Characterizing Trends of Lymphedema After Axillary Lymph Node Dissection with and Without Immediate Lymphatic Reconstruction
Simple Summary
Abstract
1. Introduction
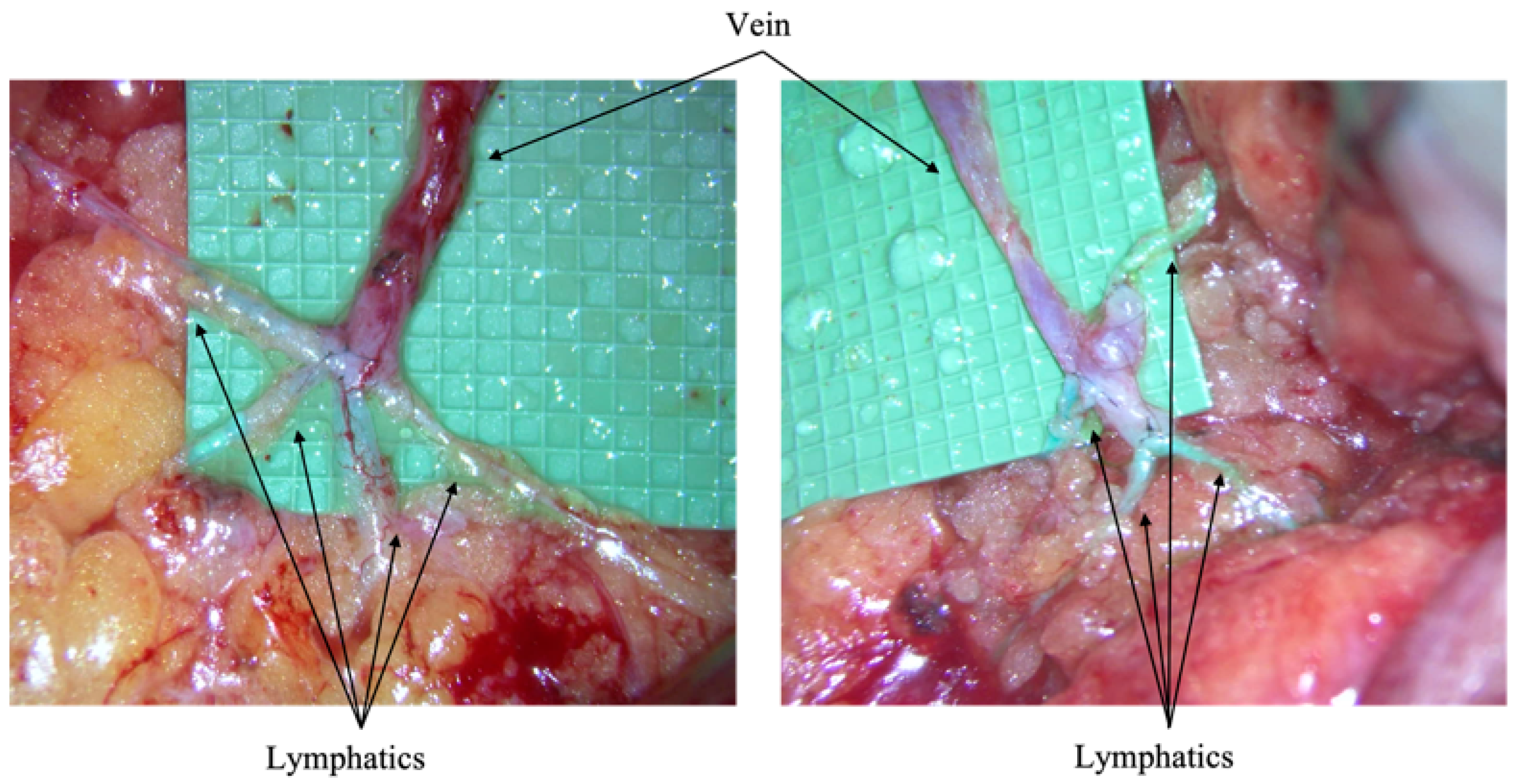
Immediate Lymphatic Reconstruction Technique
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahad Ullah, M. Breast cancer: Current perspectives on the disease status. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; pp. 51–64. [Google Scholar] [CrossRef]
- Beck, A.C.; Morrow, M. Axillary lymph node dissection: Dead or still alive? Breast 2023, 69, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Otsubo, R.; Shinohara, S.; Morita, M.; Kuba, S.; Matsumoto, M.; Nagayasu, T. Lymphedema after axillary lymph node dissection in breast cancer: Prevalence and risk factors-a single-center retrospective study. Lymphat. Res. Biol. 2022, 20, 600–606. [Google Scholar] [CrossRef]
- Bittar, S.; Simman, R.; Lurie, F. Lymphedema: A practical approach and clinical update. Wounds 2020, 32, 86–92. [Google Scholar] [PubMed]
- Liao, S.; von der Weid, P.Y. Lymphatic system: An active pathway for immune protection. Semin. Cell Dev. Biol. 2015, 38, 83–89. [Google Scholar] [CrossRef]
- Engin, O.; Sahin, E.; Saribay, E.; Dilek, B.; Akalin, E. Risk factors for developing upper limb cellulitis after breast cancer treatment. Lymphology 2022, 55, 77–83. [Google Scholar] [CrossRef]
- Vignes, S.; Poizeau, F.; Dupuy, A. Cellulitis risk factors for patients with primary or secondary lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2022, 10, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, B.A.J.; Padera, T.P.; Lund, A.W. Editorial: Regulation of immune function by the lymphatic vasculature. Front. Immunol. 2019, 10, 2597. [Google Scholar] [CrossRef]
- Johnson, L.A. In Sickness and in health: The immunological roles of the lymphatic system. Int. J. Mol. Sci. 2021, 22, 4458. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Rivera, K.K. Estimating the population burden of lymphedema. Ann. N. Y. Acad. Sci. 2008, 1131, 147–154. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.A.; Wright, M.J.; Morris, K.T.; Sampson, M.R.; Brockway, J.P.; Hurley, K.E.; Riedel, E.R.; Van Zee, K.J. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Patient perceptions and precautionary behaviors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 5220–5226. [Google Scholar] [CrossRef]
- Kalemikerakis, I.; Evaggelakou, A.; Kavga, A.; Vastardi, M.; Konstantinidis, T.; Govina, O. Diagnosis, treatment and quality of life in patients with cancer-related lymphedema. J. Buon. 2021, 26, 1735–1741. [Google Scholar]
- Taghian, N.R.; Miller, C.L.; Jammallo, L.S.; O’Toole, J.; Skolny, M.N. Lymphedema following breast cancer treatment and impact on quality of life: A review. Crit. Rev. Oncol. Hematol. 2014, 92, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.A.; Sinha, M.; Lester, M.; Fisher, C.S.; Sen, C.K.; Hassanein, A.H. Immediate lymphatic reconstruction to prevent breast cancer-related lymphedema: A systematic review. Adv. Wound Care 2022, 11, 382–391. [Google Scholar] [CrossRef]
- Coriddi, M.; Mehrara, B.; Skoracki, R.; Singhal, D.; Dayan, J.H. Immediate lymphatic reconstruction: Technical points and literature review. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3431. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Toyserkani, N.M.; Sørensen, J.A. The effect of prophylactic lymphovenous anastomosis and shunts for preventing cancer-related lymphedema: A systematic review and meta-analysis. Microsurgery 2018, 38, 576–585. [Google Scholar] [CrossRef]
- Spiguel, L.; Shaw, C.; Katz, A.; Guo, L.; Chen, H.C.; Lee, B.T.; Singhal, D. Fluorescein isothiocyanate: A novel application for lymphatic surgery. Ann. Plast. Surg. 2017, 78 (Suppl. 5), S296–S298. [Google Scholar] [CrossRef] [PubMed]
- Coroneos, C.J.; Wong, F.C.; DeSnyder, S.M.; Shaitelman, S.F.; Schaverien, M.V. Correlation of L-Dex Bioimpedance spectroscopy with limb volume and lymphatic function in lymphedema. Lymphat. Res. Biol. 2019, 17, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wiser, I.; Mehrara, B.J.; Coriddi, M.; Kenworthy, E.; Cavalli, M.; Encarnacion, E.; Dayan, J.H. Preoperative Assessment of upper extremity secondary lymphedema. Cancers 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Whitworth, P.; Valente, S.; Schwarz, G.S.; Kruse, M.; Kohli, M.; Brownson, K.; Lawson, L.; Dupree, B.; Vicini, F.A. Bioimpedance spectroscopy for breast cancer-related lymphedema assessment: Clinical practice guidelines. Breast Cancer Res. Treat. 2023, 198, 1–9. [Google Scholar] [CrossRef]
- Norman, S.A.; Localio, A.R.; Kallan, M.J.; Weber, A.L.; Torpey, H.A.; Potashnik, S.L.; Miller, L.T.; Fox, K.R.; DeMichele, A.; Solin, L.J. Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2734–2746. [Google Scholar] [CrossRef]
- Koelmeyer, L.A.; Gaitatzis, K.; Dietrich, M.S.; Shah, C.S.; Boyages, J.; McLaughlin, S.A.; Taback, B.; Stolldorf, D.P.; Elder, E.; Hughes, T.M.; et al. Risk factors for breast cancer-related lymphedema in patients undergoing 3 years of prospective surveillance with intervention. Cancer 2022, 128, 3408–3415. [Google Scholar] [CrossRef]
- Ren, Y.; Kebede, M.A.; Ogunleye, A.A.; Emerson, M.A.; Evenson, K.R.; Carey, L.A.; Troester, M.A. Burden of lymphedema in long-term breast cancer survivors by race and age. Cancer 2022, 128, 4119–4128. [Google Scholar] [CrossRef]
- Kwan, M.L.; Darbinian, J.; Schmitz, K.H.; Citron, R.; Partee, P.; Kutner, S.E.; Kushi, L.H. Risk factors for lymphedema in a prospective breast cancer survivorship study: The Pathways Study. Arch. Surg. 2010, 145, 1055–1063. [Google Scholar] [CrossRef]
- Fu, J.; Chen, R.; He, L.; Bao, L.; Lin, Z.; Jiang, W.; Zhang, J.; Wang, C.; Lin, Y. Factors affecting lymphedema after neoadjuvant chemotherapy and axillary dissection in female breast cancer patients: A retrospective cohort study based on the Chinese population. Front. Oncol. 2024, 14, 1436748. [Google Scholar] [CrossRef]
- Jia, M.; Pan, L.; Yang, H.; Gao, J.; Guo, F. Impact of neoadjuvant chemotherapy on breast cancer-related lymphedema after axillary lymph node dissection: A retrospective cohort study. Breast Cancer Res. Treat. 2024, 204, 223–235. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lim, Y.C.; Yeo, J.; Kim, S.Y.; Lee, Y.J.; Ha, I.H. Risk of Lymphedema and Death after Lymph Node Dissection with Neoadjuvant and Adjuvant Treatments in Patients with Breast Cancer: An Eight-Year Nationwide Cohort Study. Healthcare 2023, 11, 1833. [Google Scholar] [CrossRef]
- Boccardo, F.; Casabona, F.; De Cian, F.; Friedman, D.; Murelli, F.; Puglisi, M.; Campisi, C.C.; Molinari, L.; Spinaci, S.; Dessalvi, S.; et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: Over 4 years follow-up. Microsurgery 2014, 34, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.J.; Saeg, F.; Meade, A.; Kumar, T.; Toraih, E.A.; Chaffin, A.E.; Homsy, C. Immediate lymphatic reconstruction for prevention of secondary lymphedema: A meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.S.; Murphy, A.I.; Ishtihar, S.; Peysakhovich, A.; Taback, B.; Grant, R.T.; Ascherman, J.A.; Feldman, S.; Rohde, C.H. Lymphatic microsurgical preventive healing approach for the primary prevention of lymphedema: A 4-year follow-up. Plast. Reconstr. Surg. 2023, 151, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Asban, A.; Granoff, M.D.; Kang, C.O.; Lee, B.T.; Chatterjee, A.; Singhal, D. Is immediate lymphatic reconstruction cost-effective? Ann. Surg. 2021, 274, e581–e588. [Google Scholar] [CrossRef]


| Category | Subcategory | ILR n = 44 | Non-ILR n = 142 |
|---|---|---|---|
| Age (years) | 48 ± 11 | 55 ± 12 | |
| Menopausal status | Premenopausal | 29 | 52 |
| Postmenopausal | 15 | 90 | |
| Clinical tumor stage | cT1 | 4 | 21 |
| cT2 | 23 | 80 | |
| cT3 | 12 | 26 | |
| cT4 | 5 | 15 | |
| Clinical nodal stage | cN1 | 33 | 120 |
| cN2 | 5 | 16 | |
| cN3 | 6 | 6 | |
| Histology | Invasive ductal carcinoma | 40 | 126 |
| Invasive lobular carcinoma | 4 | 16 | |
| Grade | 1 | 0 | 5 |
| 2 | 24 | 78 | |
| 3 | 20 | 59 | |
| Biomarkers | ER-positive HER2-negative | 28 | 87 |
| HER2-positive | 11 | 31 | |
| Triple negative | 5 | 24 | |
| Neoadjuvant chemotherapy | 38 | 102 | |
| Initial axillary operation | Targeted axillary dissection | 15 | 37 |
| Axillary lymph node dissection | 29 | 105 | |
| Pathologic tumor stage | pT0 | 10 | 33 |
| pT1 | 16 | 47 | |
| pT2 | 13 | 50 | |
| pT3 | 5 | 10 | |
| pT4 | 0 | 2 | |
| Pathologic nodal stage | pN0 | 12 | 31 |
| pN1 | 17 | 67 | |
| pN2 | 13 | 31 | |
| pN3 | 2 | 13 | |
| Residual cancer burden | Class 0 | 6 | 27 |
| Class I | 2 | 9 | |
| Class II | 11 | 25 | |
| Class III | 15 | 33 | |
| Number of positive lymph nodes | 4 ± 5 | 4 ± 3 | |
| Number of lymph nodes removed | 16 ± 7 | 15 ± 6 | |
| Extranodal Extension | 12 | 59 | |
| Adjuvant systemic therapy | 44 | 134 | |
| Adjuvant radiation | 42 | 139 | |
| Lymphedema | 8 | 44 | |
| Distant recurrence | 3 | 16 | |
| Mean follow-up (months) | 33 ± 9 | 39 ± 5 | |
| Death | 2 | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vangsness, K.L.; Sam, A.-P.; Chang, J.; Mehta, Y.A.; Chu, M.W.; Agko, M.; Carré, A.L. Characterizing Trends of Lymphedema After Axillary Lymph Node Dissection with and Without Immediate Lymphatic Reconstruction. Cancers 2025, 17, 2964. https://doi.org/10.3390/cancers17182964
Vangsness KL, Sam A-P, Chang J, Mehta YA, Chu MW, Agko M, Carré AL. Characterizing Trends of Lymphedema After Axillary Lymph Node Dissection with and Without Immediate Lymphatic Reconstruction. Cancers. 2025; 17(18):2964. https://doi.org/10.3390/cancers17182964
Chicago/Turabian StyleVangsness, Kella L., Andre-Philippe Sam, Jeff Chang, Yash A. Mehta, Michael W. Chu, Mouchammed Agko, and Antoine L. Carré. 2025. "Characterizing Trends of Lymphedema After Axillary Lymph Node Dissection with and Without Immediate Lymphatic Reconstruction" Cancers 17, no. 18: 2964. https://doi.org/10.3390/cancers17182964
APA StyleVangsness, K. L., Sam, A.-P., Chang, J., Mehta, Y. A., Chu, M. W., Agko, M., & Carré, A. L. (2025). Characterizing Trends of Lymphedema After Axillary Lymph Node Dissection with and Without Immediate Lymphatic Reconstruction. Cancers, 17(18), 2964. https://doi.org/10.3390/cancers17182964






