Insight into the Wnt Pathway in Sporadic Small Bowel Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
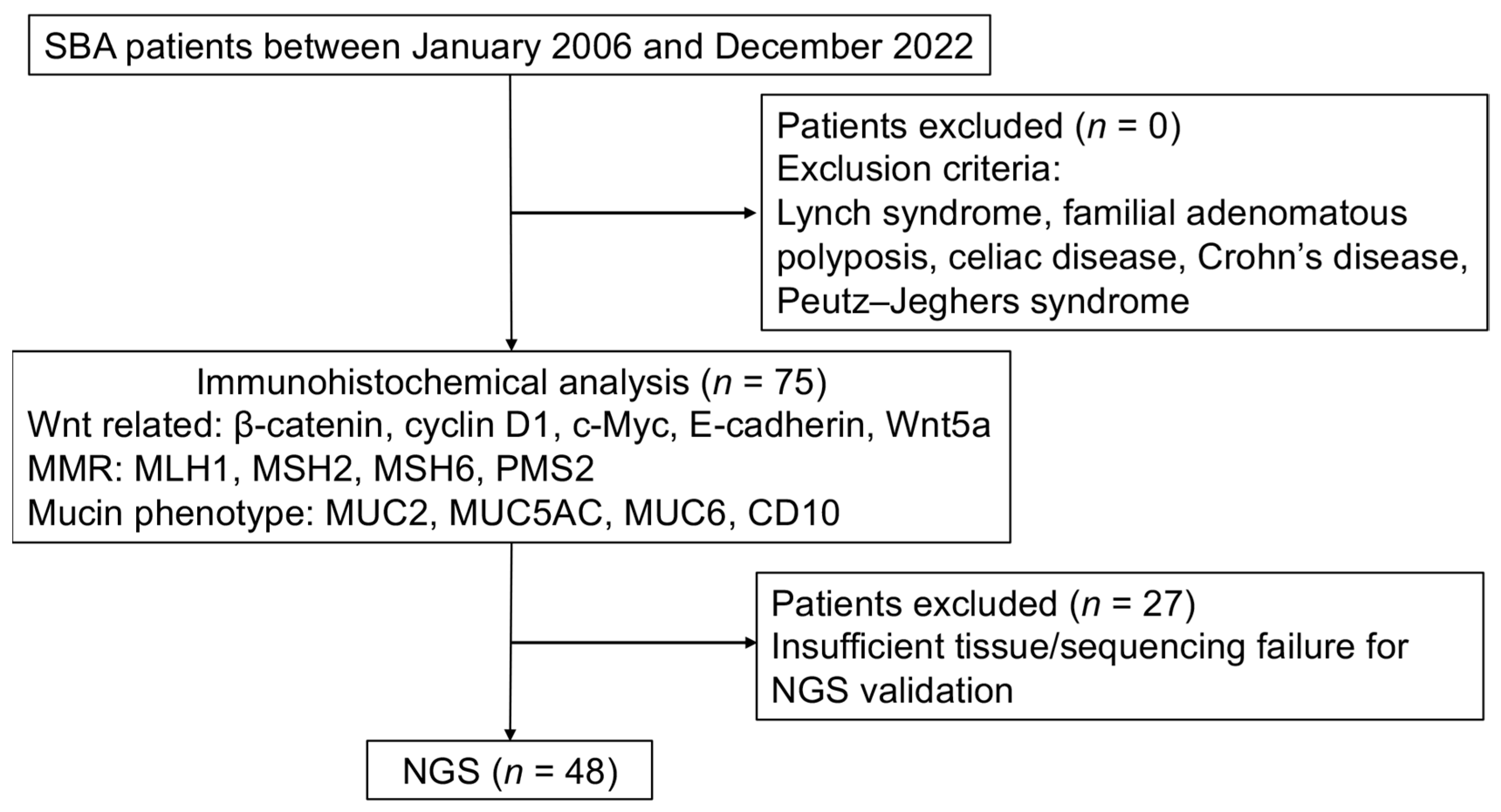
2.1. Patients and Tissue Samples
2.2. Immunohistochemical Analysis
2.3. Evaluation of Immunohistochemical Staining
2.4. Next-Generation Sequencing (NGS)
2.5. Statistical Analysis
3. Results
3.1. Demographic Data of Patients
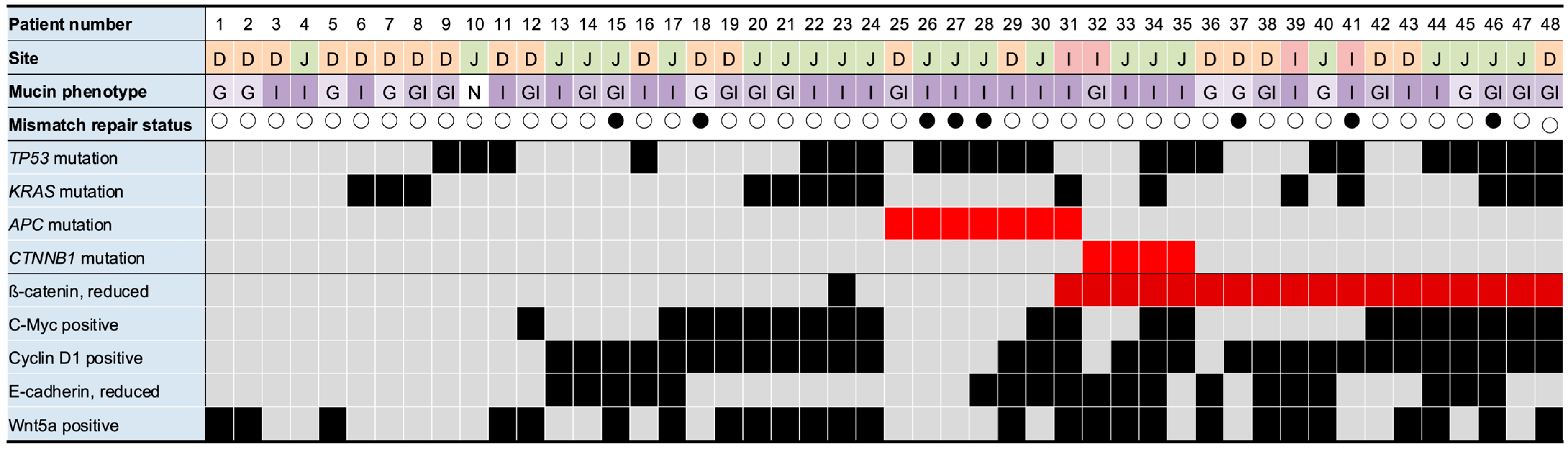
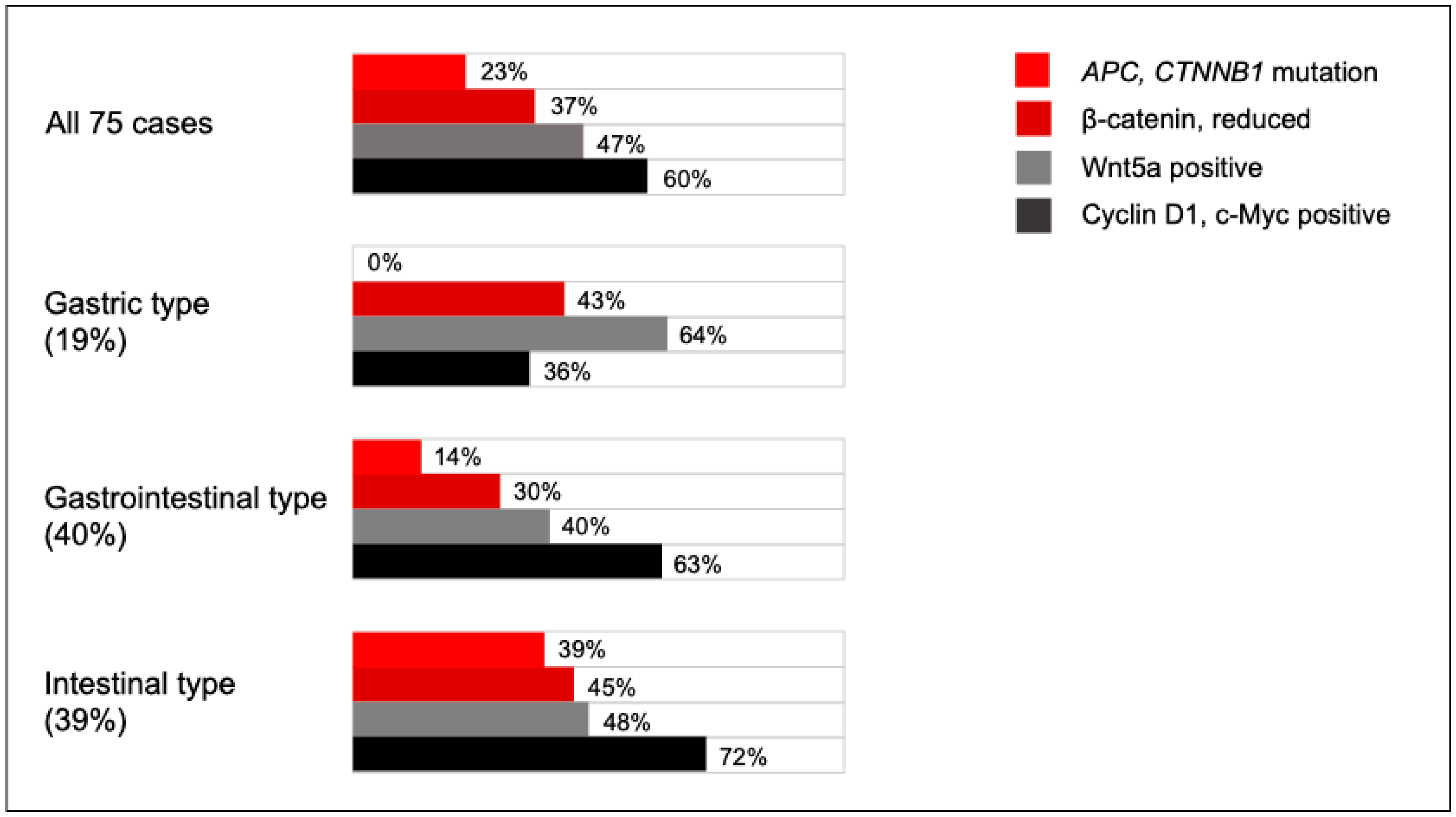
3.2. Localization of β-Catenin, Cyclin D1, c-Myc, E-Cadherin, and Wnt5a in SBA
3.3. Association Between Marker Expression and Clinicopathological Factors
3.4. Interrelationship Between Marker Expressions
3.5. Association Between Gene Mutations and Marker Expression
3.6. Comparative Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FFPE | Formalin-fixed paraffin-embedded |
| CSS | Cancer-specific survival |
| MD | Moderately differentiated tubular adenocarcinoma |
| MMR | DNA mismatch repair |
| NGS | Next-generation sequencing |
| NS | Not significantly different |
| PD | Poorly differentiated tubular adenocarcinoma |
| pT | Depth of invasion |
| SBA | Small bowel adenocarcinoma |
| WD | Well-differentiated tubular adenocarcinoma |
References
- Pedersen, K.S.; Raghav, K.; Overman, M.J. Small Bowel Adenocarcinoma: Etiology, Presentation, and Molecular Alterations. J. Natl. Compr. Cancer Netw. 2019, 17, 1135–1141. [Google Scholar] [CrossRef]
- Bouvier, A.M.; Robaszkiewicz, M.; Jooste, V.; Cariou, M.; Drouillard, A.; Bouvier, V.; Nousbaum, J.B. Trends in incidence of small bowel cancer according to histology: A population-based study. J. Gastroenterol. 2020, 55, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Falcone, R.; Romiti, A.; Filetti, M.; Roberto, M.; Righini, R.; Botticelli, A.; Pilozzi, E.; Ghidini, M.; Pizzo, C.; Mazzuca, F.; et al. Impact of tumor site on the prognosis of small bowel adenocarcinoma. Tumori J. 2019, 105, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Pandya, K.; Overman, M.J.; Gulhati, P. Molecular Landscape of Small Bowel Adenocarcinoma. Cancers 2022, 14, 1287. [Google Scholar] [CrossRef] [PubMed]
- Schrock, A.B.; Devoe, C.E.; McWilliams, R.; Sun, J.; Aparicio, T.; Stephens, P.J.; Ross, J.S.; Wilson, R.; Miller, V.A.; Ali, S.M.; et al. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017, 3, 1546–1553. [Google Scholar] [CrossRef]
- Lee, T.C.; Wima, K.; Morris, M.C.; Winer, L.K.; Sussman, J.J.; Ahmad, S.A.; Wilson, G.C.; Patel, S.H. Small Bowel Adenocarcinomas: Impact of Location on Survival. J. Surg. Res. 2020, 252, 116–124. [Google Scholar] [CrossRef]
- Wilhelm, A.; Galata, C.; Beutner, U.; Schmied, B.M.; Warschkow, R.; Steffen, T.; Brunner, W.; Post, S.; Marti, L. Duodenal localization is a negative predictor of survival after small bowel adenocarcinoma resection: A population-based, propensity score-matched analysis. J. Surg. Oncol. 2018, 117, 397–408. [Google Scholar] [CrossRef]
- Fujimori, S.; Hamakubo, R.; Hoshimoto, A.; Nishimoto, T.; Omori, J.; Akimoto, N.; Tanaka, S.; Tatsuguchi, A.; Iwakiri, K. Risk factors for small intestinal adenocarcinomas that are common in the proximal small intestine. World J. Gastroenterol. 2022, 28, 5658–5665. [Google Scholar] [CrossRef]
- Laforest, A.; Aparicio, T.; Zaanan, A.; Silva, F.P.; Didelot, A.; Desbeaux, A.; Le Corre, D.; Benhaim, L.; Pallier, K.; Aust, D.; et al. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur. J. Cancer 2014, 50, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Mackey, R.; Brown, N.; Church, J.; Burke, C.; Walsh, R.M. Outcome based on management for duodenal adenomas: Sporadic versus familial disease. J. Gastrointest. Surg. 2010, 14, 229–235. [Google Scholar] [CrossRef]
- Vanoli, A.; Grillo, F.; Furlan, D.; Arpa, G.; Grami, O.; Guerini, C.; Riboni, R.; Mastracci, L.; Di Sabatino, A. Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations. Int. J. Mol. Sci. 2021, 22, 4388. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Fujisaki, J.; Kasuga, A.; Omae, M.; Kubota, M.; Hirasawa, T.; Ishiyama, A.; Inamori, M.; Chino, A.; Yamamoto, Y.; et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: A study of follow-up surveillance. Am. J. Gastroenterol. 2011, 106, 357–364. [Google Scholar] [CrossRef]
- Wu, X.; Que, H.; Li, Q.; Wei, X. Wnt/β-catenin mediated signaling pathways in cancer: Recent advances, and applications in cancer therapy. Mol. Cancer 2025, 24, 171. [Google Scholar] [CrossRef]
- Li, C.; Furth, E.E.; Rustgi, A.K.; Klein, P.S. When You Come to a Fork in the Road, Take It: Wnt Signaling Activates Multiple Pathways Through the APC/Axin/GSK-3 Complex. Cells 2023, 12, 2256. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Schussler, O.; Hébert, J.L.; Vallée, A. Multiple Targets of the Canonical WNT/β-Catenin Signaling in Cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef]
- Kohn, A.D.; Moon, R.T. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium 2005, 38, 439–446. [Google Scholar] [CrossRef]
- Wong, S.C.C.; Lo, E.S.F.; Lee, K.C.; Chan, J.K.C.; Hsiao, W.L.W. Prognostic and diagnostic significance of β-catenin nuclear immunostaining in colorectal cancer. Clin. Cancer Res. 2004, 10, 1401–1408. [Google Scholar] [CrossRef]
- Hoshimoto, A.; Tatsuguchi, A.; Yamada, T.; Kuriyama, S.; Hamakubo, R.; Nishimoto, T.; Omori, J.; Akimoto, N.; Gudis, K.; Mitsui, K.; et al. Relationship Between Immunophenotypes, Genetic Profiles, and Clinicopathologic Characteristics in Small Bowel Adenocarcinoma. Am. J. Surg. Pathol. 2024, 48, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Tatsuguchi, A.; Yamada, T.; Ueda, K.; Furuki, H.; Hoshimoto, A.; Nishimoto, T.; Omori, J.; Akimoto, N.; Gudis, K.; Tanaka, S.; et al. Genetic analysis of Japanese patients with small bowel adenocarcinoma using next-generation sequencing. BMC Cancer 2022, 22, 723. [Google Scholar] [CrossRef]
- Furuki, H.; Yamada, T.; Takahashi, G.; Iwai, T.; Koizumi, M.; Shinji, S.; Yokoyama, Y.; Takeda, K.; Taniai, N.; Uchida, E. Evaluation of liquid biopsies for detection of emerging mutated genes in metastatic colorectal cancer. Eur. J. Surg. Oncol. 2018, 44, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.M.D.; Warren, B.F.; Mortensen, N.J.M.; Kim, H.C.; Biddolph, S.C.; Elia, G.; Beck, N.E.; Williams, G.T.; Shepherd, N.A.; Bateman, A.C.; et al. An insight into the genetic pathway of adenocarcinoma of the small intestine. Gut 2002, 50, 218–223. [Google Scholar] [CrossRef]
- Bläker, H.; Helmchen, B.; Bönisch, A.; Aulmann, S.; Penzel, R.; Otto, H.F.; Rieker, R.J. Mutational activation of the RAS-RAF-MAPK and the Wnt pathway in small intestinal adenocarcinomas. Scand. J. Gastroenterol. 2004, 39, 748–753. [Google Scholar] [CrossRef]
- Ota, R.; Sawada, T.; Tsuyama, S.; Sasaki, Y.; Suzuki, H.; Kaizaki, Y.; Hasatani, K.; Yamamoto, E.; Nakanishi, H.; Inagaki, S.; et al. Integrated genetic and epigenetic analysis of cancer-related genes in non-ampullary duodenal adenomas and intramucosal adenocarcinomas. J. Pathol. 2020, 252, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Ishizu, K.; Hashimoto, T.; Naka, T.; Yatabe, Y.; Kojima, M.; Kuwata, T.; Nonaka, S.; Oda, I.; Esaki, M.; Kudo, M.; et al. APC mutations are common in adenomas but infrequent in adenocarcinomas of the non-ampullary duodenum. J. Gastroenterol. 2021, 56, 988–998. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, O.J.; Jang, K.T.; Bae, Y.K.; Chung, J.Y.; Eom, D.W.; Kim, J.M.; Yu, E.; Hong, S.M. Combined loss of E-cadherin and aberrant β-catenin protein expression correlates with a poor prognosis for small intestinal adenocarcinomas. Am. J. Clin. Pathol. 2013, 139, 167–176. [Google Scholar] [CrossRef]
- Jun, S.Y.; Hong, S.M.; Jang, K.T. Prognostic Significance of Cyclin D1 Expression in Small Intestinal Adenocarcinoma. Cancers 2023, 15, 5032. [Google Scholar] [CrossRef]
- Bueno, M.L.P.; Saad, S.T.O.; Roversi, F.M. WNT5A in tumor development and progression: A comprehensive review. Biomed. Pharmacother. 2022, 155, 113599. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Wu, C. WNT5A: A double-edged sword in colorectal cancer progression. Mutat. Res. Rev. Mutat. Res. 2023, 792, 108465. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernández, E.; Velázquez, D.M.; Castañeda-Patlán, M.C.; Fuentes-García, G.; Fonseca-Camarillo, G.; Yamamoto-Furusho, J.K.; Romero-Avila, M.T.; García-Sáinz, J.A.; Robles-Flores, M. Canonical and non-canonical Wnt signaling are simultaneously activated by Wnts in colon cancer cells. Cell Signal 2020, 72, 109636. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Mehdawi, L.M.; Ghatak, S.; Chakraborty, P.; Sjölander, A.; Andersson, T. LGR5 Expression Predicting Poor Prognosis Is Negatively Correlated with WNT5A in Colon Cancer. Cells 2023, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Y.; Wang, Z.; Shen, H.; Xu, L.; Huang, C.; Tong, Y.; Shao, Y.; Zhang, H.; Fu, Z. Single-cell and spatial transcriptome profiling reveal CTHRC1+ fibroblasts promote EMT Through WNT5A signaling in colorectal cancer. J. Transl. Med. 2025, 23, 282. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Yahia, M.; Ahmed, Y.T.; Alfaki, M. WNT5A in Cancer: A Pan-Cancer Analysis Revealing Its Diagnostic and Prognostic Biomarker Potential. Cureus 2024, 16, e65190. [Google Scholar] [CrossRef]
- Howe, J.R.; Karnell, L.H.; Menck, H.R.; Scott-Conner, C. The American College of Surgeons Commission on Cancer and the American Cancer Society. Adenocarcinoma of the small bowel: Review of the National Cancer Data Base, 1985-1995. Cancer 1999, 86, 2693–2706. [Google Scholar] [CrossRef]
- Overman, M.J.; Hu, C.Y.; Wolff, R.A.; Chang, G.J. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: Analysis of the surveillance, epidemiology, and end results database. Cancer 2010, 116, 5374–5382. [Google Scholar] [CrossRef] [PubMed]

| Cyclin D1 | C-Myc | Reduced β-Catenin | Reduced E-Cadherin | Wnt5a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | No. (%) | p | No. (%) | p | No. (%) | p | No. (%) | p | No. (%) | p | |
| Age | |||||||||||
| <69 | 35 | 22 (63) | NS | 19 (58) | 0.038 | 15 (43) | NS | 14 (40) | NS | 18 (51) | NS |
| ≥69 | 40 | 23 (58) | 12 (30) | 13 (33) | 19 (48) | 17 (43) | |||||
| Sex | |||||||||||
| Female | 22 | 12 (55) | NS | 9 (41) | NS | 8 (36) | NS | 11 (50) | NS | 11 (50) | NS |
| Male | 53 | 33 (62) | 22 (42) | 20 (38) | 22 (42) | 24 (45) | |||||
| Site | |||||||||||
| Duodenum | 40 | 18 (45) | 0.018 | 13 (33) | NS | 11 (28) | 0.006 | 11 (28) | 0.009 | 15 (38) | NS |
| Jejunum | 30 | 23 (77) | 17 (57) | 12 (40) | 19 (63) | 18 (60) | |||||
| Ileum | 5 | 4 (80) | 1 (20) | 5 (100) | 3 (60) | 2 (40) | |||||
| Histologic Type | |||||||||||
| WD, MD | 60 | 36 (60) | NS | 24 (40) | NS | 20 (33) | NS | 22 (37) | 0.029 | 27 (45) | NS |
| PD | 10 | 7 (70) | 6 (60) | 5 (50) | 8 (80) | 4 (40) | |||||
| Mucinous | 5 | 2 (50) | 1 (20) | 3 (60) | 3 (60) | 4 (80) | |||||
| Depth of invasion (pT Factor) | |||||||||||
| pT1–3 | 62 | 36 (58) | NS | 24 (39) | NS | 20 (32) | NS | 23 (37) | 0.013 | 24 (39) | 0.004 |
| pT4 | 13 | 9 (69) | 7 (54) | 8 (62) | 10 (77) | 11 (85) | |||||
| Lymph Node Metastasis | |||||||||||
| N0 | 47 | 24 (51) | 0.053 | 17 (36) | NS | 10 (21) | <0.001 | 9 (19) | <0.001 | 12 (26) | <0.001 |
| Nx | 28 | 21 (75) | 14 (50) | 18 (64) | 24 (86) | 23 (82) | |||||
| Distant Metastasis | |||||||||||
| M0 | 59 | 33 (56) | NS | 24 (41) | NS | 16 (27) | 0.001 | 17 (29) | <0.001 | 23 (39) | 0.013 |
| Mx | 16 | 12 (75) | 7 (44) | 12 (75) | 16 (100) | 12 (75) | |||||
| TNM Stage | |||||||||||
| l | 26 | 13 (50) | NS | 7 (27) | NS | 6 (23) | 0.001 | 4 (15) | <0.001 | 3 (12) | <0.001 |
| II | 21 | 11 (52) | 10 (48) | 4 (19) | 5 (24) | 9 (43) | |||||
| III | 12 | 9 (75) | 7 (58) | 6 (50) | 8 (67) | 11 (92) | |||||
| IV | 16 | 12 (75) | 7 (44) | 12 (75) | 16 (100) | 12 (75) | |||||
| MMR Status | |||||||||||
| Proficient | 65 | 39 (60) | NS | 27 (42) | NS | 25 (39) | NS | 28 (43) | NS | 31 (28) | NS |
| Deficient | 10 | 6 (60) | 4 (40) | 3 (33) | 5 (50) | 4 (40) | |||||
| Mucin Phenotype | |||||||||||
| Gastric type | 14 | 5 (36) | 0.038 | 4 (29) | NS | 6 (43) | NS | 7 (50) | NS | 9 (64) | NS |
| Gastrointestinal type | 30 | 19 (63) | 14 (47) | 9 (30) | 8 (27) | 12 (40) | |||||
| Intestinal type | 29 | 21 (72) | 13 (45) | 13 (45) | 17 (59) | 14 (48) | |||||
| Null type | 2 | 0 | 0 | 0 | 1 (50) | 0 | |||||
| Cyclin D1 | C-Myc | E-Cadherin | Wnt5a | |
|---|---|---|---|---|
| β-Catenin | 0.002 | 0.022 | 0.003 | NS |
| Cyclin D1 | 0.001 | 0.001 | 0.021 | |
| C-Myc | NS | NS | ||
| E-cadherin | 0.003 |
| Variables | Categories | Hazard Ratio (95% CI) | p Value |
|---|---|---|---|
| Univariate Analysis | |||
| Histologic Type | PD, mucinous vs. WD, MD | 2.248 (0.915–5.523) | 0.077 |
| pT Factor | pT4 vs. pT1–3 | 4.021 (1.676–9.646) | 0.002 |
| Lymph Node Metastasis | positive vs. negative | 26.87 (6.253–115.47) | <0.001 |
| β-Catenin | reduced vs. preserved | 3.720 (1.558–8.886) | 0.003 |
| Cyclin D1 | positive vs. negative | 1.256 (0.527–2.995) | NS |
| C-Myc | positive vs. negative | 1.275 (0.549–2.958) | NS |
| Wnt5a | positive vs. negative | 4.207 (1.640–10.79) | 0.003 |
| E-Cadherin | reduced vs. preserved | 10.56 (3.122–35.75) | <0.001 |
| β-Catenin/E-Cadherin | both reduced vs. others | 8.155 (3.398–19.58) | <0.001 |
| Multivariate Analysis | |||
| pT Factor | pT4 vs. pT1–3 | 1.258 (0.512–3.096) | NS |
| Lymph Node Metastasis | positive vs. negative | 16.52 (3.494–78.12) | <0.001 |
| β-Catenin/E-cadherin | both reduced vs. others | 2.576 (1.020–6.507) | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimoto, T.; Tatsuguchi, A.; Yamada, T.; Kuriyama, S.; Hoshimoto, A.; Omori, J.; Akimoto, N.; Gudis, K.; Mitsui, K.; Tanaka, S.; et al. Insight into the Wnt Pathway in Sporadic Small Bowel Adenocarcinoma. Cancers 2025, 17, 2965. https://doi.org/10.3390/cancers17182965
Nishimoto T, Tatsuguchi A, Yamada T, Kuriyama S, Hoshimoto A, Omori J, Akimoto N, Gudis K, Mitsui K, Tanaka S, et al. Insight into the Wnt Pathway in Sporadic Small Bowel Adenocarcinoma. Cancers. 2025; 17(18):2965. https://doi.org/10.3390/cancers17182965
Chicago/Turabian StyleNishimoto, Takayoshi, Atsushi Tatsuguchi, Takeshi Yamada, Sho Kuriyama, Aitoshi Hoshimoto, Jun Omori, Naohiko Akimoto, Katya Gudis, Keigo Mitsui, Shu Tanaka, and et al. 2025. "Insight into the Wnt Pathway in Sporadic Small Bowel Adenocarcinoma" Cancers 17, no. 18: 2965. https://doi.org/10.3390/cancers17182965
APA StyleNishimoto, T., Tatsuguchi, A., Yamada, T., Kuriyama, S., Hoshimoto, A., Omori, J., Akimoto, N., Gudis, K., Mitsui, K., Tanaka, S., Fujimori, S., Hatori, T., Shimizu, A., & Atsukawa, M. (2025). Insight into the Wnt Pathway in Sporadic Small Bowel Adenocarcinoma. Cancers, 17(18), 2965. https://doi.org/10.3390/cancers17182965






