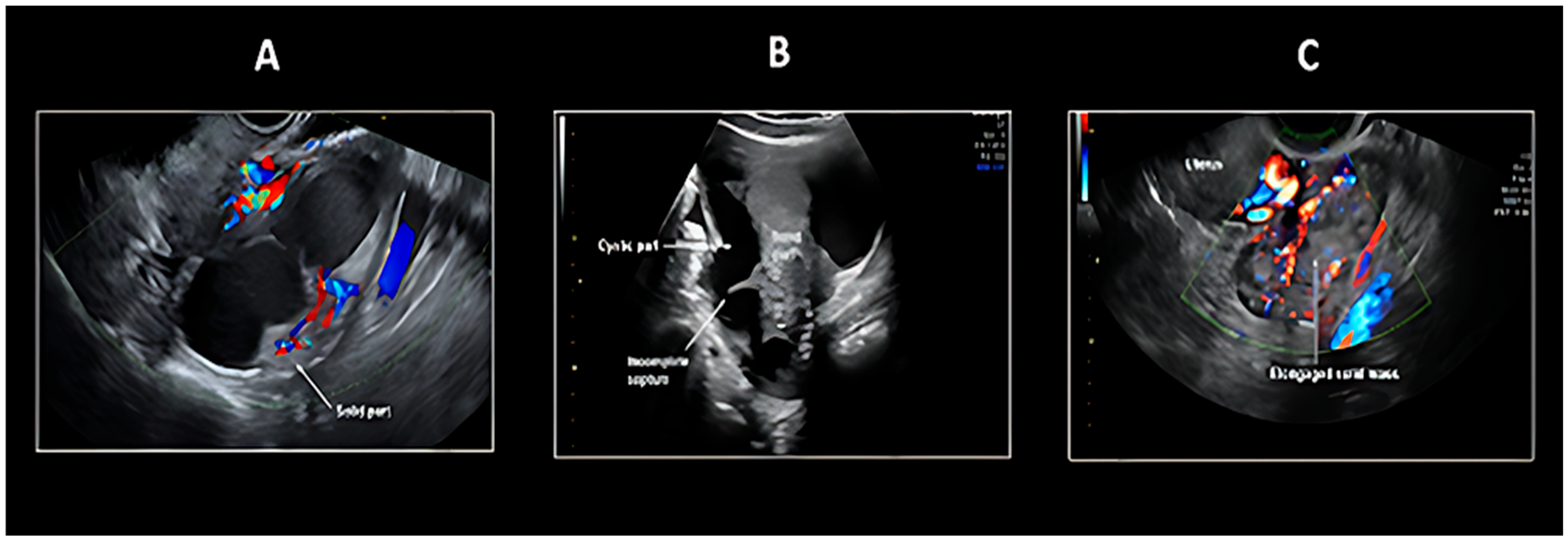

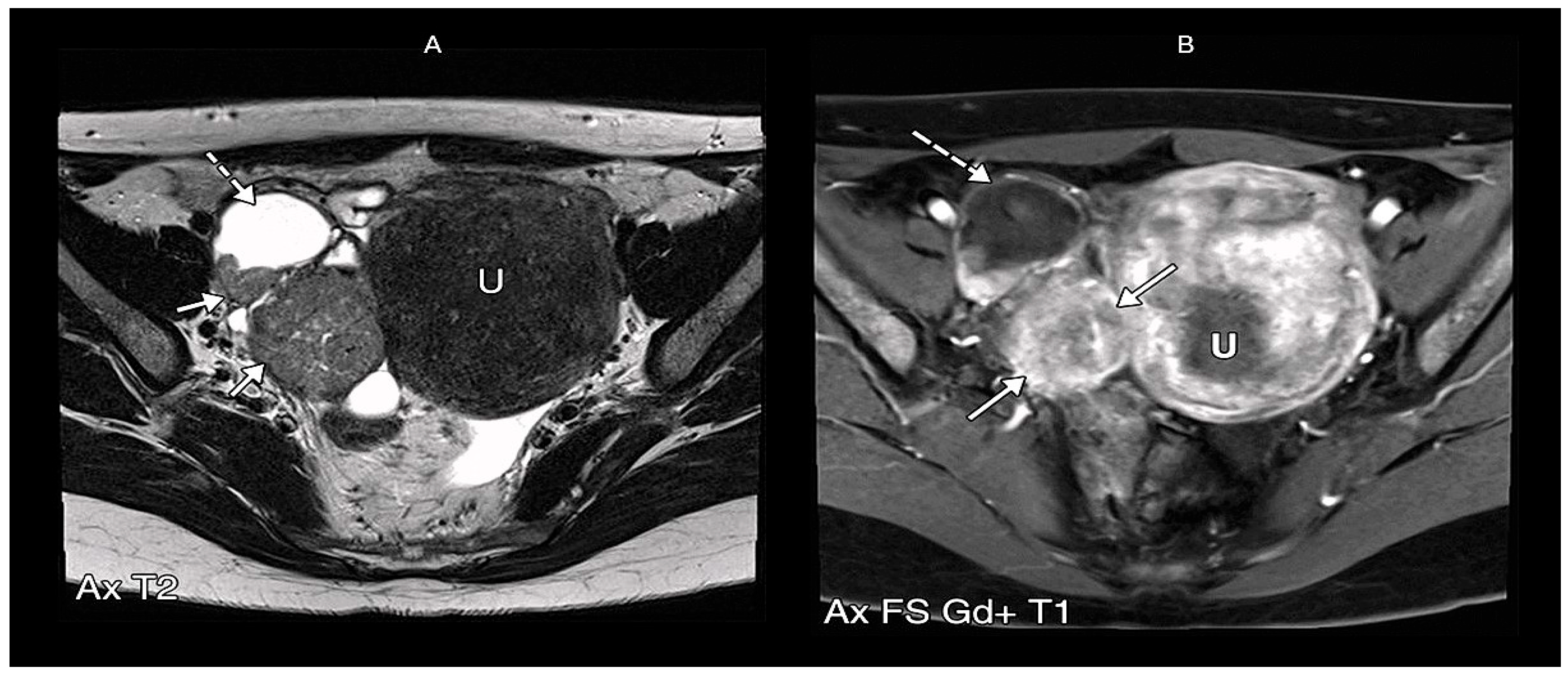

| Histological Subtype | Etiology/Genetic Mutation | Origin | Frequency |
|---|---|---|---|
| Benign Neoplasms | |||
| Papilloma | Local tubal hyperplasia in response to hormonal hyperstimulation or inflammation [18] | Epithelial Tumor | Rare |
| Cystadenoma | von Hippel-Lindau Disease (VHL gene mutations) [19] | Epithelial Tumor | Rare |
| Adenofibroma | Embryological remnant originated from the Müllerian duct [20] | Epithelial Tumor | Rare |
| Cystadenofibroma | Unknown [21] | Epithelial Tumor | Rare |
| Metaplastic papillary tumor (MPTFT) | KRAS e BRAF detected incidentally upon examination of Fallopian tube segments removed for sterilisation postpartum [22] | Epithelial Tumor | Extremely rare |
| Endometroid papilloma | Estrogen-driven hyperplasia [23] | Epithelial Tumor | Extremely rare |
| Leiomyoma | The pathogenesis of this disease is still unclear, but the nodules are thought to originate from sub-mesothelial multipotential cells located in the female pelvic peritoneum [24] | Soft Tissue Tumor | Extremely rare |
| Adenomatoid tumor | BRCA1 may be involved TRAF7 [25] | Mesothelial Tumor | Although Rare the Most Common among Benign Neoplasm |
| Mature Teratoma | Unknown [26] | Germ Cell Tumor | Extremely rare |
| Placental site nodule | Non involuted placental site from remote gestations in the uterus [27] | Trophoblastic Tumor | Rare |
| Hydatiform mole | Androgenetic diploidy (46, XX) or triploidy (69, XXY) [28] | Trophoblastic Tumor | Exceptionally rare in the Fallopian tube, usually associated with molar ectopic pregnancy |
| Malignant Neoplasms | |||
| Serous Adenocarcinoma (Low and high grade) | TP53 (high grade) BRAF/KRAS BRCA1/BRCA2 | Epithelial Tumor | The most common among malignant neoplasms (80%) [22] |
| Endometroid Adenocarcinoma | BRCA1/BRCA2 c-erbB-2 | Epithelial Tumor | 7% [22] |
| Mucinous Adenocarcinoma | BRCA1/BRCA2 | Epithelial Tumor | 2% [22] |
| Clear cell Adenocarcinoma | BRCA1/BRCA2 [29] | Epithelial Tumor | 2% [22] |
| Undifferentiated Carcinoma | BRCA1/BRCA2 [30] | Epithelial Tumor | 1% [22] |
| Transitional Cell Carcinoma | BRCA1/BRCA2 [30] | Epithelial Tumor | Rare |
| Squamous Cell Carcinoma | HPV-associated [31] | Epithelial Tumor | Extremely rare |
| Adenosarcoma | DICER1 TP53 [32] | Epithelial–Mesenchymal Tumor | Rare |
| Malignant Müllerian mixed tumor (Metaplastic Carcinoma, Carcinosarcoma) | Unknown [33] | Epithelial–Mesenchymal Tumor | Rare |
| Immature Teratoma | Unknown [34] | Germ Cell Tumor | Extremely rare |
| Leiomyosarcoma | Prior pelvic radiation Tamoxifen use for >5 years From a pre-existing leiomyoma Hereditary retinoblastoma and Li-Fraumeni syndrome [35] | Soft Tissue Tumor | Extremely rare |
| Choriocarcinoma | After complete hydatidiform mole [36] | Trophoblastic Tumor | Extremely rare |
| Invasive Mole | From complete hydatidiform moles [36] | Trophoblastic Tumor | Extremely rare |
| Placental Site Trophoblastic Tumor | Unknown [37] | Trophoblastic Tumor | Extremely rare |
| Lymphoma and Leukemia | Pathogen infection [38] | Lymphatic and hematopoietic Tumor | Extremely rare |
| Metastases | Primary tumors: Ovary, endometrium, GI tract [39] | Secondary Tumor | Variable |
| Rules for Predicting a Malignant Tumor (M-Rules) | Rules for Predicting a Benign Tumor (B-Rules) | ||
|---|---|---|---|
| M1 | Irregular solid tumor | B1 | Unilocular |
| M2 | Presence of ascites | B2 | Presence of solid components where the largest Solid component has a largest diameter < 7 mm |
| M3 | At least four papillary structures | B3 | Presence of acoustic shadows |
| M4 | Irregular multilocular solid tumor with largest diameter ≥ 100 mm | B4 | Smooth multilocular tumor with largest diameter < 100 mm |
| M5 | Very strong blood flow (color score 4) | B5 | No blood flow (color score 1) |
| Imaging Feature | PFTC |
|---|---|
| Morphology | Tubular/sausage-shaped mass |
| Laterality | Tipically, Unilateral |
| Size | Smaller (mean ~6 cm) |
| Internal Architecture | Homogeneous solid component |
| Enhancement Pattern | Continuous thick rim enhancement (>2.3 mm) |
| Hydrosalpinx | Present in ~30–50% of cases |
| Intrauterine Fluid | Present in ~30% of cases |
| T2 Signal | Hyperintense tubular structure |
| Diffusion Restriction | Non-specific ADC values |
| Doppler Flow | Moderate vascularity in solid portions |
| Clinical features | |
| Latzko triad | 15% [9,10] |
| Hidrops tubae profluens | 5% pathognomonic |
| O-RADS | Description |
|---|---|
| 0 | Incomplete exam |
| 1. Normal ovaries | No ovarian lesion |
Physiological:
| |
| 2. Almost certainly benign < 0.5% | Cyst: Unilocular—simple or endometriotic fluid
|
Cyst: Unilocular/multilocular—any type of fluid
| |
Cyst: Unilocular/multilocular—lipid content
| |
| Lesions with Solid tissue: homogeneously hypointense on T2 and DWI (dark/dark) | |
Para ovarian cyst—simple fluid
| |
Dilated Fallopian tube simple fluid
| |
| 3. Low risk < 5% | Cyst: Unilocular—hemorrhagic mucinous or proteinaceous fluid
|
| Cyst: Multilocular—any type of fluid Smooth septae and enhanced wall
| |
Lesion with solid tissue (excluding T2 dark/DWI dark)
| |
Dilated Fallopian tube—non-simple fluid
| |
| 4. Intermediate risk 5–90% | Lesion with solid tissue (excluding T2 dark/DWI dark)
|
Lesion with lipid content
| |
| 5. High risk > 90% | Lesion with solid tissue
|
| Obvious peritoneal, mesenteric, or omental nodularity or thickening |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobellis, G.; Leggio, A.; Salzillo, C.; Imparato, A.; Marzullo, A. The Imaging of Primary Fallopian Tube Carcinoma: A Literature Review. Cancers 2025, 17, 2985. https://doi.org/10.3390/cancers17182985
Iacobellis G, Leggio A, Salzillo C, Imparato A, Marzullo A. The Imaging of Primary Fallopian Tube Carcinoma: A Literature Review. Cancers. 2025; 17(18):2985. https://doi.org/10.3390/cancers17182985
Chicago/Turabian StyleIacobellis, Giulia, Alessia Leggio, Cecilia Salzillo, Amalia Imparato, and Andrea Marzullo. 2025. "The Imaging of Primary Fallopian Tube Carcinoma: A Literature Review" Cancers 17, no. 18: 2985. https://doi.org/10.3390/cancers17182985
APA StyleIacobellis, G., Leggio, A., Salzillo, C., Imparato, A., & Marzullo, A. (2025). The Imaging of Primary Fallopian Tube Carcinoma: A Literature Review. Cancers, 17(18), 2985. https://doi.org/10.3390/cancers17182985







