Tumor Mutational Burden in Cervical Cancer as Potential Marker for Immunotherapy Responders
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and DNA Isolation
2.3. NGS Library Preparation and Sequencing
2.4. Bioinformatic Processing and Variant Annotation
2.5. Statistical and Comparative Genomic Analyses
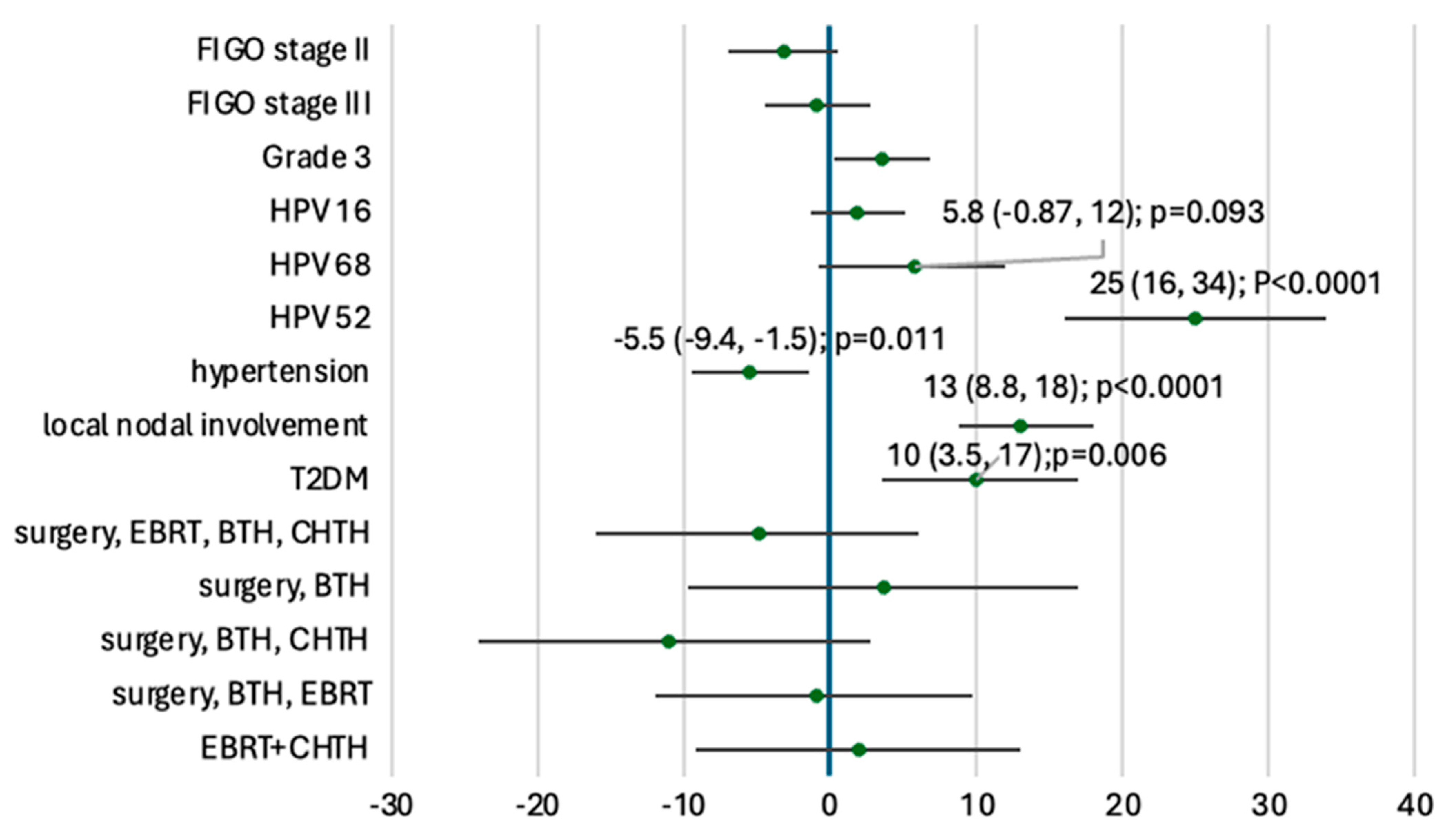
3. Results
3.1. Baseline Characteristics and Treatment Modalities
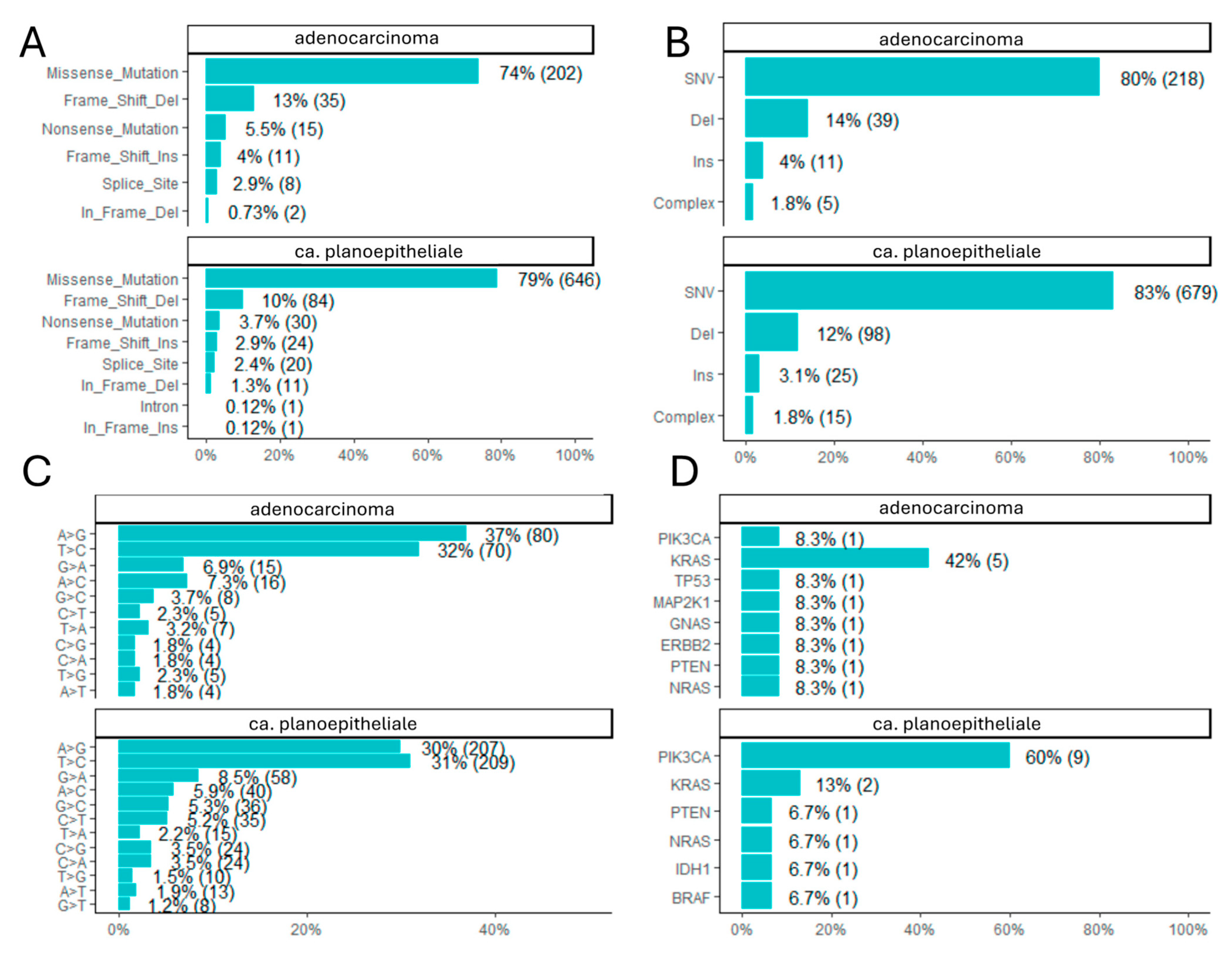
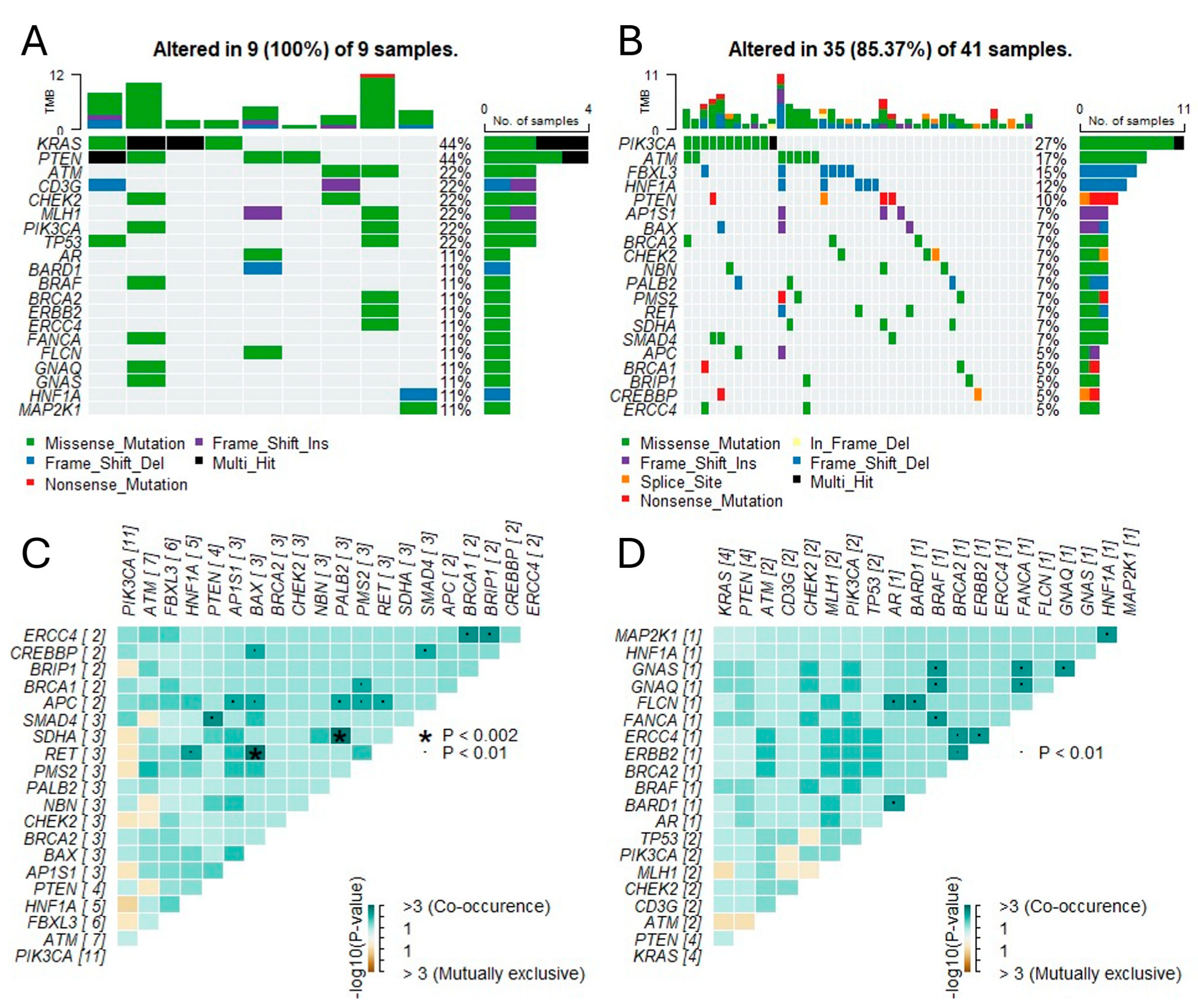
3.2. Genomic Landscape and Somatic Mutations
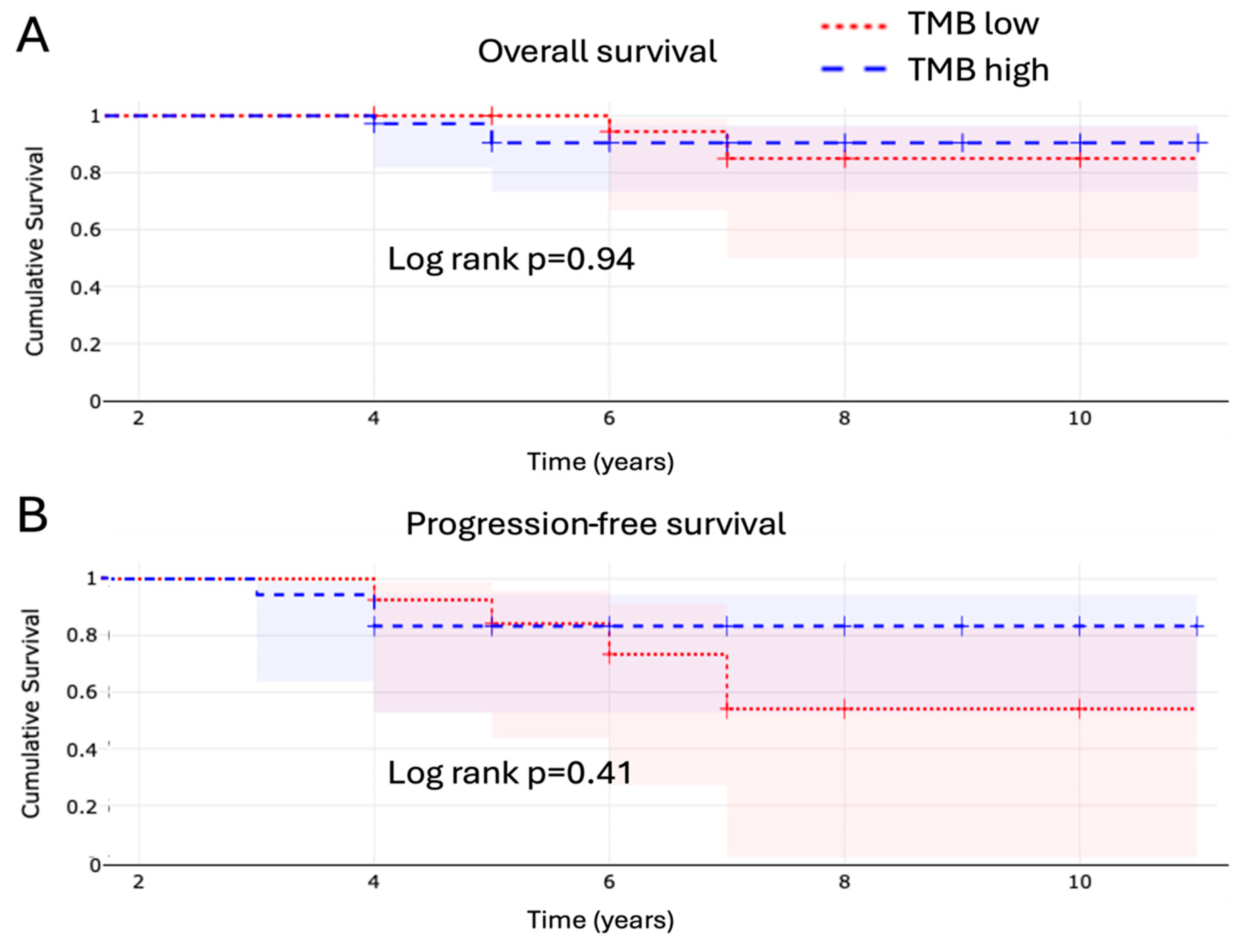
3.3. Tumor Mutational Burden and Microsatellite Instability
3.4. Homologous Recombination Deficiency
4. Discussion
4.1. Tumor Mutational Burden and Its Clinical Relevance
4.2. TMB and HRD: Disconnected Genomic Features
4.3. Clinical and Molecular Heterogeneity
4.4. Future Directions and Unanswered Questions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simms, K.T.; Steinberg, J.; Caruana, M.; Smith, M.A.; Lew, J.B.; Soerjomataram, I.; Castle, P.E.; Bray, F.; Canfell, K. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–2099: A modelling study. Lancet Oncol. 2019, 20, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203, Erratum in Lancet Glob. Health 2022, 10, e41. https://doi.org/10.1016/S2214-109X(21)00554-4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson AB3rd Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; Fakih, M.G.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefanoudakis, D.; Karopoulou, E.; Matsas, A.; Katsampoula, G.A.; Tsarna, E.; Stamoula, E.; Christopoulos, P. Immunotherapy in Cervical and Endometrial Cancer: Current Landscape and Future Directions. Life 2024, 14, 344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dyer, B.A.; Zamarin, D.; Eskandar, R.N.; Mayadev, J.M. Role of Immunotherapy in the Management of Locally Advanced and Recurrent/Metastatic Cervical Cancer. J. Natl. Compr. Canc Netw. 2019, 17, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Ma, Y.; Wu, Z.; Zeng, F.; Song, B.; Zhang, Y.; Li, J.; Lui, S.; Wu, M. Tumor Mutational Burden Predicting the Efficacy of Immune Checkpoint Inhibitors in Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 751407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ota, N.; Yoshimoto, Y.; Darwis, N.D.M.; Sato, H.; Ando, K.; Oike, T.; Ohno, T. High tumor mutational burden predicts worse prognosis for cervical cancer treated with radiotherapy. Jpn. J. Radiol. 2022, 40, 534–541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Kong, W.; Zhao, X.; Zhang, H.; Luo, D.; Chen, S. Immune checkpoint inhibitors in cervical cancer: Current status and research progress. Front. Oncol. 2022, 12, 984896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s071s090lbl.pdf (accessed on 1 July 2025).
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller WHJr Italiano, A.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; Le Tourneau, C. Keynote-158 study, FDA granted accelerated approval of pembrolizumab for the treatment of patients with advanced PD-L1-positive cervical cancer. Ann. Transl. Med. 2020, 8, 1611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merino, D.M.; McShane, L.M.; Fabrizio, D.; Funari, V.; Chen, S.J.; White, J.R.; Wenz, P.; Baden, J.; Barrett, J.C.; Chaudhary, R.; et al. Establishing guidelines to harmonize tumor mutational burden (TMB): In silico assessment of variation in TMB quantification across diagnostic platforms: Phase I of the Friends of Cancer Research TMB Harmonization Project. J. Immunother. Cancer 2020, 8, e000147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stenzinger, A.; Allen, J.D.; Maas, J.; Stewart, M.D.; Merino, D.M.; Wempe, M.M.; Dietel, M. Tumor mutational burden standardization initiatives: Recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes. Chromosomes Cancer 2019, 58, 578–588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fabrizio, D.A.; George TJJr Dunne, R.F.; Frampton, G.; Sun, J.; Gowen, K.; Kennedy, M.; Greenbowe, J.; Schrock, A.B.; Hezel, A.F.; Ross, J.S.; et al. Beyond microsatellite testing: Assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J. Gastrointest. Oncol. 2018, 9, 610–617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199, Erratum in N. Engl. J. Med. 2018, 379, 2185. https://doi.org/10.1056/NEJMx180040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oaknin, A.; Monk, B.J.; de Melo, A.C.; Kim, H.S.; Kim, Y.M.; Lisyanskaya, A.S.; Samouëlian, V.; Lorusso, D.; Damian, F.; Chang, C.L.; et al. Cemiplimab in recurrent cervical cancer: Final analysis of overall survival in the phase III EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9 trial. Eur. J. Cancer 2025, 216, 115146. [Google Scholar] [CrossRef] [PubMed]
- Farajimakin, O. The Role of Immunotherapy in the Treatment of Gynecologic Cancers: A Systematic Review. Cureus 2024, 16, e65638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burk, R.D.; Harari, A.; Chen, Z. Human papillomavirus genome variants. Virology 2013, 445, 232–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362, eaar3593, Erratum in Science 2019, 363, eaax1384. https://doi.org/10.1126/science.aax1384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
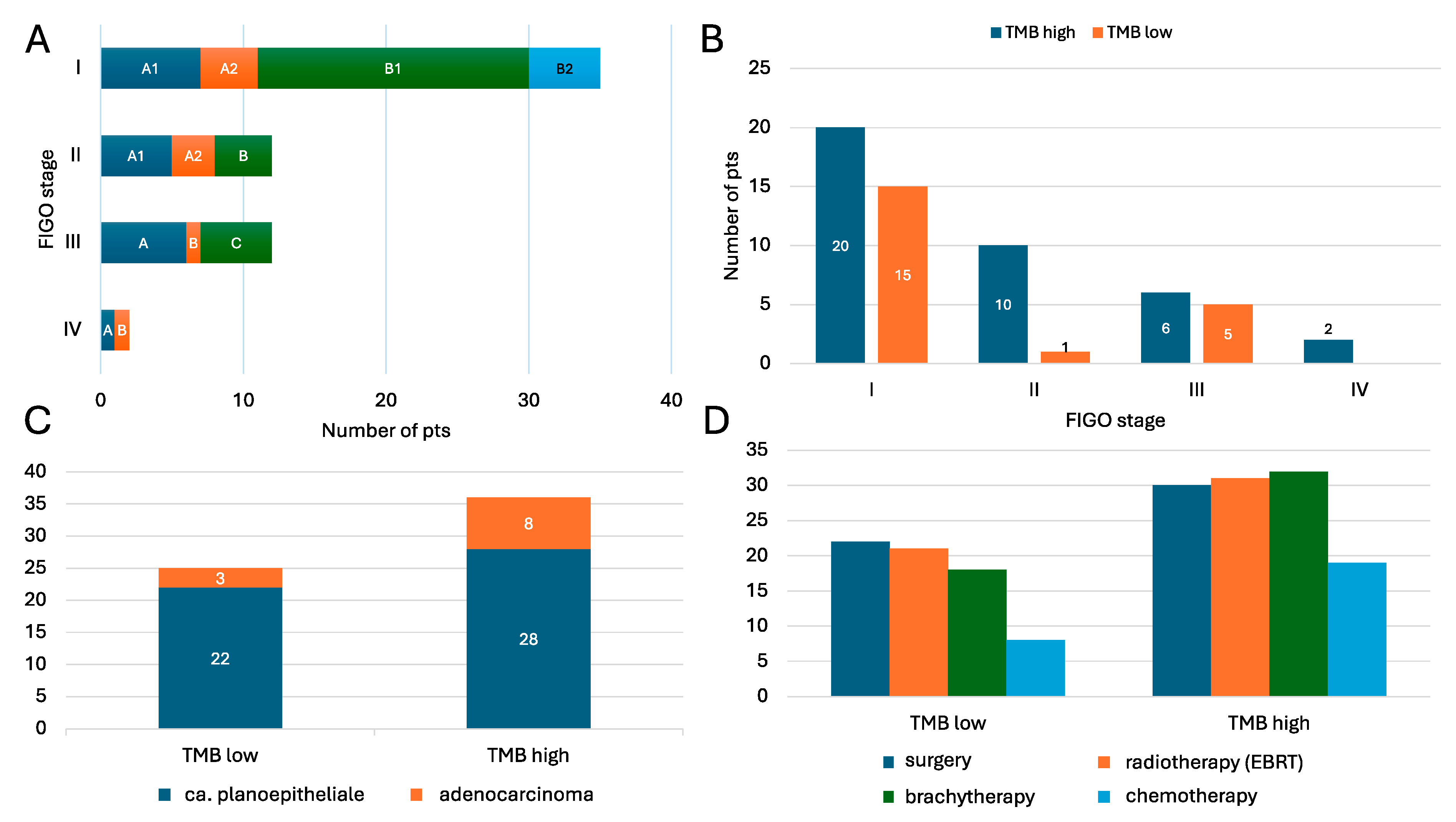

| Variable | All | TMB High | TMB Low |
|---|---|---|---|
| Mean age (±SD) | 50.6 ± 14.0 | 51.1 ± 14.6 | 49.8 ± 13.4 |
| Ca. planoepitheliale | 50 (82.0%) | 28 (77.8%) | 22 (88.0%) |
| Adenocarcinoma | 11 (18.0%) | 8 (22.2%) | 3 (12.0%) |
| Surgical treatment | 52 (85.2%) | 30 (83.3%) | 22 (88.0%) |
| Brachytherapy | 52 (85.2%) | 31 (86.1%) | 21 (84.0%) |
| Radiotherapy (EBRT) | 50 (82.0%) | 32 (88.9%) | 18 (72.0%) |
| Chemotherapy | 27 (44.3%) | 19 (52.8%) | 8 (32.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalkowska, M.E.; Kamińska, K.; Wojtysiak, J.; Koper, K.; Makarewicz, A.; Pietrzak, B.; Bomba-Opoń, D.; Dębska, M.; Wielgoś, M.; Grabiec, M.; et al. Tumor Mutational Burden in Cervical Cancer as Potential Marker for Immunotherapy Responders. Cancers 2025, 17, 2963. https://doi.org/10.3390/cancers17182963
Kowalkowska ME, Kamińska K, Wojtysiak J, Koper K, Makarewicz A, Pietrzak B, Bomba-Opoń D, Dębska M, Wielgoś M, Grabiec M, et al. Tumor Mutational Burden in Cervical Cancer as Potential Marker for Immunotherapy Responders. Cancers. 2025; 17(18):2963. https://doi.org/10.3390/cancers17182963
Chicago/Turabian StyleKowalkowska, Magdalena Ewa, Katarzyna Kamińska, Joanna Wojtysiak, Krzysztof Koper, Adrianna Makarewicz, Bronisława Pietrzak, Dorota Bomba-Opoń, Marzena Dębska, Mirosław Wielgoś, Marek Grabiec, and et al. 2025. "Tumor Mutational Burden in Cervical Cancer as Potential Marker for Immunotherapy Responders" Cancers 17, no. 18: 2963. https://doi.org/10.3390/cancers17182963
APA StyleKowalkowska, M. E., Kamińska, K., Wojtysiak, J., Koper, K., Makarewicz, A., Pietrzak, B., Bomba-Opoń, D., Dębska, M., Wielgoś, M., Grabiec, M., & Lewandowska, M. A. (2025). Tumor Mutational Burden in Cervical Cancer as Potential Marker for Immunotherapy Responders. Cancers, 17(18), 2963. https://doi.org/10.3390/cancers17182963






