Mortality Trends in Pediatric Hepatoblastoma: A Brazilian and Global Perspective
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Processing of Brazilian Data
2.2. Descriptive Statistical Analysis of Brazilian Data
2.3. Exploratory Statistical Analysis of Brazilian Data
2.4. Comparative Analysis with Global Data
3. Results
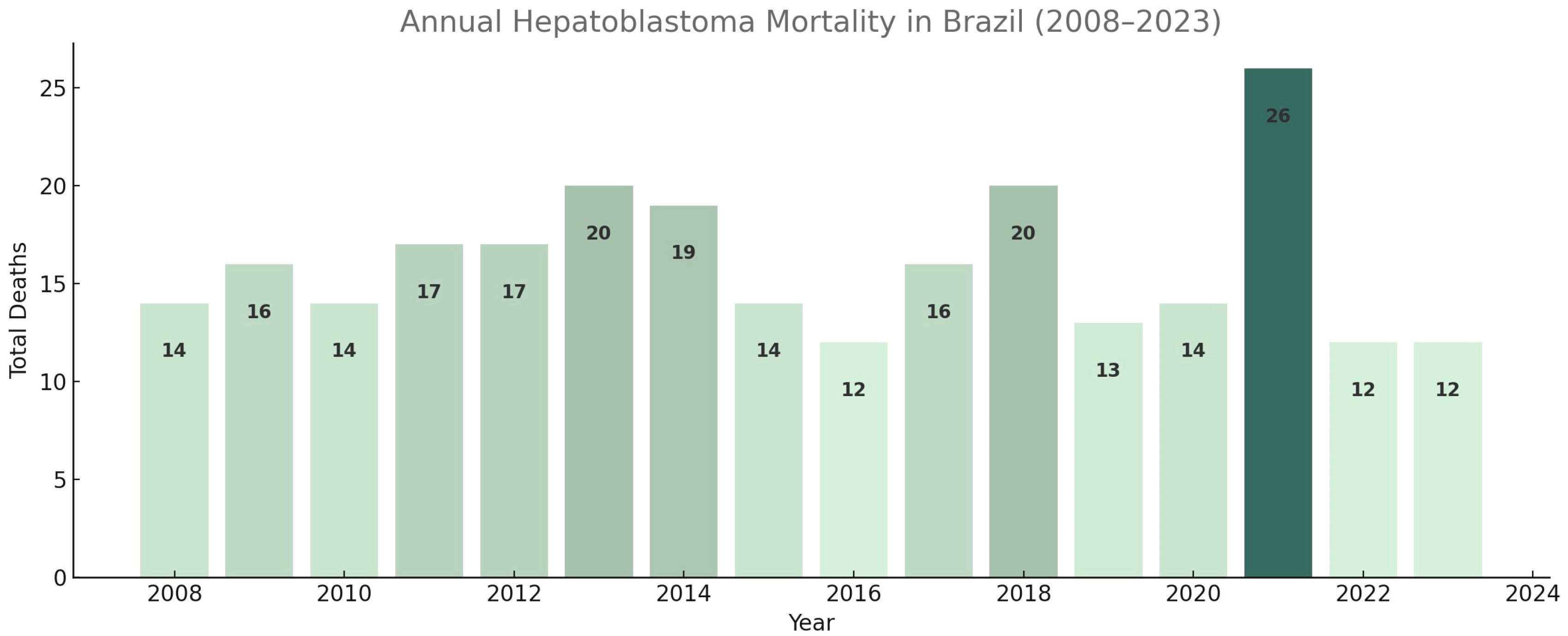
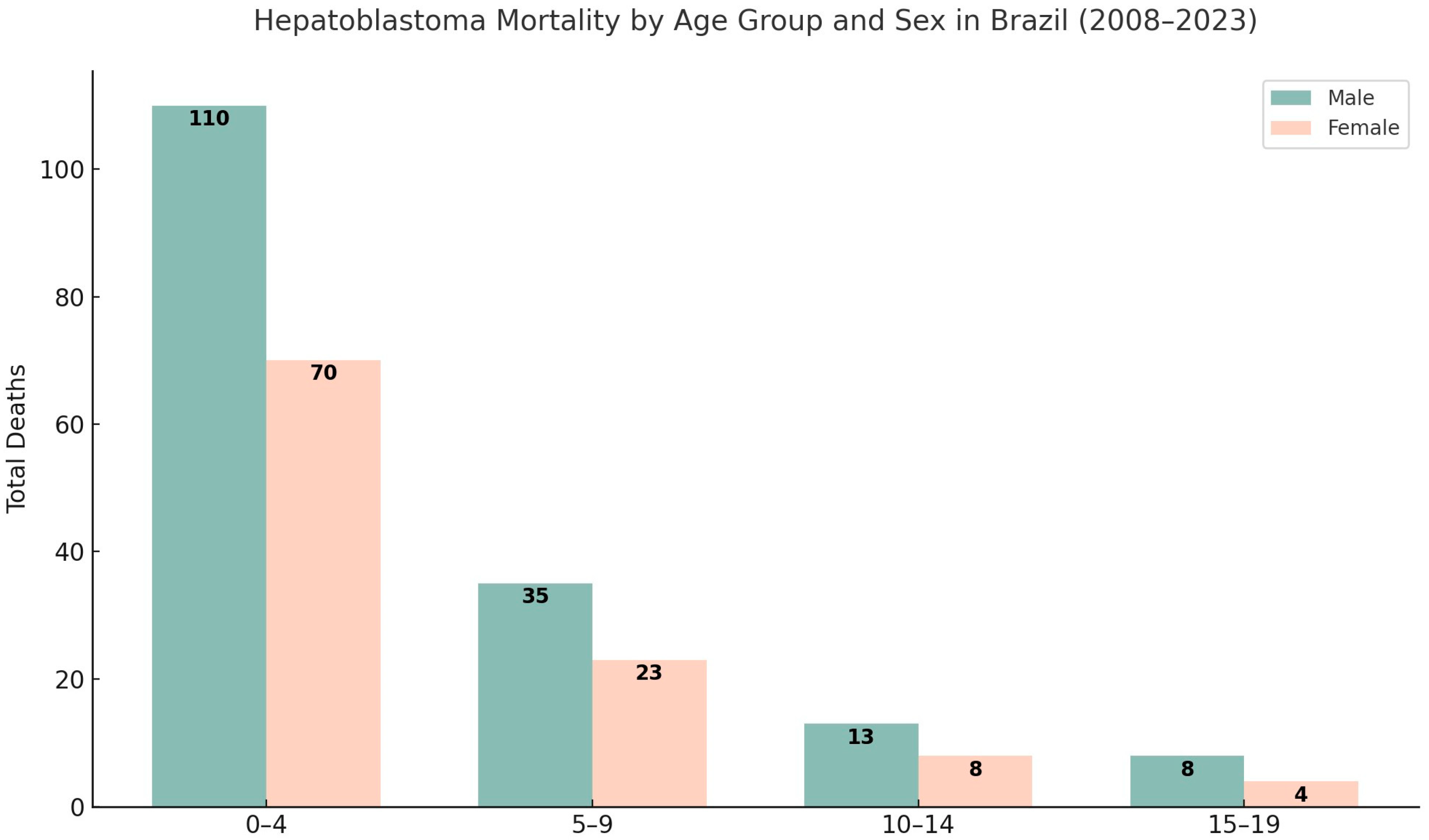
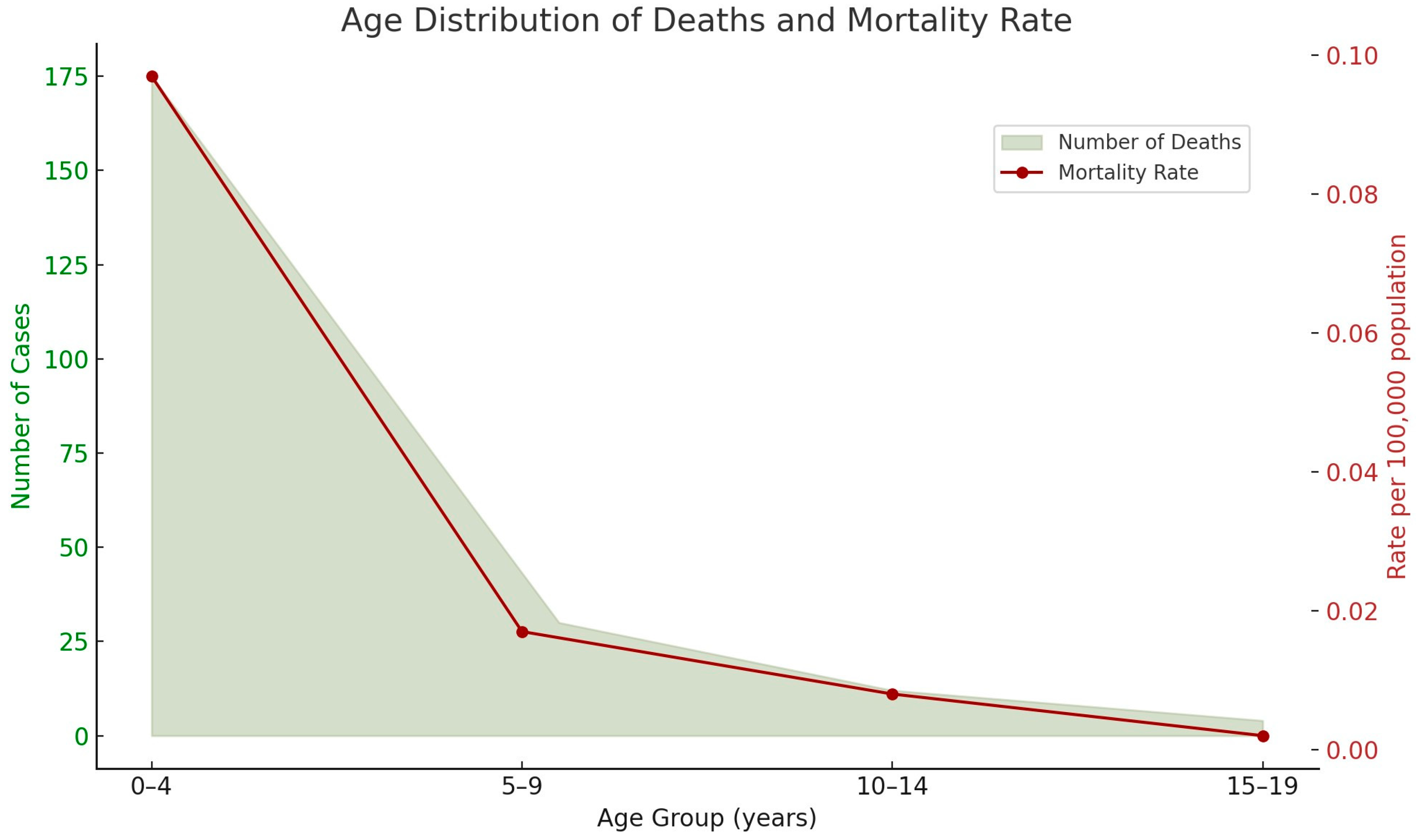
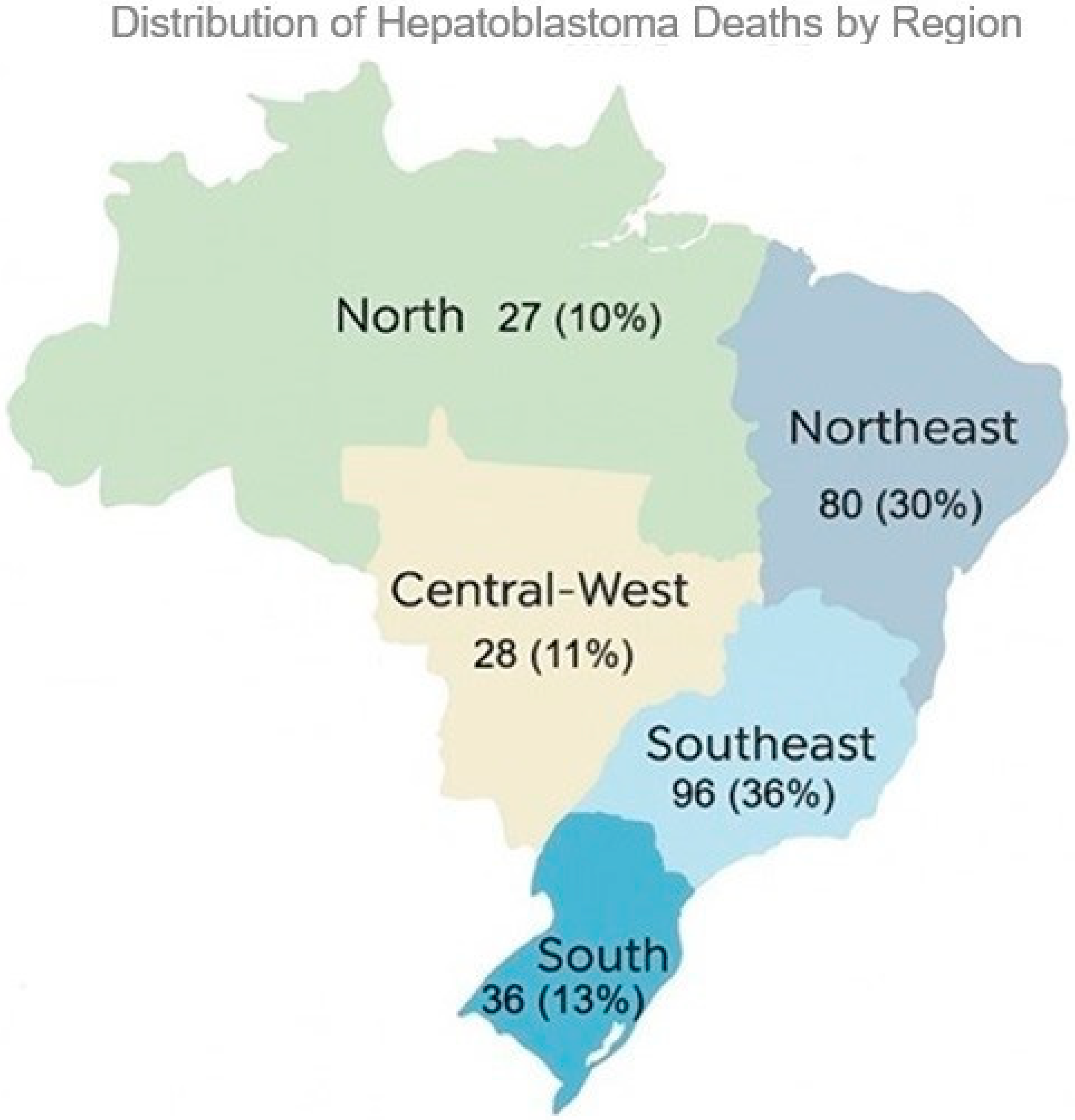
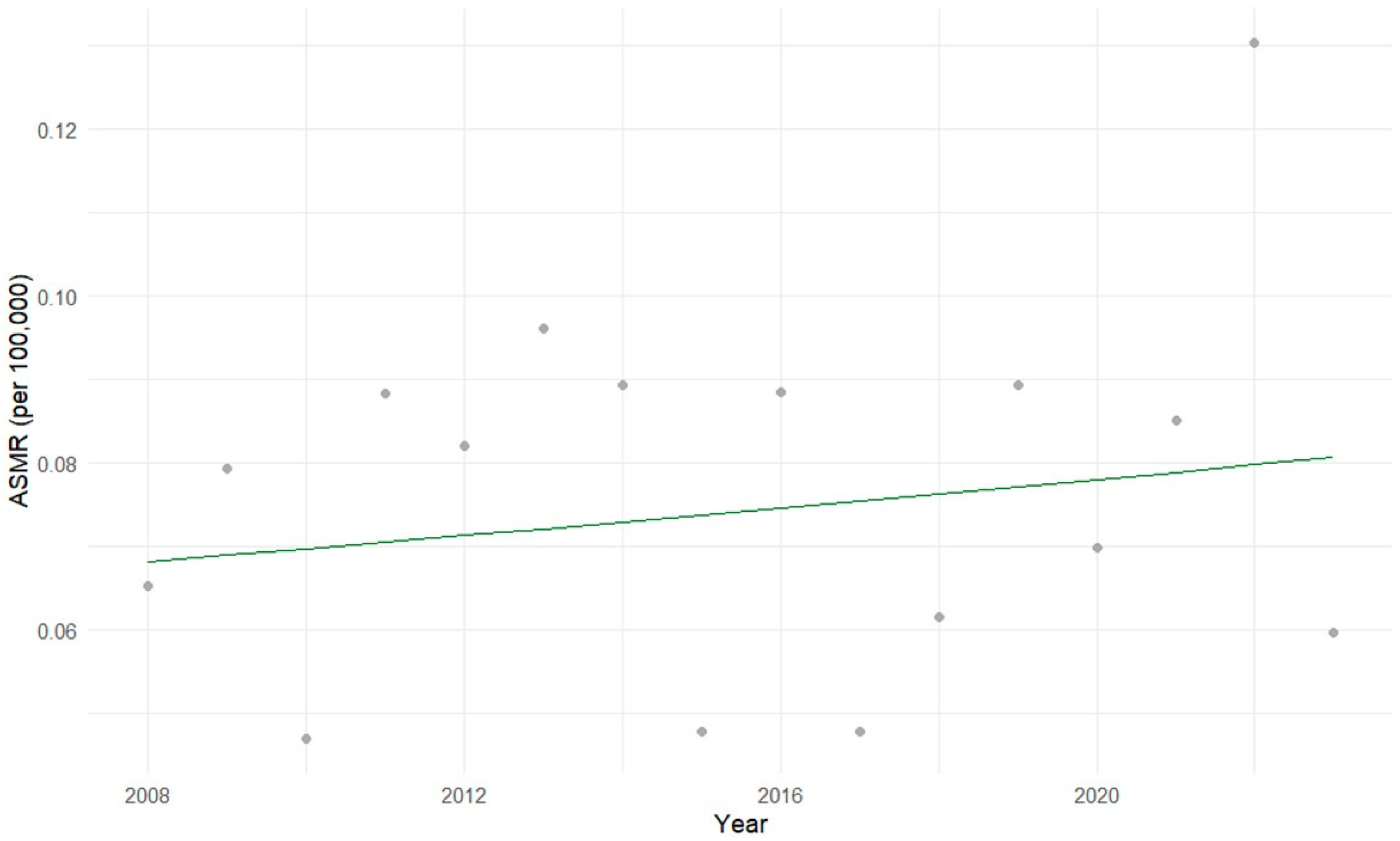
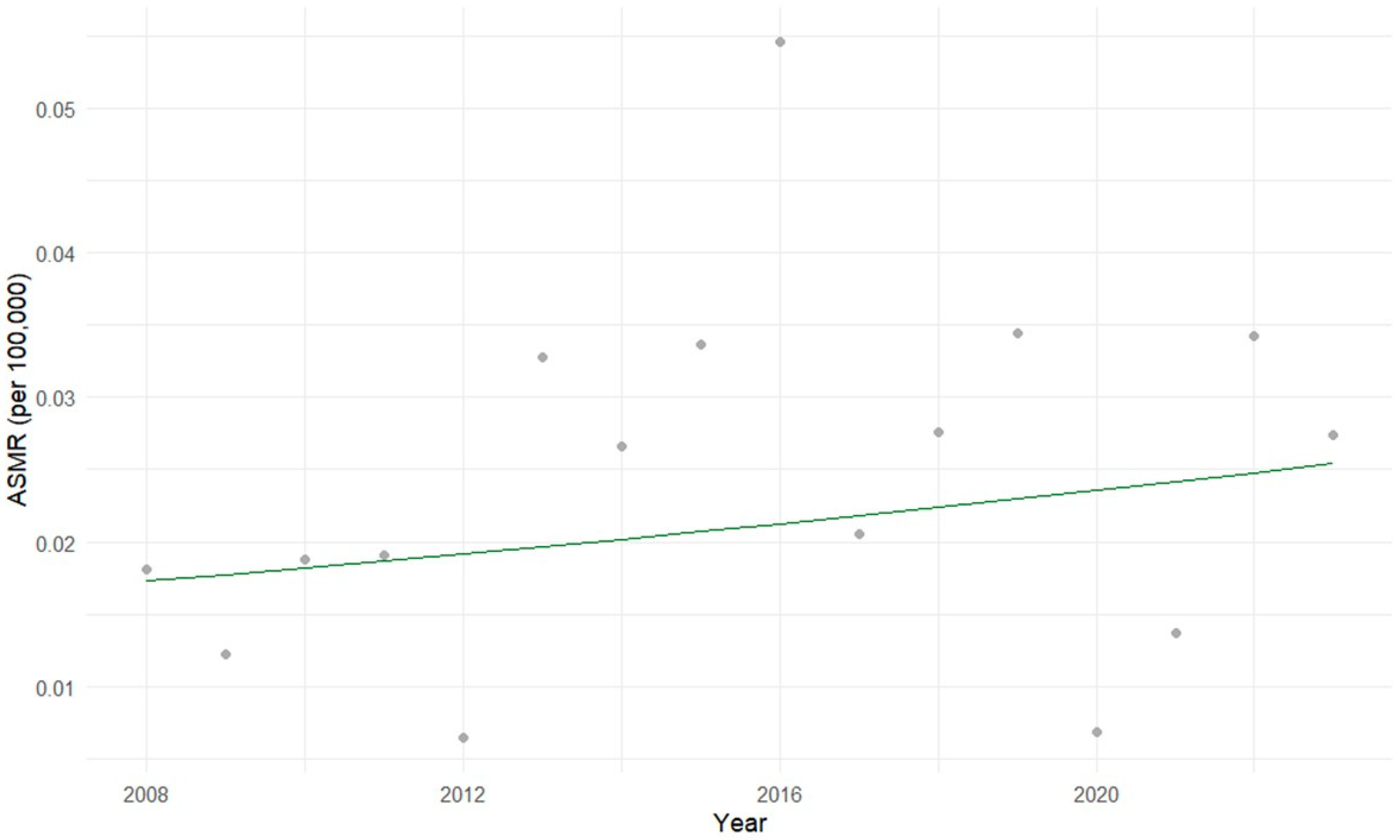
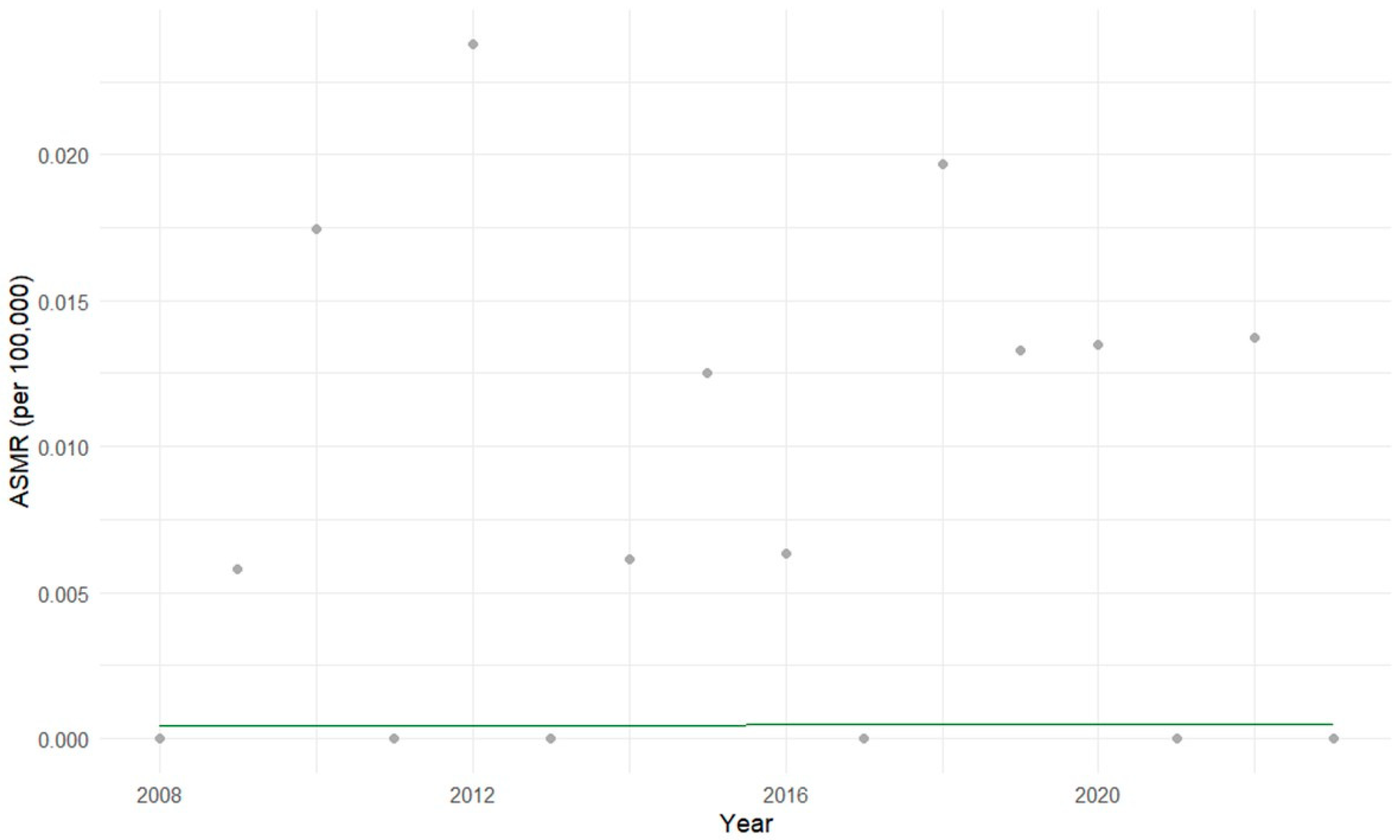
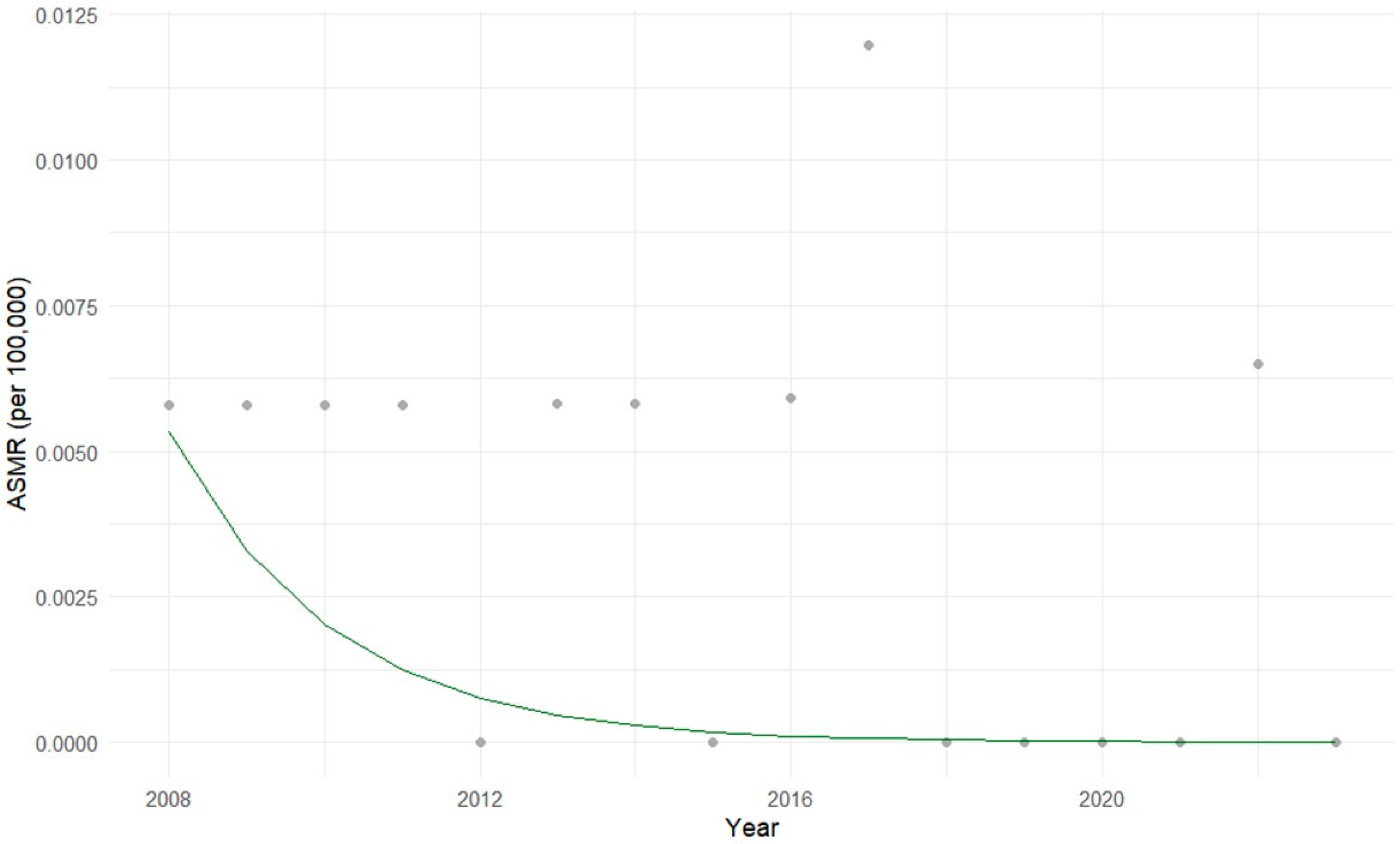
3.1. Descriptive Statistical Analysis of Brazilian Data
3.2. Exploratory Statistical Analysis of Brazilian Data
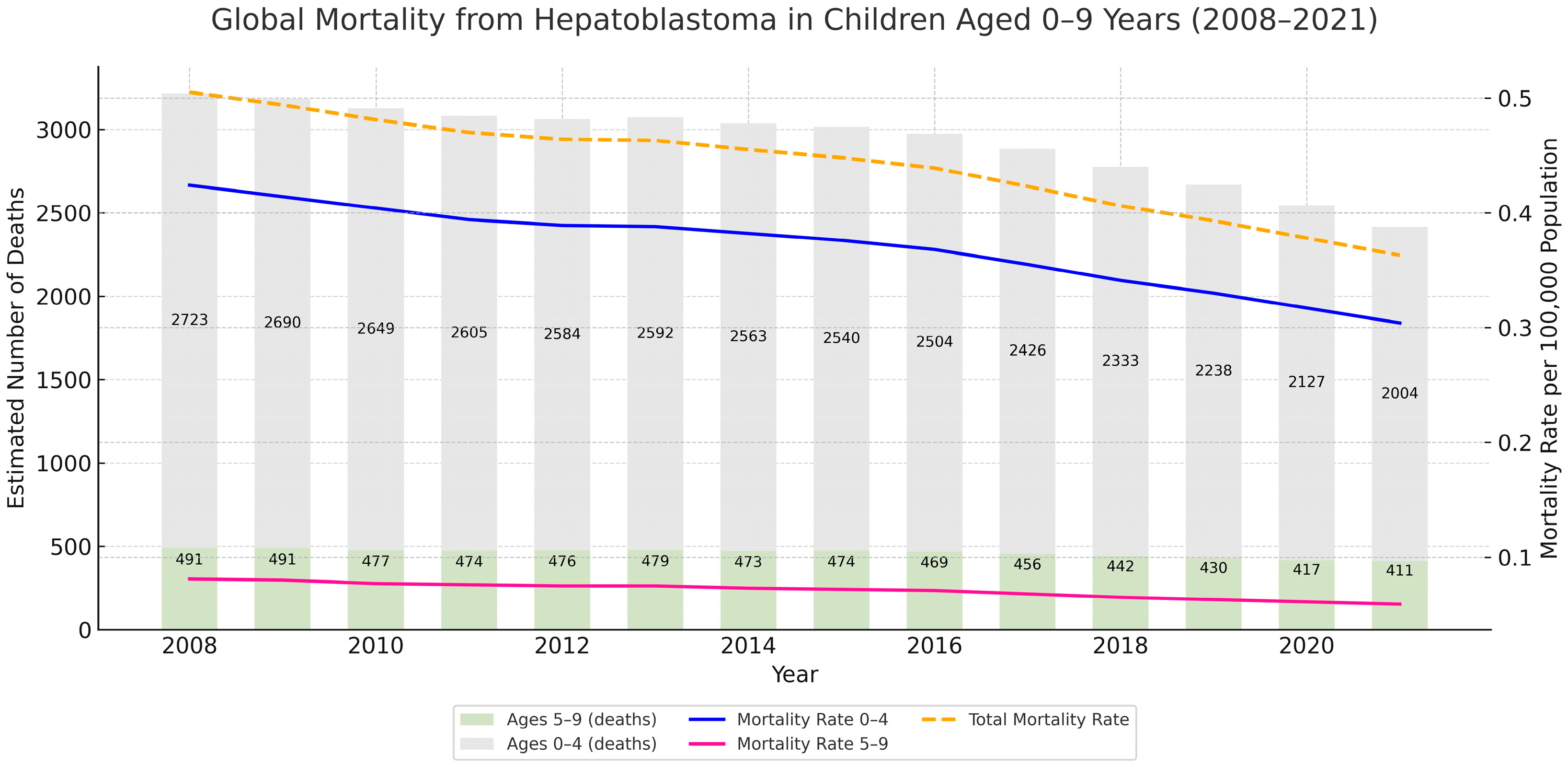
3.3. Comparative Analysis with Global Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Annual Percent Change |
| AFP | Alpha-Fetoprotein |
| WNT | Wingless-related Integration Site |
| CTNNB1 | Catenin Beta 1 |
| NFE2L2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| TERT | Telomerase Reverse Transcriptase |
| TP53 | Tumor Protein P53 |
| PRETEXT | Pretreatment Extent of Disease |
References
- Sharma, D.; Subbarao, G.; Saxena, R. Hepatoblastoma. Semin. Diagn. Pathol. 2017, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hager, J.; Sergi, C.M. Hepatoblastoma. In Liver Cancer; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar] [CrossRef]
- Ng, K.; Mogul, D.B. Pediatric liver tumors. Clin. Liver Dis. 2018, 22, 753–772. [Google Scholar] [CrossRef]
- Instituto Nacional de Câncer—INCA. Hepatoblastoma. Available online: https://www.gov.br/inca/pt-br/assuntos/cancer/tipos/infantojuvenil/especificos/hepatoblastoma (accessed on 11 June 2025).
- PDQ Pediatric Treatment Editorial Board. Childhood Liver Cancer Treatment (PDQ®): Health Professional Version; National Cancer Institute: Bethesda, MD, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65790/ (accessed on 11 June 2025).
- Eichenmüller, M.; Tripel, F.; Kreuder, M.; Beck, A.; Schwarzmayr, T.; Häberle, B.; Cairo, S.; Leuschner, I.; von Schweinitz, D.; Strom, T.M.; et al. The genomic landscape of hepatoblastoma and its progenies with hepatocellular carcinoma-like features. J. Hepatol. 2014, 61, 1312–1320. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Ranganathan, S.; Lopez-Terrada, D.; Alaggio, R. Hepatoblastoma and pediatric hepatocellular carcinoma: An update. Pediatr. Dev. Pathol. 2020, 23, 79–95. [Google Scholar] [CrossRef]
- Hiyama, E. Pediatric hepatoblastoma: Diagnosis and treatment. Transl. Pediatr. 2014, 3, 293–299. [Google Scholar] [CrossRef]
- Meyers, R.L.; Maibach, R.; Hiyama, E.; Häberle, B.; Krailo, M.; Rangaswami, A.; Aronson, D.C.; Malogolowkin, M.H.; Perilongo, G.; von Schweinitz, D.; et al. Risk-stratified staging in pediatric hepatoblastoma: A unified analysis from the Children’s Hepatic Tumors International Collaboration. Lancet Oncol. 2017, 18, 122–131. [Google Scholar] [CrossRef]
- Czauderna, P.; Häberle, B.; Hiyama, E.; Rangaswami, A.; Krailo, M.; Maibach, R.; Rinaldi, E.; Feng, Y.; Aronson, D.; Malogolowkin, M.; et al. The Children’s Hepatic Tumors International Collaboration (CHIC): Novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur. J. Cancer 2016, 52, 92–101. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2025. Available online: https://www.R-project.org/ (accessed on 11 June 2025).
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio 2025. Available online: https://posit.co/ (accessed on 11 June 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Antunes, J.L.F.; Cardoso, M.R.A. Uso da análise de séries temporais em estudos epidemiológicos. Epidemiol. Serv. Saude 2015, 24, 565–576. [Google Scholar] [CrossRef]
- Bottomley, C.; Ooko, M.; Gasparrini, A.; Keogh, R.H. In praise of Prais-Winsten: An evaluation of methods used to account for autocorrelation in interrupted time series. Stat. Med. 2023, 42, 622–640. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2023; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 12 July 2025).
- Wu, Z.; Xia, F.; Wang, W.; Zhang, K.; Fan, M.; Lin, R. Worldwide burden of liver cancer across childhood and adolescence, 2000–2021: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Reg. Health West Pac. 2024, 42, 100914. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, A.F.; Scheurer, M.E.; Chambers, T.M.; Vasudevan, S.A.; Lupo, P.J. A population-based assessment of metastatic hepatoblastoma in Texas reveals ethnic disparities. Front. Public Health 2023, 11, 1049727. [Google Scholar] [CrossRef]
- Feng, J.; Polychronidis, G.; Heger, U.; Frongia, G.; Mehrabi, A.; Hoffmann, K. Incidence trends and survival prediction of hepatoblastoma in children: A population-based study. Cancer Commun. 2019, 39, 62. [Google Scholar] [CrossRef]
- Kahla, J.A.; Siegel, D.A.; Dai, S.; Lupo, P.J.; Foster, J.H.; Scheurer, M.E.; Heczey, A.A. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr. Blood Cancer 2022, 69, e29763. [Google Scholar] [CrossRef] [PubMed]
- Mavila, N.; Thundimadathil, J. The Emerging roles of cancer stem cells and Wnt/beta-catenin signaling in hepatoblastoma. Cancers 2019, 11, 1406. [Google Scholar] [CrossRef]
- Zsíros, J.; Maibach, R.; Shafford, E.; Brugieres, L.; Brock, P.; Czauderna, P.; Roebuck, D.; Childs, M.; Zimmermann, A.; Laithier, V.; et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: Final results of the SIOPEL-3HR study. J. Clin. Oncol. 2010, 28, 2584–2590. [Google Scholar] [CrossRef]
- Pritchard, J.; Brown, J.; Shafford, E.; Perilongo, G.; Brock, P.; Dicks-Mireaux, C.; Keeling, J.; Phillips, A.; Vos, A.; Plaschkes, J. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: A successful approach—Results of the first prospective study of the International Society of Pediatric Oncology. J. Clin. Oncol. 2000, 18, 3819–3828. [Google Scholar] [CrossRef]
- Aronson, D.C.; Czauderna, P.; Maibach, R.; Perilongo, G.; Morland, B. The treatment of hepatoblastoma: Its evolution and the current status as per the SIOPEL trials. J. Indian Assoc. Pediatr. Surg. 2014, 19, 201–207. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Falqueto, L.E.; Vilar, P.R.; Campos, H.G.; Schulz, C.; Mattos e Silva, E.D. Primary malignant liver tumors: Eight-year experience in a pediatric hospital in Brazil. A cross-sectional study. Rev. Col. Bras. Cir. 2022, 49, e20223273. [Google Scholar] [CrossRef]
- Cavalcante, D.F.; Garcia, É.M.; Farias, N.S.O.; Koizumi, I.K.; Figueiredo, G.M.; Sato, A.P.S. Mortality due to hepatocellular carcinoma associated with hepatitis B and C viruses in the state of São Paulo, Brazil. Rev. Bras. Epidemiol. 2022, 25, e220004. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Furutani, E.; Ribeiro, K.B.; Rodriguez Galindo, C. Death within 1 month of diagnosis in childhood cancer: An analysis of risk factors and scope of the problem. J. Clin. Oncol. 2017, 35, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; He, E.; Sun, W.; Cui, H.; Zhao, H.; Zhao, W.; Liu, W.; Guo, Z.; Wang, Y.; Feng, W. Hepatoblastoma regional trends: Dynamic SDI & joinpoint regression analysis. BMC Cancer 2025, 25, 1148. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, R.F.L.; Barbosa, C.; Pickler, J.G.; Pedack, F.R.; Garcia, C.E.; Fronza Junior, H.; Silva, B.L.; de França, P.H.C.; Sanches, B.S.; Land, M.G.P.; et al. Mortality Trends in Pediatric Hepatoblastoma: A Brazilian and Global Perspective. Cancers 2025, 17, 2970. https://doi.org/10.3390/cancers17182970
Garcia RFL, Barbosa C, Pickler JG, Pedack FR, Garcia CE, Fronza Junior H, Silva BL, de França PHC, Sanches BS, Land MGP, et al. Mortality Trends in Pediatric Hepatoblastoma: A Brazilian and Global Perspective. Cancers. 2025; 17(18):2970. https://doi.org/10.3390/cancers17182970
Chicago/Turabian StyleGarcia, Raquel Francine Liermann, Camila Barbosa, José Guilherme Pickler, Francis Rosseti Pedack, Christian Evangelista Garcia, Hercilio Fronza Junior, Bruna Louise Silva, Paulo Henrique Condeixa de França, Bárbara Sarni Sanches, Marcelo Gerardin Poirot Land, and et al. 2025. "Mortality Trends in Pediatric Hepatoblastoma: A Brazilian and Global Perspective" Cancers 17, no. 18: 2970. https://doi.org/10.3390/cancers17182970
APA StyleGarcia, R. F. L., Barbosa, C., Pickler, J. G., Pedack, F. R., Garcia, C. E., Fronza Junior, H., Silva, B. L., de França, P. H. C., Sanches, B. S., Land, M. G. P., Roesler, R., & de Paula Alves Coelho, K. M. (2025). Mortality Trends in Pediatric Hepatoblastoma: A Brazilian and Global Perspective. Cancers, 17(18), 2970. https://doi.org/10.3390/cancers17182970







