Simple Summary
Small kidney tumors (4 cm or less) could be managed with partial kidney removal, active monitoring, thermal ablation, or, less frequently, complete kidney removal, depending on the tumor characteristics and patient preference. Thermal ablation offers a minimally invasive approach for treating small kidney tumors and could be performed using heat (radiofrequency ablation) or cold (cryoablation). This research aimed to evaluate the long-term effectiveness of cryoablation in achieving (1) tumor control and (2) preserving kidney function, specifically in these patients. Our findings indicate that tumor control outcomes are largely achievable in patients with small kidney masses attributed to Renal Cell Carcinoma. Excluding those patients who had only one kidney, renal function was acceptable among the patients with a small kidney mass.
Abstract
Background/Objectives: Cryoablation is a minimally invasive treatment option for patients with a small renal mass (SRM). We aimed to present the long-term functional and oncologic outcomes of cryoablation for SRMs. Methods: We retrospectively reviewed patients treated with percutaneous or laparoscopic cryoablation for an SRM (≤4 cm in diameter) at our tertiary hospital between October 2001 and December 2011. Primary outcomes included technical failure (persistent CT enhancement post-ablation) and progression (local recurrence or metastasis). Trifecta is defined as the absence of severe complications (Clavien–Dindo > 2), no oncological progression, and ≤10% decline in eGFR. Results: A total of 129 patients with a median age of 67 (IQR 58–74) years were analyzed. The median (IQR) clinical and radiologic follow-ups across all patients were 136 (54–180) and 74 (23–147) months, respectively, with a median (IQR) tumor volume of 3.3 (1.6–6.6) cm3. Among those with available biopsy data (n = 86), 62 (72%) were diagnosed with Renal Cell Carcinoma (RCC), and 24 (28%) exhibited benign pathologies, including angiomyolipoma, oncocytic neoplasm, and non-diagnostic pathology. Of all patients, six experienced high-grade complications. Among non-solitary kidney patients with available creatinine values between 13 and 36 months post-treatment, 64% had ≤10% eGFR decline compared to baseline. Notably, 58% (26/48) of patients with RCC (non-solitary kidney) achieved our trifecta definition at 36 months. Metastasis-free, cancer-specific, and overall survival at 15-year follow-up were 85%, 96%, and 46%, respectively. Univariable regression identified tumor volume and solitary kidney status at ablation as significant predictors for oncological progression. Conclusions: Cryoablation for the SRM showed sustained oncological and functional efficacy over long-term follow-up.
1. Introduction
The Surveillance, Epidemiology, and End Results (SEER) Program estimates that approximately 80,980 individuals will be diagnosed with kidney and renal pelvis cancer in 2025, ranking it as the seventh most common cancer type and accounting for 4.0% of all new cancer diagnoses in the United States [1,2]. Since the 1990s, the growing utilization of cross-sectional diagnostic imaging has led to a substantial rise in the incidental detection of renal masses, resulting in an approximately 1.5-fold increase in incidence [1]. Consequently, the related death rates declined from 4.3% in 1992 to 3.4% in 2023 [1]. It has been hypothesized that despite the rising incidence of renal cancer, its declining mortality in developed countries is largely attributed to the timely diagnosis of the Small Renal Masses (SRMs), secondary to increased diagnostic abdominal imaging utilization [3].
Approximately 70–80% of SRMs are malignant, though most exhibit indolent behavior and pose insignificant metastatic risk at the time of diagnosis [4]. While active surveillance is acknowledged as a management option in major urology and oncology guidelines, particularly for frail patients with limited life expectancy, nephron-sparing approaches have remained the preferred treatment choice for SRMs over the past two decades, given their advantages in preserving renal function while maintaining favorable oncologic outcomes [5,6,7]. Partial Nephrectomy (PN) stands as the gold-standard treatment for clinical stage T1 renal tumors. However, thermal ablative modalities, such as cryoablation, radiofrequency, and microwave ablation, are viable alternatives in appropriately selected patients, provided thorough counseling is conducted regarding the risks of tumor persistence or recurrence and the potential need for repeat ablation [8]. Despite the growing adoption of these novel treatments, which have demonstrated efficacy and low morbidity in the short- to intermediate-term, there remains a gap in the literature regarding long-term outcomes, specifically follow-ups beyond a decade [9]. This study aims to report long-term oncologic and renal functional outcomes following SRM cryoablation.
2. Materials and Methods
2.1. Study Design and Patient Population
We retrospectively reviewed a prospectively maintained renal mass database and included 158 consecutive patients who underwent 170 renal cryoablation procedures at our tertiary referral center between October 2001 and December 2011. The institutional review board approved the study. After excluding patients with syndromic etiologies of Renal Cell Carcinoma (RCC), such as Von Hippel–Lindau syndrome (3 cases), >4 cm tumor diameter (6 cases), and those with less than six months of follow-up (20 cases), 129 patients with an SRM were included in the analysis. All cases had preoperative cross-sectional contrast-enhanced imaging with Computed Tomography (CT) and/or Magnetic Resonance Imaging (MRI).
2.2. Surgical Intervention and Follow-Up
The decision to perform a renal biopsy before or during cryoablation was made based on physician judgment. Posterior lesions were preferentially managed with percutaneous cryoablation, while anteromedial lesions were favored for laparoscopic cryoablation due to their proximity to hilar structures. The choice between the two approaches was determined by physician discretion and patient preference. The percutaneous procedures were performed by the interventional radiology team, while the urology service conducted the laparoscopic interventions, as previously reported [10,11,12]. Follow-up assessments were scheduled at 3, 6, and 12 months post-procedure and annually thereafter. Serum creatinine levels were also measured at each follow-up visit, along with a general clinical assessment and evaluation for potential complications. Cross-sectional contrast-enhanced imaging was planned within two to six months after ablation to assess technical success, followed by surveillance at month twelve, and then annually for a minimum of three years post-ablation.
2.3. Study Variables and Outcomes
Data encompassing demographics (age, gender, race, body mass index), comorbidities (coronary arterial disease, diabetes, chronic obstructive pulmonary disease, dyslipidemia, hypertension, American Society of Anesthesiology score), smoking history, surgical history, imaging details (lesion size, RENAL nephrometry score), solitary kidney (SK) status, and radiologic and clinical follow-up variables were systematically collected.
The primary outcome included oncological efficacy, while secondary outcomes focused on functional efficacy and adverse events of SRM cryoablation. The oncological outcomes of interest, among those diagnosed with RCC, were local recurrence-free survival (RFS), metastasis-free survival (MFS), cancer-specific survival (CSS), and overall survival (OS). Procedural failure (persistence) was defined as the presence of enhancement on CT within the first three months after ablation and/or a positive biopsy, if applicable. If the first post-ablation imaging was indeterminate or postponed for any reason, the next imaging within the first six months from ablation was used to assess the technical failure. Local recurrence was defined as the appearance of a new enhancing or enlarging lesion within or at the ablated area after a technically successful ablation. Disease progression was categorized as local recurrence, metastasis, or need for systemic therapy. Complications were graded using the Clavien–Dindo system [13].
Functional outcomes were evaluated by measuring changes in creatinine levels and the estimated glomerular filtration rate (eGFR), which was calculated using the Modification of Diet in Renal Disease (MDRD) Study formula [14]. Regarding functional assessment time points, the relevant data were stratified into short-term (0–3 months), intermediate-term (4–12 months), and long-term (13–36 months) based on similar studies [15]. For patients on temporary dialysis within the first three months post-ablation, we only considered the creatinine measurement recorded immediately before dialysis initiation to account for acute kidney injury in short-term follow-up. Trifecta achievement was defined as the absence of disease progression at any point of follow-up, ≤10% decline in eGFR within 36 months of follow-up (<25% for SK patients), and the absence of grade 3–4 Clavien–Dindo complications [16]. The 10% and 25% eGFR cut-offs for non-SK and SK patients, respectively, were chosen based on prior literature [16,17]. The extended long-term (>36 months) renal functional outcomes were assessed by evaluating chronic kidney disease (CKD) stage progression, categorized as G1–G5 (≥90, 60–89, 30–59, 15–29, and <15 mL/min/1.73 m2). Additionally, we defined the term “expected eGFR” to be able to report even longer-term functional outcomes. In individuals without CKD and with normal kidney parenchyma, eGFR naturally declines from normal levels (≥90 mL/min/1.73 m2) by approximately 1 mL/min/1.73 m2 annually after the third decade of life [18,19,20]. Accordingly, “expected eGFR” was calculated based on the baseline (i.e., preoperative) eGFR, adjusted for an estimated decline of 0.5% per year.
2.4. Statistical Analysis
Continuous variables were presented as the median and Interquartile Range (IQR), while categorical variables were summarized as frequencies and percentages. The survival curves were estimated using Kaplan–Meier analysis. Creatinine and eGFR change over time were evaluated via the Generalized Linear Mixed Model, and Bonferroni post hoc adjustment was used for multiple pairwise comparisons. The threshold of statistical significance was p < 0.05. All statistical analyses were conducted using SPSS version 26 (IBM Corp., Somers, NY, USA) and Firth’s Cox Regression using R 4.2.0 (http://cran.r-project.org, accessed on 10 September 2024), and graphs were created using the ggplot2 package version 3.4.2.
3. Results
3.1. General Characteristics
A total of 129 patients (140 lesions) with a median age of 67 years (IQR 58–74) were included in the study. Among these, the median clinical and radiologic follow-up duration was 136 (IQR 54–180) and 74 (IQR 23–147) months, respectively. Biopsy data was available for 86 patients (67%). Among these, 62 (72%) were histologically diagnosed with RCC, and 24 (28%) exhibited benign pathologies, including angiomyolipoma in 3 patients, oncocytic neoplasm in 9 patients, and non-diagnostic pathology in 12 cases. Patient demographics are reported in Table 1.

Table 1.
Patient demographics. Abbreviations: RCC: Renal Cell Carcinoma; BMI: Body Mass Index; CAD: Coronary Arterial Disease; DM: Diabetes Mellitus; COPD: Chronic Obstructive Pulmonary Disease; IQR: Interquartile Range; PVD: Peripheral Vascular Disease.
Laparoscopic ablation was employed in 62 (48%), percutaneous ablation in 61 (48%), hand-assisted technique in 3 (2%), and robot-assisted laparoscopic technique in 3 (2%). Median preoperative creatinine and GFR were 1.0 mg/dL (IQR 0.8–1.3) and 68 mL/min/1.73 m2 (IQR 53–87), respectively. Table 2 shows tumor characteristics along with procedure-related information. Complete response to ablation (no enhancement on follow-up contrast CT) was achieved in 119/126 (94%) cases, while 7/126 (6%) of cases experienced radiologic procedure failure, and post-ablation images in three cases were not available within the first year of ablation for evaluating procedural success.

Table 2.
Tumor characteristics and details related to the cryoablation procedure. Abbreviations: IQR: Interquartile Range; RCC: Renal Cell Carcinoma.
3.2. Adverse Events
Most cryoablation procedures (100/129; 78%) were conducted without adverse events. Of 29 reported complications, 24 were Clavien–Dindo grade 1–2, while 6 had higher-grade adverse events. The complication rate for any Clavien–Dindo grade was twice as high in patients with left-sided tumors (31%, 19/61) compared to those with right-sided tumors (15%, 10/68), showing a statistically significant difference (p = 0.026). Considering only cases with Clavien–Dindo grade 3–4 complications, four of those were left-sided and one right-sided, but this difference did not reach statistical significance (p = 0.191). Appendix A details these complications, along with their related management.
3.3. Functional Outcomes
Solitary Kidney (SK) and non-SK cases were analyzed separately, once at the 36-month follow-up and once among those with follow-ups exceeding 36 months.
3.3.1. Non-SK Patients; 36-Month Follow-Up
For non-SK cases, the generalized mixed linear model with pairwise comparisons between time points showed a significant creatinine increase between baseline and 0–3 month intervals [mean difference (SE): 0.091 (0.021); p < 0.001]. Although statistically significant, the mean increment was ≤10% compared to the baseline. Of note, creatinine measures increased gradually from the first post-ablation checkpoint compared to later checkpoints, but this increment did not reach a statistically significant level (Table 3). A similar analysis revealed a statistically significant decline in eGFR measures post-ablation [mean difference (SE): −4.5 (1.46); p = 0.021] and in the third (12–24 month) checkpoint [mean difference (SE): −7.1 (2.30); p = 0.021] compared to pre-ablation (Table 3). Although statistically significant, the mean decline was ≤10% compared to the baseline. Of 116 non-SK patients, 81 had available creatinine between 13 and 36 months; 52 (64%) had a ≤10% creatinine elevation, and 51 (63%) had a ≤10% eGFR decline compared to baseline.

Table 3.
Renal functional outcomes, excluding solitary kidney cases. Abbreviations: eGFR: estimated Glomerular Filtration Rate.
3.3.2. SK Patients; 36-Month Follow-Up
Among 13 SK patients, renal function success, defined as <25% eGFR decline, was achieved in 54% (7/13). Of these, 29% (2/7) of biopsy-proven RCC patients and 83% (5/6) of the remainder of the SK cases were within the first 36-month follow-up.
3.3.3. Non-SK Patients; >36-Month Follow-Up
We also evaluated the extended long-term (>36 months) renal function outcomes. Ninety-three cases had available creatinine measurements beyond 36 months; eight were SK cases at the time of ablation and thus were excluded from the long-term analysis. The remaining 85 non-SK patients had a mean creatinine follow-up of 138 ± 58 months (median 155, range 37–253) with a mean creatinine and eGFR of 1.45 ± 1.12 (median 1.2) mg/dL and 59 ± 26 (median 59), respectively. Mean creatinine increase and eGFR decline compared to baseline were 0.38 ± 0.97 (median 0.20) and 14 ± 22 (median 13) at their last follow-up. Two patients progressed to dialysis after 49 and 144 months. Of note other two patients underwent unilateral Radical Nephrectomy (RN) after 53 (Case #3, cause: local recurrence) and 104 (Case #99, left renal artery stenosis on the contralateral side to cryoablation) months following ablation, and their last creatinine before RN was used to assess long-term functional outcomes. One-stage and two-stage CKD progression were detected in 34/85 (40%) and 7/85 (8%) patients, respectively. Based on the definition of “expected eGFR,” 40/85 cases (47%) experienced ≤10% eGFR decline.
3.3.4. SK Patients; >36-Month Follow-Up
Among non-SK patients, 9/106 (8%) eventually progressed to dialysis/transplantation after a median of 27 (IQR 7.5–78.5; range 6–144) months following ablation, with their median pre-ablation eGFR of 54 (IQR 36–102), while 2/13 (15%) of SK patients progressed to dialysis at 5 and 56 months post-ablation, with their pre-ablation eGFR levels of 36 and 30 mL/min/1.73 m2.
3.4. Oncological Outcomes (Only Biopsy-Proven RCC Cases)
Among patients with biopsy-proven RCC, the median overall survival time was 160 months (Figure 1). Within 5-, 10-, and 15-year follow-up timepoints, the OS rates were 81%, 60%, and 46%, and the MFS rates were 98%, 98%, and 91%, respectively. Local RFS was 91% at both 5- and 15-year follow-ups, as no local recurrences were identified after five years of follow-up. Of note, all three cases that developed metastasis had also been diagnosed with local recurrence simultaneously (in one case) or previously (in two cases). CSS rates were 98%, 96%, and 96% at 5, 10, and 15 years of follow-up.
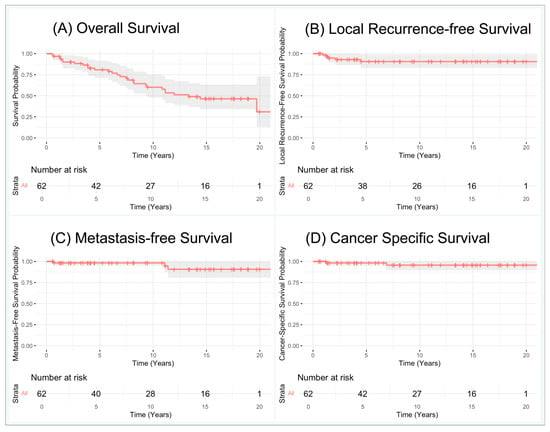
Figure 1.
Kaplan–Meier curves for biopsy-proven Renal Cell Carcinomas: (A) overall survival, (B) local recurrence-free survival, (C) metastasis-free survival, (D) cancer-specific survival.
Table 4 shows Firth’s Cox regression analysis results, which identified tumor volume (calculated by length × width2 × 0.52) and SK status at ablation as significant predictors for local recurrence. The same trend was observed in Firth’s multivariable Cox regression, although it did not reach statistical significance.

Table 4.
Cox regression analysis for local recurrence-free survival. Firth’s Penalized Likelihood method was used due to the low number of events (five progressions as events). Abbreviations: ASA: American Society of Anesthesiology; BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease; eGFR: estimated Glomerular Filtration Rate.
3.5. Trifecta Achievement
Of the 62 biopsy-proven RCC cases, 55 had data available for trifecta assessment. Considering seven SK cases, 58% (26/48) of the mentioned cohort achieved our trifecta definition at 36 months. Regarding SK patients, five RCC-proven cases experienced ≥25% eGFR decline; thus, only two of seven RCC-proven SK patients achieved the trifecta at the 36-month follow-up.
3.6. Follow-Up
Among all patients, 10 developed radiologic recurrence in the ablated part of the treated kidney; five belonged to the biopsy-proven RCC cohort, and five patients had no biopsy histology at cryoablation (Appendix B). Of note, the latter five patients were not included in the trifecta assessment, as further pathology studies showed non-malignant histology in RN/PN specimens. From these ten patients, two with radiologic recurrences were not classified as local recurrences. One had bilateral enhancing lesions at diagnosis, managed with PN (right, pathology RCC) and ablation (left). He was found to have local enhancement in the right kidney 15 years after PN, which was managed by radiofrequency ablation. One woman with a history of right RN for RCC 13 years prior developed multiple SRMs in the left kidney, managed by cryoablation (1 lesion, near hilum) and RFA (2 other polar lesions); recurrence occurred in the superior pole, for which she underwent PN and another cryoablation. Five years after the last intervention, she developed further recurrence and metastasis to the pancreatic head, ultimately succumbing to metastatic RCC. This is not an unexpected outcome given that she developed multiple distinct renal masses over 20 years from the first diagnosis of RCC. Thus, the recurrences in the two mentioned cases were not considered cryoablation-related.
4. Discussion
This study represents one of the longest follow-up series of patients undergoing cryoablation for an SRM. The primary endpoint of the present study was to evaluate the long-term oncological and renal functional outcomes of SRM cryoablation. Procedural success was achieved in 94% of cases, with minimal complications reported, most of which were low-grade. In addition, most patients clinically exhibited ≤10% eGFR decline. Extended long-term follow-up (median 155 months) showed that less than half of the non-SK patients experienced at least one stage of CKD progression. Oncological outcomes for biopsy-proven RCC were favorable, with 91% MFS and RFS at 15-year follow-up. These results underscore the effectiveness and durability of targeted cryoablation as a treatment for select patients, balancing oncological control with renal preservation.
Renal function preservation is one of the primary goals of cryoablation compared to more extensive surgeries. Although statistically significant post-ablation eGFR decline was observed, 64% of our non-SK patients experienced ≤10% decrement. Woldu et al. also showed 8.9 ± 13.4% eGFR decline within the first three months of SRM cryoablation, correlated with 8.6 ± 8.3% loss in renal parenchyma volume [21]. Similarly, Beemster and colleagues reported eGFR decline of 7 (95% CI 5–9) mL/min/1.73 m2 following SRM cryoablation and found that baseline eGFR is the sole predictor of renal insufficiency (eGFR < 30 mL/min/1.73 m2), while tumor size and SK status did not reach the statistically significant threshold [22]. Similar findings have been observed in several studies comparing ablative modalities, including cryoablation, with PN for stage cT1 renal masses, emphasizing the effectiveness of SRM cryoablation as a viable approach for preserving renal function [9]. However, the comparative functional efficacy against PN was notably lower in the SK cohort [16,23].
Assessing renal function during long-term follow-up is challenging due to the complex interplay among renal function, age, and other medical comorbidities. We were unable to find similar studies that examined renal function beyond our follow-up period. Thus, we propose the term “expected eGFR,” based on previous studies estimating the annual loss of renal function (16–18), that could serve as a creatinine-based measure for evaluating renal function in extended follow-up. However, this definition requires validation; therefore, we report CKD-stage progression to remain consistent with commonly accepted standards in the literature [24].
Regarding oncological outcomes, we found no progression (local recurrence or metastasis) after the first five years of follow-up. The 5-year OS, CSS, local RFS, and MFS rates were 81%, 98%, 91%, and 98% in our study, respectively. These values are similar to those of other studies [25,26], and the slight differences in OS measures possibly stem from the longer follow-up period in our study, the inherent selection bias, and baseline patient characteristics between the two studies [9]. Of note, the modest OS is likely related to the older age and more comorbidities in this patient population selected to undergo cryoablation. Comparative studies between ablation and PN have also shown comparable MFS and local RFS in multiple meta-analyses [9,27]. Furthermore, we found that higher tumor volume and SK status at ablation were significantly correlated development of local recurrence. This may be partially explained by the clinician’s more cautious approach to create a robust freeze with more generous margins in SK patients to minimize the risk of further deterioration in renal function. The higher risk of progression in SK patients following thermal ablation has been noted in the literature [16]. These findings highlight the excellent long-term oncological efficacy of SRM cryoablation when patients are appropriately selected.
Gill et al. introduced the trifecta concept for Partial Nephrectomy [17], which was later tailored to assess renal ablation outcomes [28]. Although the proposed model considered an eGFR decline of ≤10% as success, other studies used different cut-offs based on their studied cohort that must be considered when interpreting the results [16,28,29,30]. We used the same eGFR cut-off for non-SK cases and achieved trifecta in 58% of RCC-proven cases. Likewise, Lucignani et al. employed the same trifecta definition and evaluated 72 SRM patients treated with cryoablation with a median follow-up of 21 months (8–39) and reported a trifecta achievement rate of 59.5% [29]. Pandolfo and colleagues evaluated the outcomes of three ablation modalities, including cryoablation, and reported a trifecta achievement rate of 58.8%. However, they considered the eGFR decline of <25% as a successful renal functional outcome for completely endophytic, primarily cT1-stage renal masses [30]. This reflects the premise that tumor location is expected to impact the degree of renal function loss. The same group applied a similar cut-off when assessing outcomes following ablation of a stage cT1 renal mass in SK patients [16]. While these definitions are appropriate within the context of the studied cohorts, the decision to perform ablation remains multifactorial, wherein the achievement of the trifecta may not be possible with any treatment modality. Concerning future directions, a more standardized and comprehensive approach—potentially leveraging artificial intelligence—is still needed to integrate all contributing factors in predicting kidney function loss. This would provide a more precise benchmark for postoperative assessment while improving preoperative patient counseling and establishing realistic expectations regarding renal function.
Our study was not devoid of limitations. Despite being derived from a prospectively generated database, this study’s retrospective nature, lack of direct comparison with a PN cohort, potential selection bias, and the temporal differences in practice (modern focal therapy and imaging compared to the study period technology) during the 10-year study period should be considered when interpreting the results. Also, biopsy confirmation was not performed for all cases. However, to our knowledge, this study provides the longest follow-up in terms of renal functional outcomes following SRM cryoablation. It should also be noted that patients often struggle to return for long-term surveillance, especially when they feel cancer-free and their medical focus shifts over time. Further comparative studies with equivalent follow-up durations are necessary to validate these findings.
5. Conclusions
In this long-term report of SRM cryoablation outcomes, most patients achieved durable oncological control with low complication rates. Considering our strict criteria for functional outcomes and extended follow-up, renal function preservation was satisfactory in all cohorts, with superior long-term renal function observed in patients without solitary kidney status at ablation. These findings highlight the need for careful patient selection as well as additional research with long-term follow-up in terms of renal functional outcomes, parallel to oncological outcomes.
Author Contributions
T.J.P.: conceptualization, critical review, supervision; M.M.: conceptualization, drafting the manuscript, statistical analysis; A.G., C.Y.K. and M.T.: conceptualization, critical review, database development; S.D., E.S.A. and S.B.: database update. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Duke University Health System (protocol code Pro00111215 and date of approval 27 September 2022).
Informed Consent Statement
The consent was waived under Category 4 of the Common Rule (45 CFR 46.104(d)(4)), for secondary research, using data or specimens collected for other reasons.
Data Availability Statement
Data used in the analysis of this study is available through the corresponding author upon reasonable request and adherence to relevant institutional regulations.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SEER | Surveillance, Epidemiology, and End Results |
| SRMs | Small Renal Masses |
| PN | Partial Nephrectomy |
| RCC | Renal Cell Carcinoma |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| SK | Solitary Kidney |
| RFS | Recurrence-Free Survival |
| MFS | Metastasis-Free Survival |
| CSS | Cancer-Specific Survival |
| OS | Overall Survival |
| eGFR | Estimated Glomerular Filtration Rate |
| MDRD | Modification of Diet in Renal Disease |
| CKD | Chronic Kidney Disease |
| IQR | Interquartile Range |
| RN | Radical Nephrectomy |
Appendix A
Complications related to the cryoablation procedure. Abbreviations: BI-PAP: Bilevel Positive Airway Pressure; CDG: Clavien–Dindo Grade; RCC: Renal Cell Carcinoma; SK: Single Kidney.
| Case | Complication Description | CDG |
| #1 | Left renal cryoablation for a 2.8 cm enhancing left renal mass with subsequent left ureteral obstruction. A renal scan revealed a poorly functioning kidney even after a percutaneous nephrostomy placement. Subsequently underwent laparoscopic Radical Nephrectomy. Pathology showed no viable tumor cells. Died one month after nephrectomy due to suicide. | 4 |
| #5 | Bilateral cryoablation was planned for bilateral RCC. After the first ablation of the right kidney, the patient developed pulmonary edema requiring reintubation and Lasix administration. The left-sided lesion was ablated five months later without complication. Loss to follow-up after four years of uncomplicated follow-up. | 4 |
| #36 | Following left laparoscopic cryoablation, the patient developed intractable bleeding and later that evening underwent a completion nephrectomy. The biopsy was consistent with a clear cell RCC, and the nephrectomy showed no residual cancer. The patient was alive at last follow-up after 16 years with CKD stage II. | 3 |
| #39 | After cryoablation in left kidney, the patient underwent workup for fever of unknown origin outside Duke. He underwent drainage of a healing cryoablation. Once came back to Duke for continuation of care, the reviewed outside hospital scans were consistent with hematoma and no evidence of abscess. Patient later underwent right Radical Nephrectomy (contralateral to cryoablation site) for synchronous RCC. Nephrectomy pathology report confirmed RCC. No recurrences in the left kidney which was treated by cryoablation. The patient was alive at 83 years of age after 17 years of follow-up. | 3 |
| #41 | Following percutaneous ablation of left-sided renal mass in a case with longstanding history of diabetes, the patient developed left-sided crampy pain without fever or toxic appearance. Subsequent CT showed 10 ×10 cm perinephric abscess resulting in readmission and drainage by interventional radiologist colleagues. He remained disease-free nine years and died of intracranial hemorrhage at 81 years of age. | 3 |
| #117 | Three months after left laparoscopic cryoablation in a SK patient, developed insidious onset of left pain and fever with subsequent creatinine increase to 7.5 mg/dL. Imaging confirmed ureteropelvic junction obstruction managed with percutaneous nephrostomy and following retrograde pyelography showed urothelial slough in the pelvis removed via ureteroscopy. | 3 |
| 12 Cases (side) | Port-site wound infection managed with antibiotics (#11, left; #71, right); flank pain, hematuria with clot passage managed by readmission (#27, left; #57, right); Intraoperative splenic injury and repair, post-ablation platelet transfusion (#33, left); retroperitoneal bleeding managed conservatively (#47, right; #76, left); sensory-neural hearing loss presumed to be secondary to intra-operative Gentamicin (#66, left); presumed post-operative transient ischemic attack (#67, right); Atrial fibrillation managed with fluids and medications (#86, left); hematocrit drop managed with transfusion (#94, left; #98, left). | 2 |
| 11 Cases | Flank pain (#13, #21, #43, #46, #80); dyspnea (#18 required furosemide for pulmonary effusion; #29 asthma exacerbation; #37 desaturation requiring BI-PAP; #90 atelectasis managed by incentive spirometry); hypokalemia managed by supplements (#8); fever with negative workup, self-limited (#69). | 1 |
Appendix B
Radiologic recurrence of biopsy-proven patients and those without biopsy at cryoablation, and their subsequent management. Abbreviations: AS: Active surveillance; PN: Partial Nephrectomy; RCC: Renal Cell Carcinoma; RN: Radical Nephrectomy.
| Case(s) | Biopsy-Proven Cases | Case(s) | Without Biopsy at Cryoablation |
| #21 #100 | Repeat ablation and remained recurrence-free after seven years of follow-up. | #43 #113 | Repeat ablations. |
| #69 | Suspicious local enhancement was detected 30 months post-ablation, in the ablated zone. He underwent bilateral RN (one year on AS) outside of our center due to non-functioning kidneys, and the pathology confirmed RCC. He lived 8 years after RN and succumbed to septic shock at 72 years of age. | #3 | Left RN for multiple enhancing lesions in the tumor 50 months post-ablation; path: oncocytoma. |
| #97 | One patient who had simultaneous Gleason grade 3 + 4 prostate cancer and RCC developed metastasis after nine months of cryoablation. Subsequently, fine-needle aspiration confirmed metastatic RCC, and he succumbed to it 15 months post-ablation. | #92 | PN; path: normal kidney tissue. |
| #105 | One patient was treated with Partial Nephrectomy for ablation recurrence and later developed tumor extension to the pararenal fat and diaphragm. He was managed by excision of pararenal fat and parts of the diaphragm | #75 | Bilateral RN; non-functional kidneys path: non-diagnostic for malignancy. |
References
- National Cancer Institute. SEER Cancer Stat Facts: Kidney and Renal Pelvis Cancer. Bethesda, MD. Available online: https://seer.cancer.gov/statfacts/html/kidrp.html (accessed on 20 May 2025).
- Deivasigamani, S.; Adams, E.S.; Séguier, D.; Kotamarti, S.; Polascik, T.J. Cryoablation for the management of Small Renal Masses. Mini-Invasive Surg. 2023, 7, 9. [Google Scholar] [CrossRef]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Tsivian, M.; Mouraviev, V.; Albala, D.M.; Caso, J.R.; Robertson, C.N.; Madden, J.F.; Polascik, T.J. Clinical predictors of renal mass pathological features. BJU Int. 2011, 107, 735–740. [Google Scholar] [CrossRef]
- Campbell, S.C.; Uzzo, R.G.; Karam, J.A.; Chang, S.S.; Clark, P.E.; Souter, L. Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA guideline: Part II. J. Urol. 2021, 206, 209–218. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M. European Association of Urology guidelines on renal cell carcinoma: The 2022 update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Bagshaw, H.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; et al. Kidney cancer, version 3.2025, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Campbell, S.C.; Clark, P.E.; Chang, S.S.; Karam, J.A.; Souter, L.; Uzzo, R.G. Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA guideline: Part I. J. Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef]
- Rivero, J.R.; De La Cerda, J.; Wang, H.; Liss, M.A.; Farrell, A.M.; Rodriguez, R.; Suri, R.; Kaushik, D. Partial Nephrectomy versus Thermal Ablation for Clinical Stage T1 Renal Masses: Systematic Review and Meta-Analysis of More than 3900 Patients. J. Vasc. Interv. Radiol. 2018, 29, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Tsivian, M.; Lyne, J.C.; Mayes, J.M.; Mouraviev, V.; Kimura, M.; Polascik, T.J. Tumor size and endophytic growth pattern affect recurrence rates after laparoscopic renal cryoablation. Urology 2010, 75, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Tsivian, M.; Kim, C.Y.; Caso, J.R.; Rosenberg, M.D.; Nelson, R.C.; Polascik, T.J. Contrast enhancement on computed tomography after renal cryoablation: An evidence of treatment failure? J. Endourol. 2012, 26, 330–335. [Google Scholar] [CrossRef]
- Tsivian, M.; Caso, J.; Kimura, M.; Polascik, T.J. Renal function outcomes after laparoscopic renal cryoablation. J. Endourol. 2011, 25, 1287–1291. [Google Scholar] [CrossRef]
- Mitropoulos, D.; Artibani, W.; Graefen, M.; Remzi, M.; Rouprêt, M.; Truss, M. Reporting and Grading of Complications After Urologic Surgical Procedures: An ad hoc EAU Guidelines Panel Assessment and Recommendations. Eur. Urol. 2012, 61, 341–349. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Chlorogiannis, D.-D.; Chlorogiannis, A.; Filippiadis, D.K.; Kelekis, A.; Makris, G.C.; Georgiades, C. Impact of Percutaneous Cryoablation on Renal Function in Patients with Stage I Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2024, 35, 1278–1287.E3. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, S.D.; Loizzo, D.; Beksac, A.T.; Derweesh, I.; Celia, A.; Bianchi, L.; Elbich, J.; Costa, G.; Carbonara, U.; Lucarelli, G.; et al. Percutaneous thermal ablation for cT1 renal mass in solitary kidney: A multicenter trifecta comparative analysis versus robot-assisted partial nephrectomy. Eur. J. Surg. Oncol. 2023, 49, 486–490. [Google Scholar] [CrossRef]
- Hung, A.J.; Cai, J.; Simmons, M.N.; Gill, I.S. “Trifecta” in Partial Nephrectomy. J. Urol. 2013, 189, 36–42. [Google Scholar] [CrossRef]
- Waas, T.; Schulz, A.; Lotz, J.; Rossmann, H.; Pfeiffer, N.; Beutel, M.E.; Schmidtmann, I.; Münzel, T.; Wild, P.S.; Lackner, K.J. Distribution of estimated glomerular filtration rate and determinants of its age dependent loss in a German population-based study. Sci. Rep. 2021, 11, 10165. [Google Scholar] [CrossRef]
- Glassock, R.J.; Rule, A.D. Aging and the kidneys: Anatomy, physiology and consequences for defining chronic kidney disease. Nephron 2016, 134, 25–29. [Google Scholar] [CrossRef]
- Noronha, I.L.; Santa-Catharina, G.P.; Andrade, L.; Coelho, V.A.; Jacob-Filho, W.; Elias, R.M. Glomerular filtration in the aging population. Front. Med. 2022, 9, 769329. [Google Scholar] [CrossRef] [PubMed]
- Woldu, S.L.; Thoreson, G.R.; Okhunov, Z.; Ghandour, R.; Rothberg, M.B.; RoyChoudhury, A.; Kim, H.H.; Bozoghlanian, M.; Newhouse, J.H.; Helmy, M.A. Comparison of renal parenchymal volume preservation between partial nephrectomy, cryoablation, and radiofrequency ablation using 3D volume measurements. J. Endourol. 2015, 29, 948–955. [Google Scholar] [CrossRef]
- Beemster, P.W.; Barwari, K.; Mamoulakis, C.; Wijkstra, H.; de la Rosette, J.J.; Laguna, M.P. Laparoscopic renal cryoablation using ultrathin 17-gauge cryoprobes: Mid-term oncological and functional results. BJU Int.-Br. J. Urol. 2011, 108, 577. [Google Scholar] [CrossRef]
- Mason, R.J.; Atwell, T.D.; Lohse, C.; Bhindi, B.; Weisbrod, A.; Boorjian, S.A.; Leibovich, B.C.; Schmit, G.D.; Thompson, R.H. Renal functional outcomes in patients undergoing percutaneous cryoablation or partial nephrectomy for a solitary renal mass. BJU Int. 2017, 120, 544–549. [Google Scholar] [CrossRef]
- Britton, C.J.; Sharma, V.; Lohse, C.M.; Lieske, J.C.; Nichols, P.E.; Khanna, A.; Cheville, J.C.; Boorjian, S.A.; Leibovich, B.C.; Thompson, R.H.; et al. Progression of Chronic Kidney Disease Following Radical and Partial Nephrectomy. Urology 2022, 169, 125–133. [Google Scholar] [CrossRef]
- Stacul, F.; Sachs, C.; Giudici, F.; Bertolotto, M.; Rizzo, M.; Pavan, N.; Balestreri, L.; Lenardon, O.; Pinzani, A.; Pola, L.; et al. Cryoablation of renal tumors: Long-term follow-up from a multicenter experience. Abdom. Radiol. 2021, 46, 4476–4488. [Google Scholar] [CrossRef] [PubMed]
- Henderickx, M.; Sträter-Ruiter, A.E.C.; van der West, A.E.; Beerlage, H.P.; Zondervan, P.J.; Lagerveld, B.W. Laparoscopic cryoablation for small renal masses: Oncological outcomes at 5-year follow-up. Arab. J. Urol. 2020, 19, 159–165. [Google Scholar] [CrossRef]
- Dong, L.; Liang, W.Y.; Ya, L.; Yang, L.; Qiang, W. A Systematic Review and Meta-Analysis of Minimally Invasive Partial Nephrectomy Versus Focal Therapy for Small Renal Masses. Front. Oncol. 2022, 12, 732714. [Google Scholar] [CrossRef] [PubMed]
- Piasentin, A.; Claps, F.; Silvestri, T.; Rebez, G.; Traunero, F.; Mir, M.C.; Rizzo, M.; Celia, A.; Cicero, C.; Urbani, M.; et al. Assessing Trifecta Achievement after Percutaneous Cryoablation of Small Renal Masses: Results from a Multi-Institutional Collaboration. Medicina 2022, 58, 1041. [Google Scholar] [CrossRef]
- Lucignani, G.; Rizzo, M.; Ierardi, A.M.; Piasentin, A.; De Lorenzis, E.; Trombetta, C.; Liguori, G.; Bertolotto, M.; Carrafiello, G.; Montanari, E.; et al. A Trifecta-Based Evaluation of Patients Treated with Percutaneous Thermal Ablation of Small Renal Masses. J. Endourol. 2024, 39, 38–45. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Beksac, A.T.; Derweesh, I.; Celia, A.; Schiavina, R.; Bianchi, L.; Costa, G.; Carbonara, U.; Loizzo, D.; Lucarelli, G.; et al. Percutaneous Ablation vs Robot-Assisted Partial Nephrectomy for Completely Endophytic Renal Masses: A Multicenter Trifecta Analysis with a Minimum 3-Year Follow-Up. J. Endourol. 2023, 37, 279–285. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).