Real-World Outcomes Between Perioperative Chemotherapy (FLOT) and Preoperative Concurrent Chemoradiotherapy (CROSS) in Localized Esophageal and Esophagogastric Junction Adenocarcinoma: A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives and Endpoints
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Treatment Information
3.3. Effectiveness
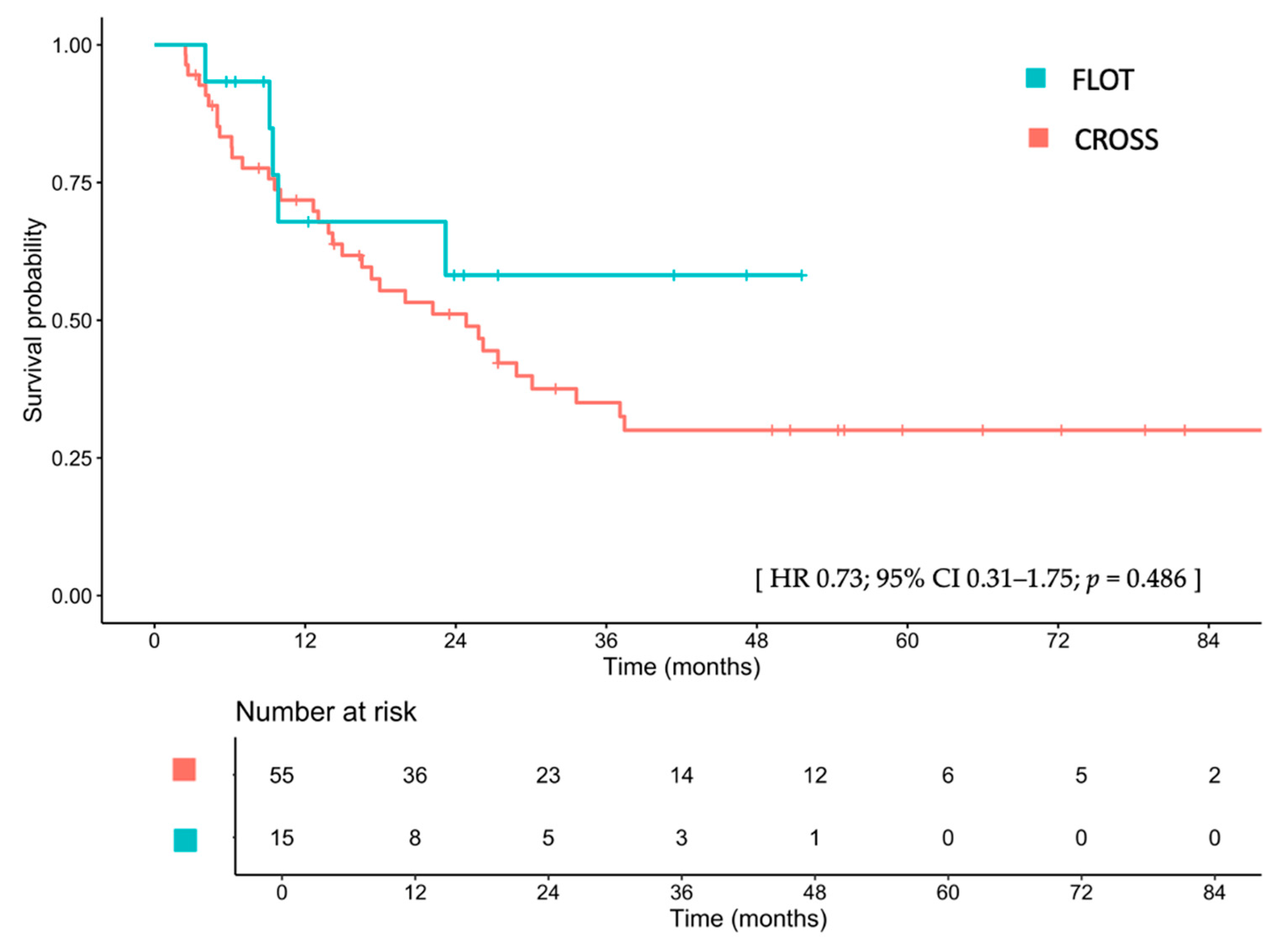
3.3.1. OS
3.3.2. EFS
3.4. Response Rates
3.5. Pathological Response Rates
3.6. Pattern of Recurrence and Subsequent Treatment
3.7. Postoperative Complications
3.8. Systemic Treatment Toxicities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CCRT | Concurrent chemoradiotherapy |
| CI | Confidence interval |
| CROSS | Chemoradiotherapy for esophageal cancer followed by surgery study CT |
| CT | Computed tomography |
| EFS | Event-free survival |
| DFS | Disease-free survival |
| ECOG | Eastern Cooperative Oncology Group; EGJ, esophagogastric junction |
| FLOT | Fluorouracil, leucovorin, oxaliplatin, and docetaxel |
| G-CSF | Granulocyte colony-stimulating factor |
| HR | Hazard ratio |
| IQR | Interquartile ranges |
| ORR | Objective response rate |
| OS | Overall survival |
| pCR | Pathological complete response |
| PET | Positron emission tomography |
| RCTs | Randomized controlled trials |
| SD | Standard deviations |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mukkamalla, S.K.R.; Singh, R.; Lyons, S. Esophageal cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459267/ (accessed on 2 March 2025).
- Arnold, M.; Ferlay, J.; Van Berge Henegouwen, M.I.; Soerjomataram, I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 2020, 69, 1564–1571. [Google Scholar] [CrossRef]
- Van Rossum, P.S.N.; Mohammad, N.H.; Vleggaar, F.P.; Van Hillegersberg, R. Treatment for unresectable or metastatic oesophageal cancer: Current evidence and trends. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 235–249. [Google Scholar] [CrossRef]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Devkota, M.; Chaudhary, M. Management of locally advanced esophageal cancer. JNMA J. Nepal Med Assoc. 2021, 59, 409–416. [Google Scholar] [CrossRef]
- Tsuji, T.; Matsuda, S.; Takeuchi, M.; Kawakubo, H.; Kitagawa, Y. Updates of perioperative multidisciplinary treatment for surgically resectable esophageal cancer. Jpn. J. Clin. Oncol. 2023, 53, 645–652. [Google Scholar] [CrossRef]
- Shoji, Y.; Koyanagi, K.; Kanamori, K.; Tajima, K.; Ogimi, M.; Yatabe, K.; Yamamoto, M.; Kazuno, A.; Nabeshima, K.; Nakamura, K.; et al. Current status and future perspectives for the treatment of resectable locally advanced esophagogastric junction cancer: A narrative review. World J. Gastroenterol. 2023, 29, 3758–3769. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Kurokawa, Y.; Doki, Y. Surgical and perioperative treatments for esophagogastric junction cancer. Ann. Thorac. Cardiovasc. Surg. 2024, 30, 24–00056. [Google Scholar] [CrossRef]
- Yanagimoto, Y.; Kurokawa, Y.; Doki, Y.; Yoshikawa, T.; Boku, N.; Terashima, M. Surgical and perioperative treatment strategy for resectable esophagogastric junction cancer. Jpn. J. Clin. Oncol. 2022, 52, 417–424. [Google Scholar] [CrossRef]
- Van Den Ende, T.; Smyth, E.; Hulshof, M.C.C.M.; Van Laarhoven, H.W.M. Chemotherapy and novel targeted therapies for operable esophageal and gastroesophageal junctional cancer. Best Pract. Res. Clin. Gastroenterol. 2018, 36–37, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Iams, W.T.; Villaflor, V.M. Neoadjuvant treatment for locally invasive esophageal cancer. World J. Surg. 2017, 41, 1719–1725. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; Van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Möhring, C.; Mańczak, A.; Timotheou, A.; Sadeghlar, F.; Zhou, T.; Mahn, R.; Monin, M.B.; Toma, M.; Feldmann, G.; Brossart, P.; et al. Perioperative therapy with FLOT4 significantly increases survival in patients with gastroesophageal and gastric cancer in a large real-world cohort. Int. J. Cancer 2023, 153, 609–622. [Google Scholar] [CrossRef]
- Sisic, L.; Crnovrsanin, N.; Nienhueser, H.; Jung, J.O.; Schiefer, S.; Haag, G.M.; Bruckner, T.; Schneider, M.; Müller-Stich, B.P.; Büchler, M.W.; et al. Perioperative chemotherapy with 5-FU, leucovorin, oxaliplatin, and docetaxel (FLOT) for esophagogastric adenocarcinoma: Ten years real-life experience from a surgical perspective. Langenbecks Arch. Surg. 2023, 408, 81. [Google Scholar] [CrossRef]
- Han, J.; Wang, Z.; Liu, C. Survival and complications after neoadjuvant chemotherapy or chemoradiotherapy for esophageal cancer: A meta-analysis. Future Oncol. 2021, 17, 2257–2274. [Google Scholar] [CrossRef]
- Ronellenfitsch, U.; Friedrichs, J.; Barbier, E.; Bass, G.A.; Burmeister, B.; Cunningham, D.; Eyck, B.M.; Grilli, M.; Hofheinz, R.D.; Kieser, M.; et al. Preoperative chemoradiotherapy vs chemotherapy for adenocarcinoma of the esophagogastric junction: A network meta-analysis. JAMA Netw. Open 2024, 7, e2425581. [Google Scholar] [CrossRef] [PubMed]
- Grizzi, G.; Petrelli, F.; Di Bartolomeo, M.; Viti, M.; Texeira Moraes, M.; Luciani, A.; Passalacqua, R.; Ghidini, M.; Tomasello, G.; Baiocchi, G.L.; et al. Preferred neoadjuvant therapy for gastric and gastroesophageal junction adenocarcinoma: A systematic review and network meta-analysis. Gastric Cancer 2022, 25, 982–987. [Google Scholar] [CrossRef]
- Reynolds, J.V. Neoadjuvant chemoradiation versus perioperative chemotherapy for oeosphageal adenocarcinoma. Br. J. Surg. 2023, 110, 1681–1682. [Google Scholar] [CrossRef]
- Shariff, B.; Mehta, R. FLOT or CROSS for gastroesophageal junction cancers-is the debate over yet? Chin. Clin. Oncol. 2023, 12, 24. [Google Scholar] [CrossRef]
- Von Döbeln, G.A.; Klevebro, F.; Jacobsen, A.B.; Johannessen, H.O.; Nielsen, N.H.; Johnsen, G.; Hatlevoll, I.; Glenjen, N.I.; Friesland, S.; Lundell, L.; et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: Long-term results of a randomized clinical trial. Dis. Esophagus 2019, 32, doy078. [Google Scholar] [CrossRef]
- Reynolds, J.V.; Preston, S.R.; O’Neill, B.; Lowery, M.A.; Baeksgaard, L.; Crosby, T.; Cunningham, M.; Cuffe, S.; Griffiths, G.O.; Parker, I.; et al. Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): An open-label, randomised, phase 3 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Walz, M.K.; Riera-Knorrenschild, J.; Stuschke, M.; Sandermann, A.; Bitzer, M.; Wilke, H.; Budach, W. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur. J. Cancer 2017, 81, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, J.; Brunner, T.; Schmoor, C.; Bronsert, P.; Kulemann, B.; Claus, R.; Utzolino, S.; Izbicki, J.R.; Gockel, I.; Gerdes, B.; et al. Perioperative chemotherapy or preoperative chemoradiotherapy in esophageal cancer. N. Engl. J. Med. 2025, 392, 323–335. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Subbiah, V.; Chuang, H.H.; Gambhire, D.; Kairemo, K. Defining clinical response criteria and early response criteria for precision oncology: Current state-of-the-art and future perspectives. Diagnostics 2017, 7, 10. [Google Scholar] [CrossRef]
- Ryan, R.; Gibbons, D.; Hyland, J.M.P.; Treanor, D.; White, A.; Mulcahy, H.E.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef]
- Leong, T.; Smithers, B.M.; Haustermans, K.; Michael, M.; Gebski, V.; Miller, D.; Zalcberg, J.; Boussioutas, A.; Findlay, M.; O’Connell, R.L.; et al. TOPGEAR: A randomized, Phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: Interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann. Surg. Oncol. 2017, 24, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- De Silva Sewastjanow, M.; Rogers, J.E.; Hofstetter, W.L.; Ajani, J.A. Esophageal cancer: Is the CROSS strategy ready for history books? J. Thorac. Cardiovasc. Surg. 2023, 165, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.J.D.; Ruurda, J.P.; van Diest, P.J.; van Hillegersberg, R.; Ten Kate, F.J.W. The significance of the HER-2 status in esophageal adenocarcinoma for survival: An immunohistochemical and an in situ hybridization study. Ann. Oncol. 2013, 24, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- SPACE-FLOT Investigators. Pathological response guides adjuvant 5-fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy in surgically resected gastro-oesophageal cancer (SPACE-FLOT): International cohort study. Br. J. Surg. 2025, 112, znaf056. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hoefnagel, S.J.M.; Krishnadath, K.K. Molecular Biology and Clinical Management of Esophageal Adenocarcinoma. Cancers 2023, 15, 5410. [Google Scholar] [CrossRef]
- Liu, K.; Jiao, Y.L.; Shen, L.Q.; Chen, P.; Zhao, Y.; Li, M.X.; Gu, B.L.; Lan, Z.J.; Ruan, H.J.; Liu, Q.W.; et al. A Prognostic Model Based on mRNA Expression Analysis of Esophageal Squamous Cell Carcinoma. Front. Bioeng. Biotechnol. 2022, 10, 823619. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Ma, Q.L.; Huang, W.; Liu, X.; Qiu, L.H.; Lin, P.; Long, H.; Zhang, L.J.; Ma, G.W. A prognostic model for stratification of stage IB/IIA esophageal squamous cell carcinoma: A retrospective study. BMC Gastroenterol. 2021, 21, 59. [Google Scholar] [CrossRef]

| Characteristics | FLOT (n = 15) | CROSS (n = 55) | p-Value |
|---|---|---|---|
| Age, years (SD) | 62.7 (9.7) | 66.8 (9.4) | 0.141 |
| Age ≥ 65 years, n (%) | 7 (46.7) | 36 (65.5) | 0.305 |
| Male, n (%) | 12 (80.0) | 49 (89.1) | 0.392 |
| BMI, n (%) | 0.809 | ||
| 18.5–24.9 kg/m2 | 6 (40.0) | 18 (32.7) | |
| 25.0–29.9 kg/m2 | 6 (40.0) | 22 (40.0) | |
| ≥30 kg/m2 | 3 (20.0) | 15 (27.3) | |
| ECOG PS, n (%) | 0.135 | ||
| 0 | 12 (80.0) | 28 (50.9) | |
| 1 | 3 (20.0) | 26 (47.2) | |
| 2 | 0 (0) | 1 (1.8) | |
| Comorbidities, n (%) # | 7 (46.7) | 41 (74.5) | 0.059 |
| Hypertension | 5 (33.3) | 34 (61.8) | |
| Diabetes mellitus | 3 (20.0) | 6 (10.9) | |
| Cirrhosis | 1 (6.7) | 0 (0) | |
| Chronic kidney disease | 0 (0) | 3 (5.5) | |
| COPD | 0 (0) | 6 (10.9) | |
| Coronary artery disease | 0 (0) | 9 (16.4) | |
| Cerebrovascular disease | 0 (0) | 1 (1.8) | |
| Others | 1 (6.7) | 11 (20.0) | |
| Clinical T stage, n (%) | 0.012 | ||
| T2 | 3 (20.0) | 4 (7.3) | |
| T3 | 10 (66.7) | 51 (92.7) | |
| T4 | 2 (13.3) | 0 (0) | |
| Clinical N stage, n (%) | 0.427 | ||
| N0 | 10 (66.7) | 28 (50.9) | |
| N+ | 5 (33.3) | 27 (49.1) | |
| Tumor location, n (%) | <0.001 | ||
| Distal esophagus | 1 (6.7) | 26 (47.3) | |
| EGJ, Siewert I | 0 (0) | 14 (25.5) | |
| EGJ, Siewert II | 14 (93.3) | 15 (27.3) | |
| Tumor differentiation, n (%) | 0.681 | ||
| Well | 0 (0) | 3 (5.5) | |
| Moderately | 5 (33.3) | 23 (41.8) | |
| Poorly | 10 (66.7) | 29 (52.7) | |
| HER2 overexpression status, n (%) | n = 15 | n = 40 | 0.091 |
| Positive | 0 (0) | 8 (20.0) | |
| Negative | 15 (100) | 32 (80.0) | |
| MMR status, n (%) | n = 15 | n = 38 | 0.568 |
| Proficient | 15 (100) | 34 (89.5) | |
| Deficient | 0 (0) | 4 (10.5) | |
| PD-L1 status, n (%) | 0.355 | ||
| CPS ≥1 | 3 (20.0) | 5 (9.1) | |
| Not tested | 12 (80.0) | 50 (90.9) | |
| Preoperative feeding tube, n (%) | 0.005 | ||
| Percutaneous jejunostomy | 2 (13.3) | 29 (52.7) | |
| Nasojejunal tube | 1 (6.7) | 1 (1.8) | |
| Nasogastric tube | 1 (6.7) | 0 (0) | |
| Baseline laboratory values | |||
| Hemoglobin, g/dL (SD) | 12.7 (2.0) | 13.5 (1.8) | 0.147 |
| Albumin, g/dL (IQR) | 3.9 (3.8, 4.2) | 3.8 (3.5, 4.0) | 0.175 |
| CrCl, mL/min (SD) | 96.5 (23) | 103.5 (29) | 0.517 |
| FLOT (n = 15) | CROSS (n = 55) | |
|---|---|---|
| Number of preoperative cycles, n (%) | ||
| Complete | 14 (93.3) | 51 (92.7) |
| Incomplete | 1 (7.7) | 4 (7.3) |
| Number of postoperative cycles, n (%) | - - - | |
| Complete | 7 (46.7) | |
| Incomplete | 1 (7.7) | |
| No | 7 (46.7) | |
| Dose reduction, n (%) | 9 (60.0) | 1 (1.8) |
| Discontinuation, n (%) * | ||
| Complete | 7 (46.7) | 52 (94.5) |
| Postoperative disease progression | 1 (6.7) | 0 (0) |
| Death | 2 (13.3) | 0 (0) |
| Declined ECOG PS | 2 (13.3) | 1 (1.8) |
| Patient’s preference | 2 (13.3) | 0 (0) |
| Infection | 0 (0) | 1 (1.8) |
| Prolonged neutropenia | 0 (0) | 1 (1.8) |
| Postoperative complication | 1 (6.7) | 0 (0) |
| Median time to surgery after preop treatment, months (range) | 2.07 (1.60–4.30) | 2.23 (0.92–5.32) |
| Resection, n (%) | 13 (86.7) | 37 (67.3) |
| Resection status, n (%) | ||
| R0 | 13 (100) | 36 (97.3) |
| R1 | 0 (0) | 1 (2.7) |
| Resection type, n (%) | ||
| Ivor Lewis esophagectomy | 2 (15.4) | 36 (97.3) |
| Esophagectomy and gastrectomy | 3 (23.1) | 0 (0) |
| Total gastrectomy | 5 (38.5) | 1 (2.7) |
| Partial gastrectomy | 3 (23.1) | 0 (0) |
| Cause of non-resection, n (%) | ||
| Progressive disease (metastasis) | 0 (0) | 5 (9.1) |
| Death | 1 (6.7) | 0 (0) |
| Patient’s denial | 0 (0) | 1 (1.8) |
| Declined ECOG PS | 0 (0) | 8 (14.5) |
| Unresectable | 1 (6.7) | 4 (7.3) |
| Adjuvant nivolumab | - | 6 (10.9) |
| Response | FLOT (n = 15) | CROSS (n = 55) |
|---|---|---|
| Objective response rate | 9 (60.0) | 45 (81.8) |
| Complete response | 1 (6.7) | 1 (1.8) |
| Partial response | 8 (53.3) | 44 (80.0) |
| Stable disease | 6 (40.0) | 8 (14.6) |
| Progressive disease | 0 (0) | 2 (3.6) |
| FLOT (n = 13) | CROSS (n = 37) | |
|---|---|---|
| Pathological T stage, n (%) | ||
| ypT0 | 0 (0) | 5 (13.5) |
| ypT1 | 4 (30.8) | 13 (35.1) |
| ypT2 | 2 (15.3) | 9 (24.3) |
| ypT3 | 4 (30.8) | 9 (24.3) |
| ypT4 | 3 (23.1) | 1 (2.7) |
| Downstaging T stage at least 1 or more stages, n (%) | 5 (38.5) | 26 (70.3) |
| Pathological N stage, n (%) | ||
| ypN0 | 6 (46.1) | 22 (59.5) |
| ypN1 | 3 (23.1) | 8 (21.6) |
| ypN2 | 1 (7.7) | 6 (16.2) |
| ypN3 | 3 (23.1) | 1 (2.7) |
| Downstaging N stage from cN+ to ypN0, n (%) | 0 (0) | 7/17 (41.2) # |
| Pathological complete response, n (%) | 0 (0) | 5 (13.5) |
| Pathological tumor regression grade, n (%) *,** | ||
| Grade 0 | 0 (0) | 5 (13.5) |
| Grade 1 | 0 (0) | 10 (27.0) |
| Grade 2 | 6 (46.2) | 20 (54.1) |
| Grade 3 | 7 (53.8) | 2 (5.4) |
| Toxicities | Perioperative Chemotherapy (n = 15) | Preoperative CCRT (n = 49) | ||
|---|---|---|---|---|
| All Grades, n (%) | Grades 3–4, n (%) | All Grade, n (%) | Grades 3–4, n (%) | |
| Hematologic | ||||
| Anemia | 3 (20.0) | 0 (0) | 4 (8.2) | 0 (0) |
| Thrombocytopenia | 0 (0) | 0 (0) | 3 (6.1) | 0 (0) |
| Leukopenia | 1 (6.7) | 1 (6.7) | 12 (24.5) | 4 (8.2) |
| Neutropenia | 1 (6.7) | 1 (6.7) | 12 (24.5) | 4 (8.2) |
| Febrile neutropenia | 1 (6.7) | 1 (6.7) | 2 (4.1) | 2 (4.1) |
| Non-hematologic | ||||
| Nausea | 11 (73.3) * | 0 (0) | 19 (38.8) | 0 (0) |
| Vomiting | 3 (20.0) | 0 (0) | 5 (10.2) | 0 (0) |
| Mucositis | 3 (20.0) * | 0 (0) | 1 (2.0) | 0 (0) |
| Diarrhea | 9 (60.0) * | 0 (0) | 3 (6.1) | 0 (0) |
| Fatigue | 15 (100) * | 0 (0) | 30 (61.2) | 1 (2.0) |
| Peripheral neuropathy | 10 (66.7) * | 0 (0) | 3 (6.1) | 0 (0) |
| Infection | 1 (6.7) | 0 (0) | 12 (24.5) | 0 (0) |
| Hand–foot syndrome | 3 (20.0) * | 0 (0) | 0 (0) | 0 (0) |
| Pneumonitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wonglhow, J.; Wong, H.-L.; Duong, C.; Spillane, J.; Liu, D.S.; Leong, T.; Chu, J.; Michael, M. Real-World Outcomes Between Perioperative Chemotherapy (FLOT) and Preoperative Concurrent Chemoradiotherapy (CROSS) in Localized Esophageal and Esophagogastric Junction Adenocarcinoma: A Retrospective Cohort Study. Cancers 2025, 17, 2962. https://doi.org/10.3390/cancers17182962
Wonglhow J, Wong H-L, Duong C, Spillane J, Liu DS, Leong T, Chu J, Michael M. Real-World Outcomes Between Perioperative Chemotherapy (FLOT) and Preoperative Concurrent Chemoradiotherapy (CROSS) in Localized Esophageal and Esophagogastric Junction Adenocarcinoma: A Retrospective Cohort Study. Cancers. 2025; 17(18):2962. https://doi.org/10.3390/cancers17182962
Chicago/Turabian StyleWonglhow, Jirapat, Hui-Li Wong, Cuong Duong, John Spillane, David S. Liu, Trevor Leong, Julie Chu, and Michael Michael. 2025. "Real-World Outcomes Between Perioperative Chemotherapy (FLOT) and Preoperative Concurrent Chemoradiotherapy (CROSS) in Localized Esophageal and Esophagogastric Junction Adenocarcinoma: A Retrospective Cohort Study" Cancers 17, no. 18: 2962. https://doi.org/10.3390/cancers17182962
APA StyleWonglhow, J., Wong, H.-L., Duong, C., Spillane, J., Liu, D. S., Leong, T., Chu, J., & Michael, M. (2025). Real-World Outcomes Between Perioperative Chemotherapy (FLOT) and Preoperative Concurrent Chemoradiotherapy (CROSS) in Localized Esophageal and Esophagogastric Junction Adenocarcinoma: A Retrospective Cohort Study. Cancers, 17(18), 2962. https://doi.org/10.3390/cancers17182962






