Radiotherapy for Locally Advanced Pancreatic Cancer in the Modern Era: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
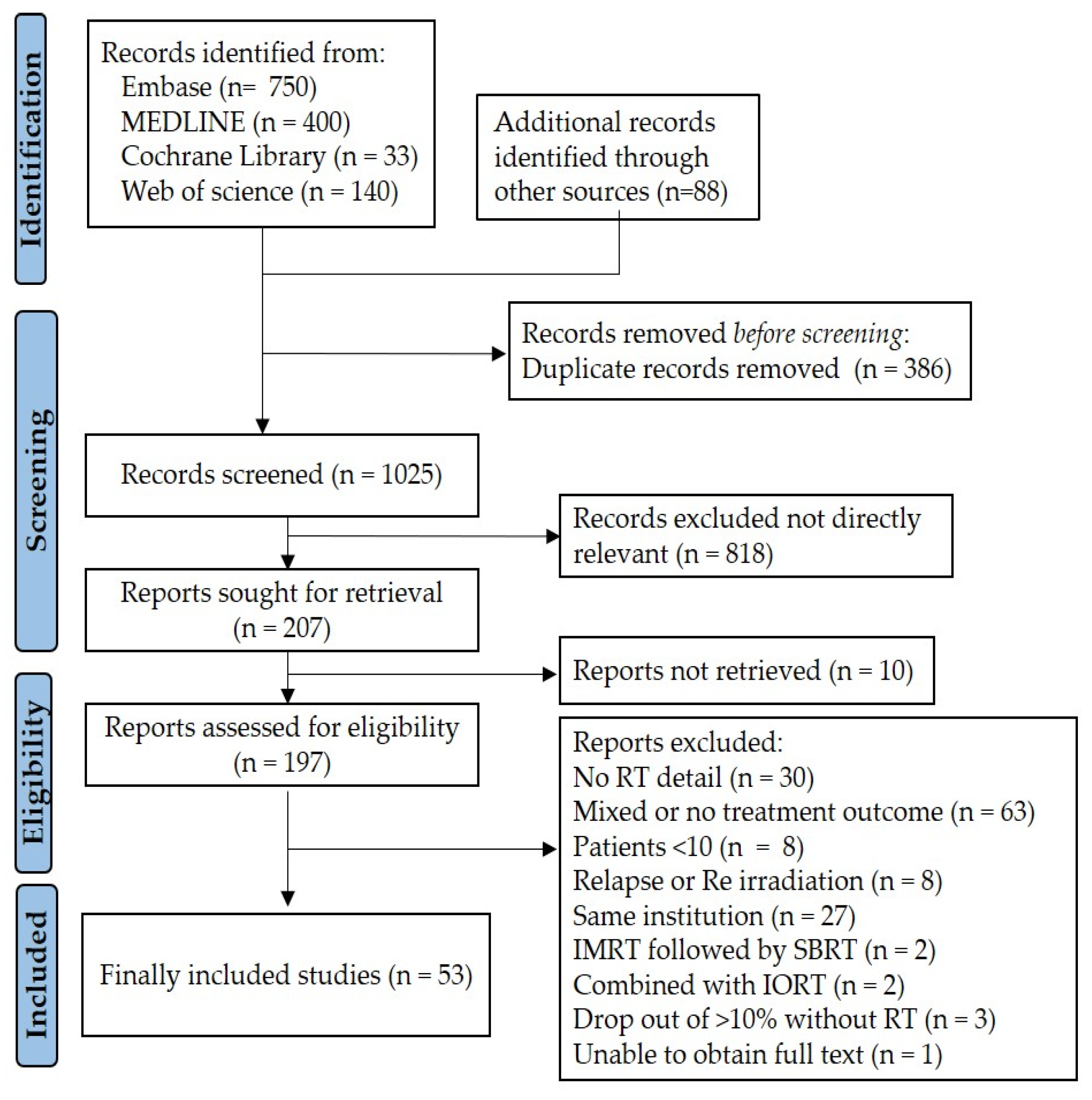
3. Results
3.1. Study Characteristics
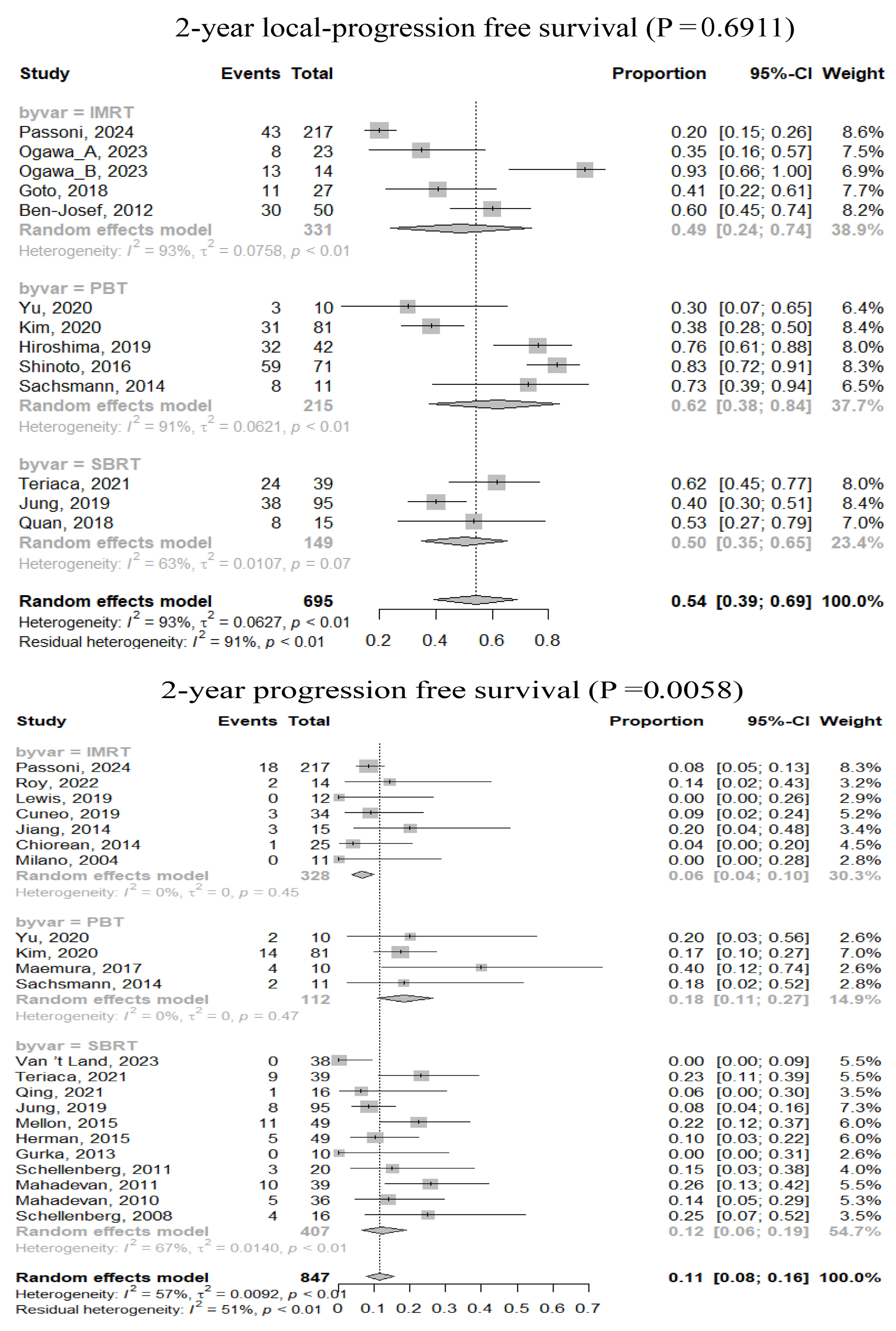
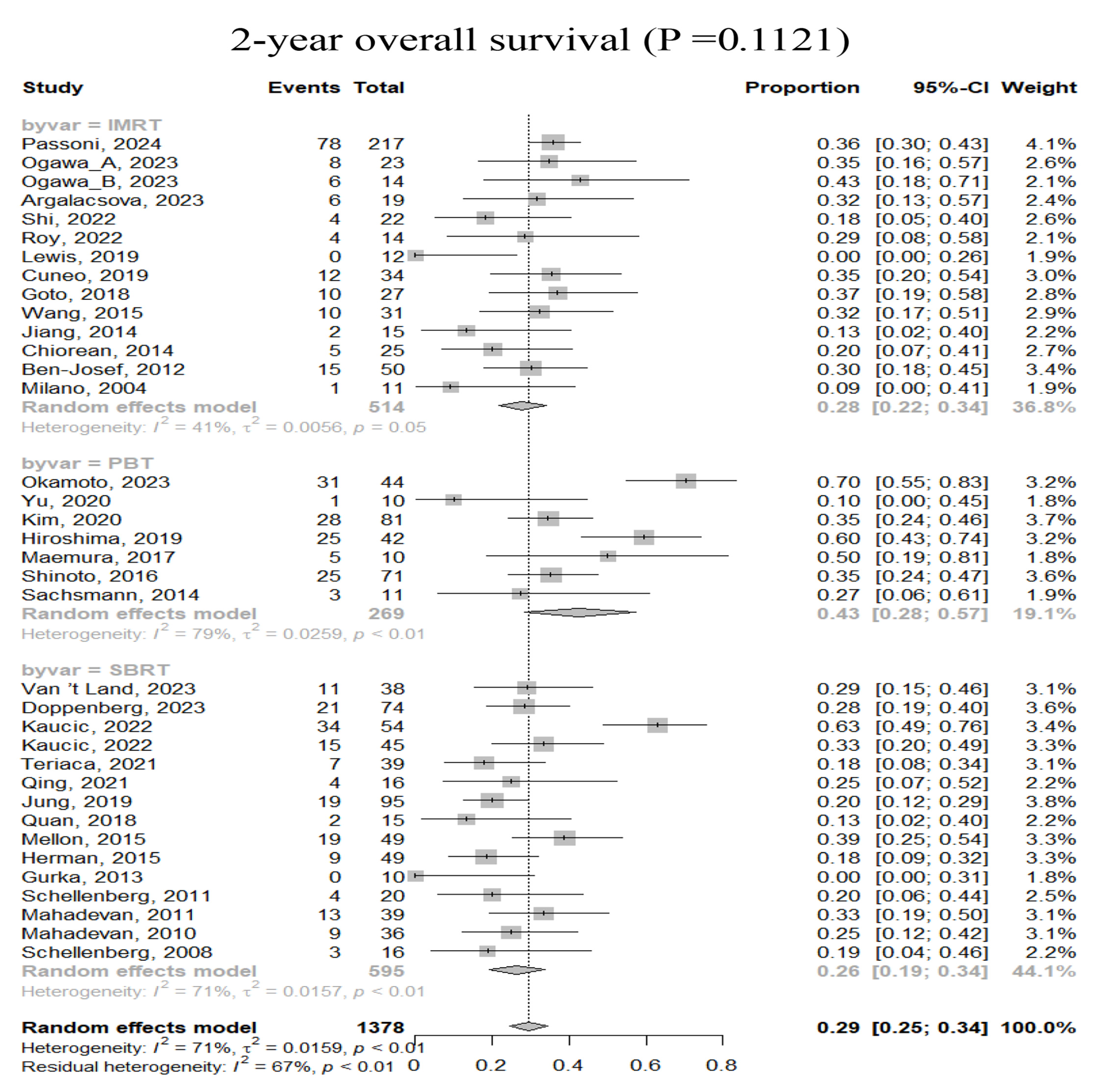
3.2. Efficacy
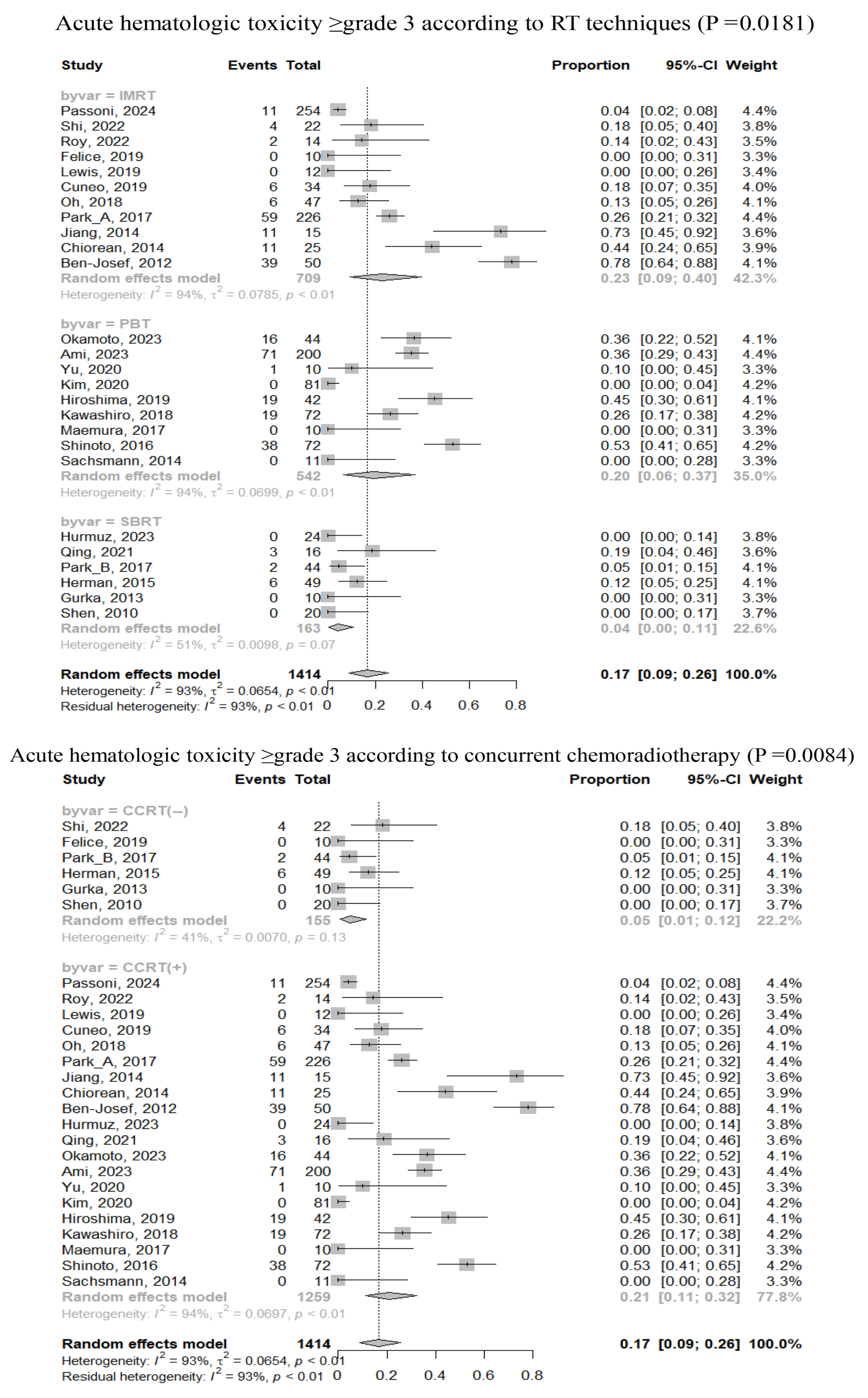
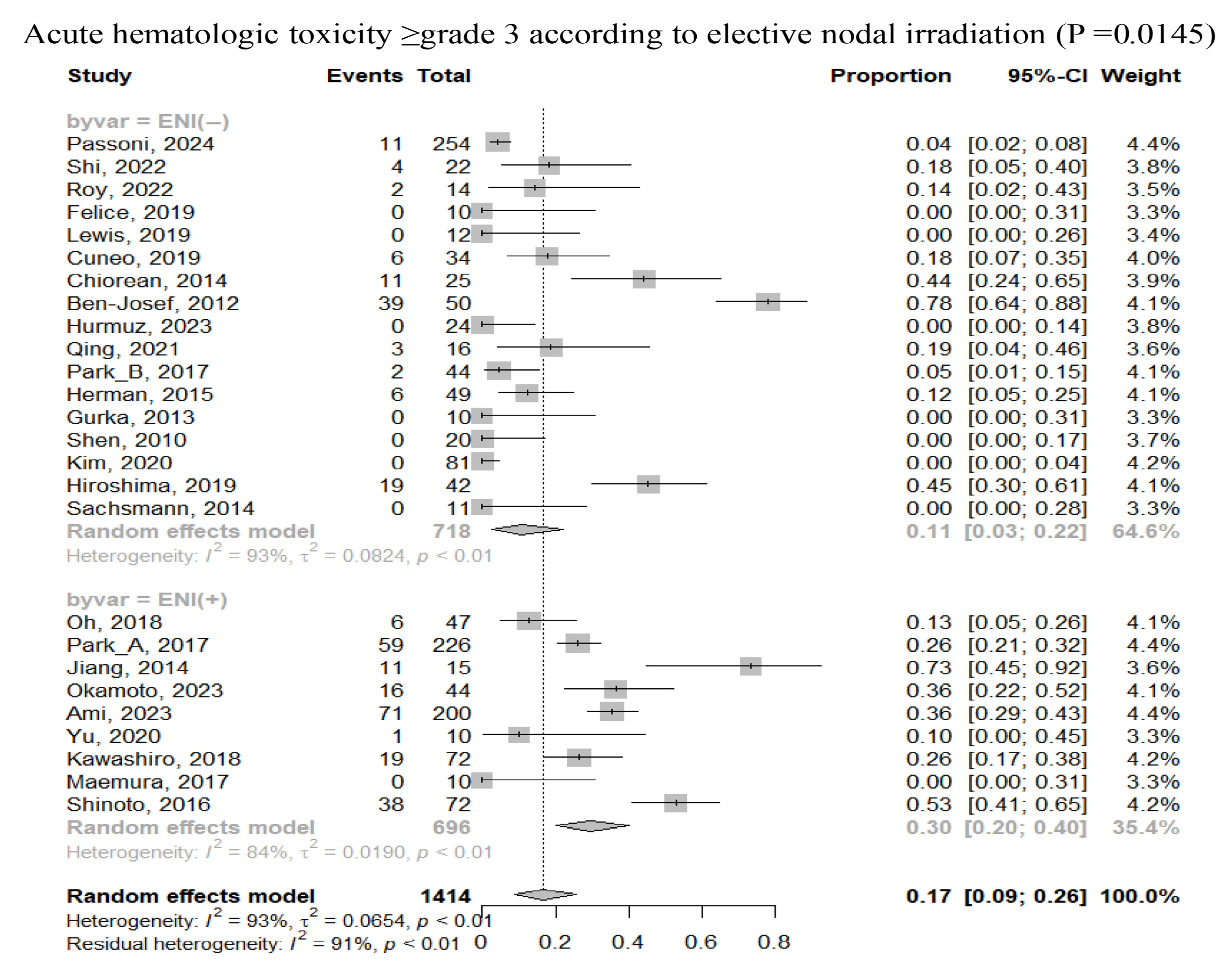
3.3. Safety
3.4. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Russo, S.; Butler, J.; Ove, R.; Blackstock, A.W. Locally advanced pancreatic cancer: A review. Semin. Oncol. 2007, 34, 327–334. [Google Scholar] [CrossRef]
- Balaban, E.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Mukherjee, S.; Crane, C.H.; Javle, M.M.; Eads, J.R.; Allen, P.; Ko, A.H.; et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2654–2668. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Guidelines: Pancreatic Adenocarcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 23 November 2024).
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, D.J.; MacIntyre, J.M.; Catton, G.E.; Engstrom, P.F.; Moertel, C.G. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—An Eastern Cooperative Oncology Group study. J. Clin. Oncol. 1985, 3, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: Comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J. Natl. Cancer Inst. 1988, 80, 751–755. [Google Scholar] [CrossRef]
- Chauffert, B.; Mornex, F.; Bonnetain, F.; Rougier, P.; Mariette, C.; Bouche, O.; Bosset, J.F.; Aparicio, T.; Mineur, L.; Azzedine, A.; et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. Oncol. 2008, 19, 1592–1599. [Google Scholar] [CrossRef]
- Loehrer, P.J., Sr.; Feng, Y.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; Alberts, S.R.; et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouche, O.; Shannon, J.; Andre, T.; et al. Effect of Chemoradiotherapy vs. Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine with or without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Wang, X.; Wang, Y.; Cha, N. Effects of chemoradiotherapy and chemotherapy on survival of patients with locally advanced pancreatic cancer: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e12260. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, J.; Santos, T.D.; Yang, L.; Li, M.; Jiang, Q.; Ma, C. Survival outcome after stereotactic body radiotherapy for locally advanced and borderline resectable pancreatic cancer: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 101139. [Google Scholar] [CrossRef]
- Shouman, M.A.; Fuchs, F.; Walter, F.; Corradini, S.; Westphalen, C.B.; Vornhulz, M.; Beyer, G.; Andrade, D.; Belka, C.; Niyazi, M.; et al. Stereotactic body radiotherapy for pancreatic cancer—A systematic review of prospective data. Clin. Transl. Radiat. Oncol. 2024, 45, 100738. [Google Scholar] [CrossRef] [PubMed]
- Arcelli, A.; Tarantino, G.; Cellini, F.; Buwenge, M.; Macchia, G.; Bertini, F.; Guido, A.; Deodato, F.; Cilla, S.; Scotti, V.; et al. Comparative Effectiveness of Chemotherapy Alone Versus Radiotherapy-Based Regimens in Locally Advanced Pancreatic Cancer: A Real-World Multicenter Analysis (PAULA-1). Curr. Oncol. 2023, 30, 5690–5703. [Google Scholar] [CrossRef]
- Veldeman, L.; Madani, I.; Hulstaert, F.; De Meerleer, G.; Mareel, M.; De Neve, W. Evidence behind use of intensity-modulated radiotherapy: A systematic review of comparative clinical studies. Lancet Oncol. 2008, 9, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Grau, C.; Durante, M.; Georg, D.; Langendijk, J.A.; Weber, D.C. Particle therapy in Europe. Mol. Oncol. 2020, 14, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Passoni, P.; Reni, M.; Broggi, S.; Slim, N.; Fodor, A.; Macchini, M.; Orsi, G.; Peretti, U.; Balzano, G.; Tamburrino, D.; et al. Hypofractionated radiotherapy concomitant to capecitabine after induction chemotherapy for advanced pancreatic adenocarcinoma. Clin. Transl. Radiat. Oncol. 2024, 47, 100778. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Yoshimura, M.; Nakamura, M.; Adachi, T.; Iwai, T.; Ashida, R.; Mizowaki, T. Impact of planning organ at risk volume margins and matching method on late gastrointestinal toxicity in moderately hypofractionated IMRT for locally advanced pancreatic ductal adenocarcinoma. Radiat. Oncol. 2023, 18, 103. [Google Scholar] [CrossRef]
- Argalacsova, S.; Vocka, M.; Petruzelka, L.; Ryska, M.; Zaruba, P.; Krska, Z.; Fryba, V.; Ulrych, J.; Cerny, V.; Tuma, T.; et al. Chemotherapy versus chemoradiotherapy in borderline resectable and locally advanced pancreatic adenocarcinoma. Neoplasma 2023, 70, 468–475. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, J.; Kong, W.; Qiu, X.; Lu, C.; Liu, J.; Liu, B.; Du, J. Use of Nab-Paclitaxel Plus Gemcitabine Followed by Hypofractionated Tomotherapy with Simultaneous Integrated Boost in Patients with Locally Advanced Pancreatic Cancer. Front. Oncol. 2022, 12, 782730. [Google Scholar] [CrossRef]
- Roy, A.C.; Abbas, M.N.; Price, T.J.; Singhal, N.; Vatandoust, S.; Leung, J.; Kichenadasse, G.; Koczwara, B.; Sukumaran, S.; Kumar, R.; et al. Phase I Trial of nab-Paclitaxel Administered Concurrently with Radiotherapy in Patients with Locally Advanced Inoperable Pancreatic Adenocarcinoma. Pancreas 2022, 51, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Reyngold, M.; O’Reilly, E.M.; Varghese, A.M.; Fiasconaro, M.; Zinovoy, M.; Romesser, P.B.; Wu, A.; Hajj, C.; Cuaron, J.J.; Tuli, R.; et al. Association of Ablative Radiation Therapy with Survival Among Patients with Inoperable Pancreatic Cancer. JAMA Oncol. 2021, 7, 735–738. [Google Scholar] [CrossRef]
- De Felice, F.; Benevento, I.; Bulzonetti, N.; Shima, B.; Rubini, F.; Marampon, F.; Musio, D.; Tombolini, V. Hypofractionated intensity-modulated radiotherapy in locally advanced unresectable pancreatic cancer: A pilot study. Curr. Probl. Cancer 2019, 43, 495–503. [Google Scholar] [CrossRef]
- Lewis, S.; Sastri, S.C.; Arya, S.; Mehta, S.; Patil, P.; Shrivastava, S.; Phurailatpam, R.; Shrikhande, S.V.; Engineer, R. Dose escalated concurrent chemo-radiation in borderline resectable and locally advanced pancreatic cancers with tomotherapy based intensity modulated radiotherapy: A phase II study. J. Gastrointest. Oncol. 2019, 10, 474–482. [Google Scholar] [CrossRef]
- Cuneo, K.C.; Morgan, M.A.; Sahai, V.; Schipper, M.J.; Parsels, L.A.; Parsels, J.D.; Devasia, T.; Al-Hawaray, M.; Cho, C.S.; Nathan, H.; et al. Dose Escalation Trial of the Wee1 Inhibitor Adavosertib (AZD1775) in Combination with Gemcitabine and Radiation for Patients with Locally Advanced Pancreatic Cancer. J. Clin. Oncol. 2019, 37, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Kim, T.H.; Woo, S.M.; Lee, W.J.; Lee, J.H.; Youn, S.H.; Han, S.S.; Park, S.J.; Kim, D.Y. Effectiveness and feasibility of concurrent chemoradiotherapy using simultaneous integrated boost-intensity modulated radiotherapy with and without induction chemotherapy for locally advanced pancreatic cancer. Radiat. Oncol. J. 2018, 36, 200–209. [Google Scholar] [CrossRef]
- Goto, Y.; Nakamura, A.; Ashida, R.; Sakanaka, K.; Itasaka, S.; Shibuya, K.; Matsumoto, S.; Kanai, M.; Isoda, H.; Masui, T.; et al. Clinical evaluation of intensity-modulated radiotherapy for locally advanced pancreatic cancer. Radiat. Oncol. 2018, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hajj, C.; Reyngold, M.; Shi, W.; Zhang, Z.; Cuaron, J.J.; Crane, C.H.; O’Reilly, E.M.; Lowery, M.A.; Yu, K.H.; et al. Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncol. 2017, 56, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Colbert, L.E.; Moningi, S.; Chadha, A.; Amer, A.; Lee, Y.; Wolff, R.A.; Varadhachary, G.; Fleming, J.; Katz, M.; Das, P.; et al. Dose escalation with an IMRT technique in 15 to 28 fractions is better tolerated than standard doses of 3DCRT for LAPC. Adv. Radiat. Oncol. 2017, 2, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, Z.G.; Ma, N.Y.; Zhao, J.D.; Zhang, Z.; Ma, X.J.; Long, J.; Xu, J.; Jiang, G.L. Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: A mono-institutional retrospective analysis. Radiat. Oncol. 2015, 10, 14. [Google Scholar] [CrossRef]
- Jiang, Y.; Mackley, H.B.; Kimchi, E.T.; Zhu, J.; Gusani, N.; Kaifi, J.; Staveley-O’Carroll, K.F.; Belani, C.P. Phase I dose escalation study of capecitabine and erlotinib concurrent with radiation in locally advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2014, 74, 205–210. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Schneider, B.P.; Akisik, F.M.; Perkins, S.M.; Anderson, S.; Johnson, C.S.; DeWitt, J.; Helft, P.; Clark, R.; Johnston, E.L.; et al. Phase 1 pharmacogenetic and pharmacodynamic study of sorafenib with concurrent radiation therapy and gemcitabine in locally advanced unresectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 284–291. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Schipper, M.; Francis, I.R.; Hadley, S.; Ten-Haken, R.; Lawrence, T.; Normolle, D.; Simeone, D.M.; Sonnenday, C.; Abrams, R.; et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 1166–1171. [Google Scholar] [CrossRef]
- Abelson, J.A.; Murphy, J.D.; Minn, A.Y.; Chung, M.; Fisher, G.A.; Ford, J.M.; Kunz, P.; Norton, J.A.; Visser, B.C.; Poultsides, G.A.; et al. Intensity-modulated radiotherapy for pancreatic adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e595–e601. [Google Scholar] [CrossRef]
- Milano, M.T.; Chmura, S.J.; Garofalo, M.C.; Rash, C.; Roeske, J.C.; Connell, P.P.; Kwon, O.H.; Jani, A.B.; Heimann, R. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 445–453. [Google Scholar] [CrossRef]
- van ‘t Land, F.R.; Latifi, D.; Moskie, M.; Homs, M.Y.V.; Bosscha, K.; Bonsing, B.A.; Mieog, S.D.; van der Harst, E.; Coene, P.L.O.; Wijsman, J.H.; et al. Feasibility, safety, and efficacy of stereotactic body radiotherapy combined with intradermal heat-killed mycobacterium obuense (IMM-101) vaccination for non-progressive locally advanced pancreatic cancer, after induction chemotherapy with (modified)FOLFIRINOX—The LAPC-2 trial. Radiother. Oncol. 2023, 183, 109541. [Google Scholar] [CrossRef]
- Reyngold, M.; Karam, S.D.; Hajj, C.; Wu, A.J.; Cuaron, J.; Lobaugh, S.; Yorke, E.D.; Dickinson, S.; Jones, B.; Vinogradskiy, Y.; et al. Phase 1 Dose Escalation Study of SBRT Using 3 Fractions for Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 53–63. [Google Scholar] [CrossRef]
- Hurmuz, P.; Cengiz, M.; Ozyigit, G.; Yuce Sari, S.; Kahvecioglu, A.; Beduk Esen, C.S.; Yalcin, S.; Zorlu, F. Stereotactic Body Radiotherapy as an Effective Treatment for Pancreatic Cancer. Cureus 2023, 15, e38255. [Google Scholar] [CrossRef] [PubMed]
- Doppenberg, D.; Lagerwaard, F.J.; van Dieren, S.; Meijerink, M.R.; van der Vliet, J.J.; Besselink, M.G.; van Tienhoven, G.; Versteijne, E.; Slotman, B.J.; Wilmink, J.W.; et al. Optimizing patient selection for stereotactic ablative radiotherapy in patients with locally advanced pancreatic cancer after initial chemotherapy—A single center prospective cohort. Front. Oncol. 2023, 13, 1149961. [Google Scholar] [CrossRef]
- Comito, T.; Massaro, M.; Teriaca, M.A.; Franzese, C.; Franceschini, D.; Navarria, P.; Clerici, E.; Di Cristina, L.; Bertolini, A.; Tomatis, S.; et al. Can STEreotactic Body Radiation Therapy (SBRT) Improve the Prognosis of Unresectable Locally Advanced Pancreatic Cancer? Long-Term Clinical Outcomes, Toxicity and Prognostic Factors on 142 Patients (STEP Study). Curr. Oncol. 2023, 30, 7073–7088. [Google Scholar] [CrossRef]
- Lee, H.I.; Kang, H.C.; Chie, E.K. Consolidatory ablative stereotactic body radiation therapy after induction chemotherapy for unresectable pancreatic cancer: A single center experience. Front. Oncol. 2022, 12, 974454. [Google Scholar] [CrossRef]
- Kaucic, H.; Kosmina, D.; Schwarz, D.; Mack, A.; Sobat, H.; Cehobasic, A.; Leipold, V.; Andrasek, I.; Avdicevic, A.; Mlinaric, M. Stereotactic Ablative Radiotherapy Using CALYPSO® Extracranial Tracking for Intrafractional Tumor Motion Management-A New Potential Local Treatment for Unresectable Locally Advanced Pancreatic Cancer? Results from a Retrospective Study. Cancers 2022, 14, 2688. [Google Scholar] [CrossRef]
- Kaucic, H.; Kosmina, D.; Schwarz, D.; Mack, A.; Cehobasic, A.; Leipold, V.; Avdicevic, A.; Mlinaric, M.; Lekic, M.; Schwarz, K.; et al. Stereotactic Body Radiotherapy for Locally Advanced Pancreatic Cancer Using Optical Surface Management System—AlignRT as an Optical Body Surface Motion Management in Deep Breath Hold Patients: Results from a Single-Arm Retrospective Study. Cancer Manag. Res. 2022, 14, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, Y.; Lu, M.; Zhao, X.; Jiang, L.; Ye, Y.; Ju, X.; Zhang, H. Stereotactic body radiation therapy with sequential S-1 for patients with locally advanced pancreatic cancer and poor performance status: An open-label, single-arm, phase 2 trial. Radiother. Oncol. 2021, 162, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Teriaca, M.A.; Loi, M.; Suker, M.; Eskens, F.; van Eijck, C.H.J.; Nuyttens, J.J. A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): Long-term outcome. Radiother. Oncol. 2021, 155, 232–236. [Google Scholar] [CrossRef]
- Qing, S.; Gu, L.; Zhang, H. Phase I study of dose-escalated stereotactic body radiation therapy for locally advanced pancreatic head cancers: Initial clinical results. Cancer Med. 2021, 10, 6736–6743. [Google Scholar] [CrossRef]
- Bouchart, C.; Engelholm, J.L.; Closset, J.; Navez, J.; Loi, P.; Gokburun, Y.; De Grez, T.; Mans, L.; Hendlisz, A.; Bali, M.A.; et al. Isotoxic high-dose stereotactic body radiotherapy integrated in a total multimodal neoadjuvant strategy for the treatment of localized pancreatic ductal adenocarcinoma. Ther. Adv. Med. Oncol. 2021, 13, 17588359211045860. [Google Scholar] [CrossRef]
- Jung, J.; Yoon, S.M.; Park, J.H.; Seo, D.W.; Lee, S.S.; Kim, M.H.; Lee, S.K.; Park, D.H.; Song, T.J.; Ryoo, B.Y.; et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS ONE 2019, 14, e0214970. [Google Scholar] [CrossRef]
- Quan, K.; Sutera, P.; Xu, K.; Bernard, M.E.; Burton, S.A.; Wegner, R.E.; Zeh, H.; Bahary, N.; Stoller, R.; Heron, D.E. Results of a prospective phase 2 clinical trial of induction gemcitabine/capecitabine followed by stereotactic ablative radiation therapy in borderline resectable or locally advanced pancreatic adenocarcinoma. Pract. Radiat. Oncol. 2018, 8, 95–106. [Google Scholar] [CrossRef]
- Jumeau, R.; Delouya, G.; Roberge, D.; Donath, D.; Beliveau-Nadeau, D.; Campeau, M.P. Stereotactic body radiotherapy (SBRT) for patients with locally advanced pancreatic cancer: A single center experience. Dig. Liver Dis. 2018, 50, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Heerkens, H.D.; van Vulpen, M.; Erickson, B.; Reerink, O.; Intven, M.P.; van den Berg, C.A.; Molenaar, I.Q.; Vleggaar, F.P.; Meijer, G.J. MRI guided stereotactic radiotherapy for locally advanced pancreatic cancer. Br. J. Radiol. 2018, 91, 20170563. [Google Scholar] [CrossRef]
- Mellon, E.A.; Hoffe, S.E.; Springett, G.M.; Frakes, J.M.; Strom, T.J.; Hodul, P.J.; Malafa, M.P.; Chuong, M.D.; Shridhar, R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015, 54, 979–985. [Google Scholar] [CrossRef]
- Herman, J.M.; Chang, D.T.; Goodman, K.A.; Dholakia, A.S.; Raman, S.P.; Hacker-Prietz, A.; Iacobuzio-Donahue, C.A.; Griffith, M.E.; Pawlik, T.M.; Pai, J.S.; et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015, 121, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.K.; Collins, S.P.; Slack, R.; Tse, G.; Charabaty, A.; Ley, L.; Berzcel, L.; Lei, S.; Suy, S.; Haddad, N.; et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat. Oncol. 2013, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, D.; Kim, J.; Christman-Skieller, C.; Chun, C.L.; Columbo, L.A.; Ford, J.M.; Fisher, G.A.; Kunz, P.L.; Van Dam, J.; Quon, A.; et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 181–188. [Google Scholar] [CrossRef]
- Mahadevan, A.; Miksad, R.; Goldstein, M.; Sullivan, R.; Bullock, A.; Buchbinder, E.; Pleskow, D.; Sawhney, M.; Kent, T.; Vollmer, C.; et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e615–e622. [Google Scholar] [CrossRef]
- Shen, Z.T.; Wu, X.H.; Li, B.; Wang, L.; Zhu, X.X. Preliminary efficacy of CyberKnife radiosurgery for locally advanced pancreatic cancer. Chin. J. Cancer 2010, 29, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Jain, S.; Goldstein, M.; Miksad, R.; Pleskow, D.; Sawhney, M.; Brennan, D.; Callery, M.; Vollmer, C. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 735–742. [Google Scholar] [CrossRef]
- Schellenberg, D.; Goodman, K.A.; Lee, F.; Chang, S.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Quon, A.; Desser, T.S.; Norton, J.; et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 678–686. [Google Scholar] [CrossRef]
- Okamoto, M.; Shiba, S.; Kobayashi, D.; Miyasaka, Y.; Okazaki, S.; Shibuya, K.; Ohno, T. Carbon-Ion Radiotherapy Combined with Concurrent Chemotherapy for Locally Advanced Pancreatic Cancer: A Retrospective Case Series Analysis. Cancers 2023, 15, 2857. [Google Scholar] [CrossRef]
- Lautenschlaeger, S.; Dumke, C.; Exeli, L.; Hauswald, H.; Engenhart-Cabillic, R.; Eberle, F. Treatment of primary or recurrent non-resectable pancreatic cancer with proton beam irradiation combined with gemcitabine-based chemotherapy. Strahlenther. Onkol. 2023, 199, 982–991. [Google Scholar] [CrossRef]
- Ami, K.; Terashima, K.; Ishida, J.; Suga, M.; Okawa, T.; Takahashi, D.; Park, S.; Matsuo, Y.; Nanno, Y.; Tokumaru, S.; et al. Proton radiotherapy as a treatment strategy to increase survival in locally advanced pancreatic cancer in the body and tail: A retrospective study. Radiat. Oncol. 2023, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hong, Z.; Zhang, Q.; Lin, L.C.; Shahnazi, K.; Wu, X.; Lu, J.; Jiang, G.; Wang, Z. Proton and carbon ion radiation therapy for locally advanced pancreatic cancer: A phase I dose escalation study. Pancreatology 2020, 20, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, W.J.; Woo, S.M.; Oh, E.S.; Youn, S.H.; Jang, H.Y.; Han, S.S.; Park, S.J.; Suh, Y.G.; Moon, S.H.; et al. Efficacy and feasibility of proton beam radiotherapy using the simultaneous integrated boost technique for locally advanced pancreatic cancer. Sci. Rep. 2020, 10, 21712. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Fukumitsu, N.; Saito, T.; Numajiri, H.; Murofushi, K.N.; Ohnishi, K.; Nonaka, T.; Ishikawa, H.; Okumura, T.; Sakurai, H. Concurrent chemoradiotherapy using proton beams for unresectable locally advanced pancreatic cancer. Radiother. Oncol. 2019, 136, 37–43. [Google Scholar] [CrossRef]
- Kawashiro, S.; Yamada, S.; Okamoto, M.; Ohno, T.; Nakano, T.; Shinoto, M.; Shioyama, Y.; Nemoto, K.; Isozaki, Y.; Tsuji, H.; et al. Multi-institutional Study of Carbon-ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1212–1221. [Google Scholar] [CrossRef]
- Maemura, K.; Mataki, Y.; Kurahara, H.; Kawasaki, Y.; Iino, S.; Sakoda, M.; Ueno, S.; Arimura, T.; Higashi, R.; Yoshiura, T.; et al. Comparison of proton beam radiotherapy and hyper-fractionated accelerated chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology 2017, 17, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Shinoto, M.; Yamada, S.; Terashima, K.; Yasuda, S.; Shioyama, Y.; Honda, H.; Kamada, T.; Tsujii, H.; Saisho, H.; Working Group for Pancreas, C. Carbon Ion Radiation Therapy With Concurrent Gemcitabine for Patients With Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Sachsman, S.; Nichols, R.C.; Morris, C.G.; Zaiden, R.; Johnson, E.A.; Awad, Z.; Bose, D.; Ho, M.W.; Huh, S.N.; Li, Z.; et al. ProtonTherapyandConcomitant CapecitabineforNon-Metastatic UnresectablePancreatic Adenocarcinoma. Int. J. Part. Ther. 2014, 1, 692–701. [Google Scholar] [CrossRef]
- Jin, L.; Wang, R.; Jiang, S.; Yue, J.; Liu, T.; Dou, X.; Zhu, K.; Feng, R.; Xu, X.; Chen, D.; et al. Dosimetric and clinical toxicity comparison of critical organ preservation with three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and RapidArc for the treatment of locally advanced cancer of the pancreatic head. Curr. Oncol. 2016, 23, e41–e48. [Google Scholar] [CrossRef]
- Bittner, M.I.; Grosu, A.L.; Brunner, T.B. Comparison of toxicity after IMRT and 3D-conformal radiotherapy for patients with pancreatic cancer—A systematic review. Radiother. Oncol. 2015, 114, 117–121. [Google Scholar] [CrossRef]
- Chang, C.-N.; Hsu, W.-T.; Li, G. Effectiveness and acute toxicity of intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in pancreatic cancer: A systematic review and a meta-analy. Ther. Radiol. Oncol. 2018, 2, 4–13. [Google Scholar] [CrossRef]
- Shi, C.; De, B.; Tran Cao, H.S.; Liu, S.; Florez, M.A.; Kouzy, R.; Grippin, A.J.; Katz, M.H.G.; Tzeng, C.D.; Ikoma, N.; et al. Escalated-dose radiotherapy for unresected locally advanced pancreatic cancer: Patterns of care and survival in the United States. Cancer Med. 2024, 13, e7434. [Google Scholar] [CrossRef]
- Burkon, P.; Trna, J.; Slavik, M.; Nemecek, R.; Kazda, T.; Pospisil, P.; Dastych, M.; Eid, M.; Novotny, I.; Prochazka, T.; et al. Stereotactic Body Radiotherapy (SBRT) of Pancreatic Cancer-A Critical Review and Practical Consideration. Biomedicines 2022, 10, 2480. [Google Scholar] [CrossRef]
- Chang, D.T.; Schellenberg, D.; Shen, J.; Kim, J.; Goodman, K.A.; Fisher, G.A.; Ford, J.M.; Desser, T.; Quon, A.; Koong, A.C. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009, 115, 665–672. [Google Scholar] [CrossRef]
- Hoyer, M.; Roed, H.; Sengelov, L.; Traberg, A.; Ohlhuis, L.; Pedersen, J.; Nellemann, H.; Kiil Berthelsen, A.; Eberholst, F.; Engelholm, S.A.; et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother. Oncol. 2005, 76, 48–53. [Google Scholar] [CrossRef]
- Sanford, N.N.; Narang, A.K.; Aguilera, T.A.; Bassetti, M.F.; Chuong, M.D.; Erickson, B.A.; Goodman, K.A.; Herman, J.M.; Intven, M.; Kilcoyne, A.; et al. NRG Oncology International Consensus Contouring Atlas on Target Volumes and Dosing Strategies for Dose-Escalated Pancreatic Cancer Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2024, 121, 918–929. [Google Scholar] [CrossRef]
- Tchelebi, L.T.; Lehrer, E.J.; Trifiletti, D.M.; Sharma, N.K.; Gusani, N.J.; Crane, C.H.; Zaorsky, N.G. Conventionally fractionated radiation therapy versus stereotactic body radiation therapy for locally advanced pancreatic cancer (CRiSP): An international systematic review and meta-analysis. Cancer 2020, 126, 2120–2131. [Google Scholar] [CrossRef]
- de Geus, S.W.L.; Eskander, M.F.; Kasumova, G.G.; Ng, S.C.; Kent, T.S.; Mancias, J.D.; Callery, M.P.; Mahadevan, A.; Tseng, J.F. Stereotactic body radiotherapy for unresected pancreatic cancer: A nationwide review. Cancer 2017, 123, 4158–4167. [Google Scholar] [CrossRef] [PubMed]
- Seshacharyulu, P.; Baine, M.J.; Souchek, J.J.; Menning, M.; Kaur, S.; Yan, Y.; Ouellette, M.M.; Jain, M.; Lin, C.; Batra, S.K. Biological determinants of radioresistance and their remediation in pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, O.; Sishc, B.J.; Saha, J.; Pompos, A.; Rahimi, A.; Story, M.D.; Davis, A.J.; Kim, D.W.N. Carbon Ion Radiotherapy: A Review of Clinical Experiences and Preclinical Research, with an Emphasis on DNA Damage/Repair. Cancers 2017, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Rutenberg, M.S.; Nichols, R.C. Proton beam radiotherapy for pancreas cancer. J. Gastrointest. Oncol. 2020, 11, 166–175. [Google Scholar] [CrossRef]
- Bae, S.H.; Jang, W.I.; Mortensen, H.R.; Weber, B.; Kim, M.S.; Hoyer, M. Recent update of proton beam therapy for hepatocellular carcinoma: A systematic review and meta-analysis. J. Liver Cancer 2024, 24, 286–302. [Google Scholar] [CrossRef]
- Huguet, F.; Mukherjee, S.; Javle, M. Locally advanced pancreatic cancer: The role of definitive chemoradiotherapy. Clin. Oncol. 2014, 26, 560–568. [Google Scholar] [CrossRef]
- Huguet, F.; Andre, T.; Hammel, P.; Artru, P.; Balosso, J.; Selle, F.; Deniaud-Alexandre, E.; Ruszniewski, P.; Touboul, E.; Labianca, R.; et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J. Clin. Oncol. 2007, 25, 326–331. [Google Scholar] [CrossRef]
- Krishnan, S.; Rana, V.; Janjan, N.A.; Varadhachary, G.R.; Abbruzzese, J.L.; Das, P.; Delclos, M.E.; Gould, M.S.; Evans, D.B.; Wolff, R.A.; et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007, 110, 47–55. [Google Scholar] [CrossRef]
- Torgeson, A.; Lloyd, S.; Boothe, D.; Tao, R.; Whisenant, J.; Garrido-Laguna, I.; Cannon, G.M. Multiagent induction chemotherapy followed by chemoradiation is associated with improved survival in locally advanced pancreatic cancer. Cancer 2017, 123, 3816–3824. [Google Scholar] [CrossRef]
- Maggino, L.; Malleo, G.; Marchegiani, G.; Viviani, E.; Nessi, C.; Ciprani, D.; Esposito, A.; Landoni, L.; Casetti, L.; Tuveri, M.; et al. Outcomes of Primary Chemotherapy for Borderline Resectable and Locally Advanced Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2019, 154, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; Jager, D.; Buchler, M.W.; Strobel, O.; Hackert, T.; Palmer, D.H.; Neoptolemos, J.P. Chemotherapy for pancreatic cancer. Presse Med. 2019, 48, e159–e174. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Theijse, R.T.; Seelen, L.W.F.; Groot Koerkamp, B.; van Eijck, C.H.J.; Wolfgang, C.L.; van Tienhoven, G.; van Santvoort, H.C.; Molenaar, I.Q.; Wilmink, J.W.; et al. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Hess, C.F. The impact of gross tumor volume (GTV) and clinical target volume (CTV) definition on the total accuracy in radiotherapy theoretical aspects and practical experiences. Strahlenther. Onkol. 2003, 179, 21–30. [Google Scholar] [CrossRef]
- Goodman, K.A.; Regine, W.F.; Dawson, L.A.; Ben-Josef, E.; Haustermans, K.; Bosch, W.R.; Turian, J.; Abrams, R.A. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 901–908. [Google Scholar] [CrossRef]
- Murphy, J.D.; Adusumilli, S.; Griffith, K.A.; Ray, M.E.; Zalupski, M.M.; Lawrence, T.S.; Ben-Josef, E. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 801–808. [Google Scholar] [CrossRef]
- Brunner, T.B.; Haustermans, K.; Huguet, F.; Morganti, A.G.; Mukherjee, S.; Belka, C.; Krempien, R.; Hawkins, M.A.; Valentini, V.; Roeder, F. ESTRO ACROP guidelines for target volume definition in pancreatic cancer. Radiother. Oncol. 2021, 154, 60–69. [Google Scholar] [CrossRef]
- Palta, M.; Godfrey, D.; Goodman, K.A.; Hoffe, S.; Dawson, L.A.; Dessert, D.; Hall, W.A.; Herman, J.M.; Khorana, A.A.; Merchant, N.; et al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2019, 9, 322–332. [Google Scholar] [CrossRef]
- Blettner, M.; Sauerbrei, W.; Schlehofer, B.; Scheuchenpflug, T.; Friedenreich, C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int. J. Epidemiol. 1999, 28, 1–9. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardiere, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Grimbergen, G.; Eijkelenkamp, H.; Snoeren, L.M.W.; Bahij, R.; Bernchou, U.; van der Bijl, E.; Heerkens, H.D.; Binda, S.; Ng, S.S.W.; Bouchart, C.; et al. Treatment planning for MR-guided SBRT of pancreatic tumors on a 1.5 T MR-Linac: A global consensus protocol. Clin. Transl. Radiat. Oncol. 2024, 47, 100797. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Study | No. of pts | ICT (%) | CC RT (%) | ACT (%) | RT | mRT Dose (Gy) | mNo. of fx (Range) | ENI | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Passoni [22] | 2024 | P/S | 217 | 100 | 93 | 0 | IMRT | 44.3 | 15 | No | 5 |
| Ogawa_A [23] | 2023 | R/S | 23 | 100 | 100 | IMRT | 48 | 15 | No | 5 | |
| Ogawa_B [23] | 2023 | R/S | 14 | 100 | 100 | IMRT | 48 | 15 | No | 5 | |
| Argalacsova [24] | 2023 | R/M | 19 | 100 | 0 | IMRT | 39.9 | 15 | No | 8 | |
| Shi [25] | 2022 | R/S | 22 | 100 | 0 | IMRT | SIB: 50/30 | 10 | No | 5 | |
| Roy [26] | 2022 | P/M | 14 | 0 | 100 | 0 | IMRT | 50.4 | 28 | No | 6 |
| Reyngold [27] | 2021 | P/S | 119 | 98 | 93 | 16 | IMRT | 75 | 25 (15 or 25) | Yes | 5 |
| Felice [28] | 2019 | P/S | 10 | 80 | 0 | 0 | IMRT | 52/13 | 13 | No | 4 |
| Lewis [29] | 2019 | P/S | 12 | 0 | 100 | IMRT | SIB: 57/45 | 25 | No | 5 | |
| Cuneo [30] | 2019 | P/S | 34 | 100 | 100 | 100 | IMRT | 52.5 | 25 | No | 5 |
| Oh [31] | 2018 | R/S | 47 | 79 | 100 | 49 | IMRT | SIB: 55/44 | 22 | Yes | 7 |
| Goto [32] | 2018 | R/S | 27 | 100 | 100 | IMRT | 48 | 15 | Yes | 7 | |
| Park_A [33] | 2017 | R/S | 226 | 100 | 97 | IMRT | NR (25–28) | Yes | 8 | ||
| Colbert [34] | 2017 | R/S | 59 | 100 | 100 | IMRT | 63 | 28 (15–28) | No | 5 | |
| Wang [35] | 2015 | R/S | 31 | IMRT | 46 | 23 | No | 5 | |||
| Jiang [36] | 2014 | P/S | 15 | 0 | 100 | IMRT | 50.4 | 28 | Yes | 5 | |
| Chiorean [37] | 2014 | P/S | 25 | 100 | 100 | 100 | IMRT | SIB: 50/45 | 25 | No | 5 |
| Ben-Josef [38] | 2012 | P/M | 50 | 100 | 100 | 84 | IMRT | 55 | 25 (24–25) | No | 5 |
| Abelson [39] | 2012 | R/S | 18 | 28 | 100 | 17 | IMRT | 54 | 30 (22–33) | Yes | 6 |
| Milano [40] | 2004 | R/S | 11 | 100 | IMRT | 59.4 | 33 (28–33) | No | 4 | ||
| Van ’t Land [41] | 2023 | P/S | 38 | 100 | 100 | 100 | SBRT | 40 | 5 | No | 5 |
| Reyngold [42] | 2023 | P/M | 24 | 100 | 0 | 0 | SBRT | 30 | 3 | No | 5 |
| Hurmuz [43] | 2023 | R/S | 24 | 67 | 34 | 67 | SBRT | 35 | 5 (3–5) | No | 5 |
| Doppenberg [44] | 2023 | R/S | 74 | 100 | 0 | 30 | SBRT | 40 | 5 (4–5) | No | 5 |
| Comito [45] | 2023 | R/S | 142 | 54 | 0 | 30 | SBRT | 45 | 6 | No | 5 |
| Lee [46] | 2022 | R/S | 33 | 100 | 0 | SBRT | 5 | No | 5 | ||
| Kaucic [47] | 2022 | R/S | 54 | 0 | SBRT | 45 | 3 (1–5) | No | 5 | ||
| Kaucic [48] | 2022 | R/S | 45 | 0 | SBRT | 40 | 5 (3–5) | No | 5 | ||
| Zhu [49] | 2021 | P/S | 63 | 0 | 0 | 100 | SBRT | 36 | 5 | No | 5 |
| Teriaca [50] | 2021 | P/M | 39 | 100 | 0 | 0 | SBRT | 40 | 5 | No | 6 |
| Qing [51] | 2021 | P/S | 16 | 0 | 100 | 87 | SBRT | 40 | 5 | No | 5 |
| Bouchart [52] | 2021 | P/M | 16 | 100 | 0 | SBRT | 5 | No | 5 | ||
| Jung [53] | 2019 | R/S | 95 | 14 | 0 | 81 | SBRT | 28 | 4 (4–5) | No | 5 |
| Quan [54] | 2018 | P/S | 15 | 100 | 0 | 47 | SBRT | 36 | 3 | No | 5 |
| Jumeau [55] | 2018 | R/S | 17 | 29 | 0 | SBRT | 30 | 5 (5–6) | No | 4 | |
| Heerkens [56] | 2018 | P/S | 20 | 0 | 0 | 0 | SBRT | 24 | 3 | No | 5 |
| Park_B [33] | 2017 | R/S | 44 | 95 | 0 | SBRT | 5 | No | 8 | ||
| Mellon [57] | 2015 | R/S | 49 | 100 | 0 | SBRT | SIB: 40/30 | 5 | No | 5 | |
| Herman [58] | 2015 | P/M | 49 | 90 | 0 | 100 | SBRT | 33 | 5 | No | 6 |
| Gurka [59] | 2013 | P/S | 10 | 100 | 0 | 100 | SBRT | 25 | 5 | No | 5 |
| Schellenberg [60] | 2011 | P/S | 20 | 100 | 0 | 100 | SBRT | 25 | 1 | No | 5 |
| Mahadevan [61] | 2011 | R/S | 39 | 100 | 0 | 95 | SBRT | 24 | 3 | No | 5 |
| Shen [62] | 2010 | R/S | 20 | 0 | SBRT | 45 | 4 (3–6) | No | 4 | ||
| Mahadevan [63] | 2010 | R/S | 36 | 0 | 0 | 86 | SBRT | 30 | 3 | No | 5 |
| Schellenberg [64] | 2008 | P/S | 16 | 100 | 0 | SBRT | 25 | 1 | No | 5 | |
| Okamoto [65] | 2023 | R/S | 44 | 84 | 100 | 100 | PBT | 55.2 | 12 | Yes | 5 |
| Lautenschlaeger [66] | 2023 | R/S | 15 | 73 | PBT | No | 4 | ||||
| Ami [67] | 2023 | R/S | 200 | 53 | 93 | PBT | 67.5 | 25 | Yes | 5 | |
| Yu [68] | 2020 | P/S | 10 | 90 | 60 | PBT | 65.4 | 33 (32–34) | Yes | 5 | |
| Kim [69] | 2020 | R/S | 81 | 24 | 90 | 74 | PBT | SIB: 45/30 | 10 | No | 7 |
| Hiroshima [70] | 2019 | R/S | 42 | 76 | 100 | 81 | PBT | 60 | 25 (25–33) | No | 5 |
| Kawashiro [71] | 2018 | R/M | 72 | 74 | 78 | 0 | PBT | 55.2 | 12 | Yes | 6 |
| Maemura [72] | 2017 | R/S | 10 | 100 | 100 | 100 | PBT | 50 | 25 | Yes | 7 |
| Shinoto [73] | 2016 | P/S | 72 | 0 | 99 | 0 | PBT | 12 | Yes | 5 | |
| Sachsmann [74] | 2014 | P/S | 11 | 73 | 100 | PBT | 59.4 | 33 | No | 5 |
| Author | RT | 1-yr LPFS (%) | 2-yr LPFS (%) | 3-yr LPFS (%) | 1-yr PFS (%) | 2-yr PFS (%) | 3-yr PFS (%) | mOS (mo) | 1-yr OS (%) | 2-yr OS (%) | 3-yr OS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Passoni [22] | IMRT | 71 | 20 | 12 | 51 | 8 | 3 | 20 | 85 | 36 | 13 |
| Ogawa_A [23] | IMRT | 73 | 33 | 25 | 18 | 78 | 35 | 10 | |||
| Ogawa_B [23] | IMRT | 90 | 90 | 90 | 17 | 65 | 43 | 37 | |||
| Argalacsova [24] | IMRT | 14 | 58 | 32 | 5 | ||||||
| Shi [25] | IMRT | 52 | 16 | 73 | 16 | ||||||
| Roy [26] | IMRT | 35 | 18 | 18 | 11 | 50 | 27 | 18 | |||
| Reyngold [27] | IMRT | 27 | |||||||||
| Felice [28] | IMRT | 69 | 83 | ||||||||
| Lewis [29] | IMRT | 42 | 0 | 0 | 12 | 50 | 0 | 0 | |||
| Cuneo [30] | IMRT | 68 | 22 | 10 | 10 | 22 | 72 | 34 | 19 | ||
| Oh [31] | IMRT | 14 | |||||||||
| Goto [32] | IMRT | 73 | 43 | 22 | 92 | 38 | 28 | ||||
| Park_A [33] | IMRT | ||||||||||
| Colbert [34] | IMRT | ||||||||||
| Wang [35] | IMRT | 16 | 62 | 32 | 9 | ||||||
| Jiang [36] | IMRT | 29 | 19 | 19 | 13 | 68 | 11 | 11 | |||
| Chiorean [37] | IMRT | 34 | 4 | 0 | 13 | 52 | 20 | 16 | |||
| Ben-Josef [38] | IMRT | 87 | 59 | 59 | 15 | 74 | 30 | 9 | |||
| Abelson [39] | IMRT | 64 | 16 | 8 | 24 | ||||||
| Milano [40] | IMRT | 0 | 0 | 13 | 58 | 12 | |||||
| Van ’t Land [41] | SBRT | 47 | 0 | 19 | 82 | 29 | |||||
| Reyngold [42] | SBRT | ||||||||||
| Hurmuz [43] | SBRT | ||||||||||
| Doppenberg [44] | SBRT | 20 | 79 | 29 | 13 | ||||||
| Comito [45] | SBRT | ||||||||||
| Lee [46] | SBRT | ||||||||||
| Kaucic [47] | SBRT | 100 | 72 | 24 | 91 | 63 | 40 | ||||
| Kaucic [48] | SBRT | 96 | 69 | 17 | 71 | 33 | 18 | ||||
| Zhu [49] | SBRT | 12 | 14 | 73 | |||||||
| Teriaca [50] | SBRT | 81 | 62 | 53 | 43 | 23 | 15 | 18 | 77 | 18 | 13 |
| Qing [51] | SBRT | 69 | 38 | 6 | 15 | 69 | 25 | 19 | |||
| Bouchart [52] | SBRT | 25 | 88 | ||||||||
| Jung [53] | SBRT | 80 | 40 | 15 | 43 | 8 | 3 | 17 | 67 | 20 | 5 |
| Quan [54] | SBRT | 78 | 52 | 14 | 60 | 16 | |||||
| Jumeau [55] | SBRT | 22 | |||||||||
| Heerkens [56] | SBRT | 9 | |||||||||
| Park_B [33] | SBRT | ||||||||||
| Mellon [57] | SBRT | 78 | 58 | 22 | 15 | 15 | 78 | 38 | 9 | ||
| Herman [58] | SBRT | 78 | 32 | 10 | 14 | 59 | 18 | ||||
| Gurka [59] | SBRT | 20 | 0 | 12 | 50 | 0 | |||||
| Schellenberg [60] | SBRT | 37 | 17 | 12 | 50 | 20 | 7 | ||||
| Mahadevan [61] | SBRT | 55 | 25 | 20 | 69 | 33 | |||||
| Shen [62] | SBRT | ||||||||||
| Mahadevan [63] | SBRT | 30 | 14 | 14 | 51 | 25 | |||||
| Schellenberg [64] | SBRT | 51 | 25 | 11 | 50 | 18 | |||||
| Okamoto [65] | PBT | 35 | 95 | 71 | 49 | ||||||
| Lautenschlaeger [66] | PBT | ||||||||||
| Ami [67] | PBT | ||||||||||
| Yu [68] | PBT | 67 | 27 | 27 | 60 | 20 | 20 | 17 | 80 | 13 | 13 |
| Kim [69] | PBT | 79 | 38 | 24 | 45 | 18 | 11 | 19 | 73 | 35 | 7 |
| Hiroshima [70] | PBT | 90 | 77 | 28 | 85 | 59 | |||||
| Kawashiro [71] | PBT | ||||||||||
| Maemura [72] | PBT | 60 | 41 | 22 | 80 | 45 | 23 | ||||
| Shinoto [73] | PBT | 92 | 83 | 78 | 20 | 73 | 35 | 16 | |||
| Sachsmann [74] | PBT | 86 | 69 | 55 | 14 | 18 | 61 | 31 |
| Group | Cohorts | N | p, Heterogeneity | I2 | Random Event Rate (95% CI) | p (Between Groups) |
|---|---|---|---|---|---|---|
| 1-year OS | 40 | 1485 | <0.0001 | 69.29% | 0.71 (0.66–0.76) | |
| IMRT | 16 | 542 | <0.0001 | 76.64% | 0.67 (0.57–0.76) | 0.1111 |
| PBT | 7 | 269 | 0.0175 | 60.99% | 0.80 (0.71–0.89) | |
| SBRT | 17 | 674 | 0.0005 | 61.55% | 0.71 (0.65–0.77) | |
| mBED ≤ 60 Gy10 | 16 | 724 | <0.0001 | 71.13% | 0.66 (0.58–0.73) | 0.1317 |
| mBED > 60 Gy10 | 22 | 674 | <0.0001 | 70.22% | 0.74 (0.67–0.80) | |
| mICT > 1 mo | 9 | 523 | 0.0025 | 66.41% | 0.80 (0.72–0.86) | 0.0031 |
| mICT ≤ 1 mo | 14 | 424 | 0.0040 | 57.39% | 0.64 (0.56–0.72) | |
| ENI (−) | 33 | 1290 | <0.0001 | 61.58% | 0.70 (0.66–0.75) | 0.6109 |
| ENI (+) | 7 | 195 | <0.0001 | 85.61% | 0.76 (0.56–0.91) | |
| 2-year OS | 36 | 1378 | <0.0001 | 70.57% | 0.29 (0.25–0.34) | |
| IMRT | 14 | 514 | 0.0522 | 41.46% | 0.28 (0.22–0.34) | 0.1121 |
| PBT | 7 | 269 | <0.0001 | 78.73% | 0.43 (0.28–0.57) | |
| SBRT | 15 | 595 | <0.0001 | 71.09% | 0.26 (0.19–0.34) | |
| mBED ≤ 60 Gy10 | 16 | 724 | 0.0009 | 60.49% | 0.25 (0.19–0.31) | 0.1363 |
| mBED > 60 Gy10 | 19 | 583 | <0.0001 | 75.07% | 0.33 (0.25–0.41) | |
| mICT > 1 mo | 8 | 507 | <0.0001 | 78.13% | 0.34 (0.25–0.45) | 0.0439 |
| mICT ≤ 1 mo | 13 | 361 | 0.0350 | 46.03% | 0.22 (0.16–0.28) | |
| ENI (−) | 30 | 1201 | <0.0001 | 65.75% | 0.28 (0.23–0.33) | 0.3641 |
| ENI (+) | 6 | 177 | <0.0001 | 81.20% | 0.37 (0.19–0.56) | |
| 3-year OS | 25 | 1089 | <0.0001 | 68.51% | 0.14 (0.10–0.19) | |
| IMRT | 12 | 481 | 0.1987 | 24.95% | 0.13 (0.09–0.17) | 0.7400 |
| PBT | 5 | 216 | <0.0001 | 85.16% | 0.19 (0.05–0.37) | |
| SBRT | 8 | 392 | <0.0001 | 78.37% | 0.14 (0.07–0.23) | |
| mBED ≤ 60 Gy10 | 11 | 568 | 0.4707 | 0% | 0.09 (0.06–0.11) | 0.0040 |
| mBED > 60 Gy10 | 13 | 450 | <0.0001 | 72.58% | 0.20 (0.12–0.28) | |
| mICT > 1 mo | 5 | 415 | <0.0001 | 86.35% | 0.18 (0.08–0.30) | 0.5665 |
| mICT ≤ 1 mo | 9 | 250 | 0.2772 | 18.62% | 0.13 (0.08–0.19) | |
| ENI (−) | 19 | 912 | 0.0005 | 59.73% | 0.12 (0.08–0.16) | 0.0670 |
| ENI (+) | 6 | 177 | 0.0066 | 68.91% | 0.23 (0.11–0.37) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.H.; Jang, W.I.; Yu, J.I.; Park, H.C.; Moon, J.E.; Haustermans, K.; Scorsetti, M.; Høyer, M.; Kim, M.S. Radiotherapy for Locally Advanced Pancreatic Cancer in the Modern Era: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 2959. https://doi.org/10.3390/cancers17182959
Bae SH, Jang WI, Yu JI, Park HC, Moon JE, Haustermans K, Scorsetti M, Høyer M, Kim MS. Radiotherapy for Locally Advanced Pancreatic Cancer in the Modern Era: A Systematic Review and Meta-Analysis. Cancers. 2025; 17(18):2959. https://doi.org/10.3390/cancers17182959
Chicago/Turabian StyleBae, Sun Hyun, Won Il Jang, Jeong Il Yu, Hee Chul Park, Ji Eun Moon, Karin Haustermans, Marta Scorsetti, Morten Høyer, and Mi Sook Kim. 2025. "Radiotherapy for Locally Advanced Pancreatic Cancer in the Modern Era: A Systematic Review and Meta-Analysis" Cancers 17, no. 18: 2959. https://doi.org/10.3390/cancers17182959
APA StyleBae, S. H., Jang, W. I., Yu, J. I., Park, H. C., Moon, J. E., Haustermans, K., Scorsetti, M., Høyer, M., & Kim, M. S. (2025). Radiotherapy for Locally Advanced Pancreatic Cancer in the Modern Era: A Systematic Review and Meta-Analysis. Cancers, 17(18), 2959. https://doi.org/10.3390/cancers17182959






