Role of Qualified Exercise Professionals in Medical Clearance for Exercise: Alberta Cancer Exercise Hybrid Effectiveness-Implementation Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Co-Design
2.3. Eligibility Criteria
2.4. Settings
2.5. Participant Screening for Exercise Safety
2.6. Data Collection Related to Screening and Triage
2.7. Fitness Assessments (In-Person, Virtual, and Optional)
2.8. Planned Exercise Intervention
3. Results
4. Discussion
4.1. Limitations
4.2. Patient Partner Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Shan, T.; Zhang, D.; Ma, F. Nowcasting and forecasting global aging and cancer burden: Analysis of data from the GLOBOCAN and Global Burden of Disease Study. J. Natl. Cancer Cent. 2024, 4, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Caswell-Jin, J.L.; Sun, L.P.; Munoz, D.; Lu, Y.; Li, Y.; Huang, H.; Hampton, J.M.; Song, J.; Jayasekera, J.; Schechter, C.; et al. Analysis of Breast Cancer Mortality in the US-1975 to 2019. JAMA 2024, 331, 233–241. [Google Scholar] [CrossRef]
- Firkins, J.; Hansen, L.; Driessnack, M.; Dieckmann, N. Quality of life in “chronic” cancer survivors: A meta-analysis. J. Cancer Surviv. 2020, 14, 504–517. [Google Scholar] [CrossRef]
- Tometich, D.B.; Hyland, K.A.; Soliman, H.; Jim, H.S.L.; Oswald, L. Living with Metastatic Cancer: A Roadmap for Future Research. Cancers 2020, 12, 3684. [Google Scholar] [CrossRef]
- Smith, A.K.; White, D.B.; Arnold, R.M. Uncertainty—The other side of prognosis. N. Engl. J. Med. 2013, 368, 2448–2450. [Google Scholar] [CrossRef] [PubMed]
- Pituskin, E.; Joy, A.A.; Fairchild, A. Advanced Cancer as a Chronic Disease: Introduction. Semin. Oncol. Nurs. 2021, 37, 151176. [Google Scholar] [CrossRef]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [CrossRef]
- Speck, R.M.; Courneya, K.S.; Masse, L.C.; Duval, S.; Schmitz, K.H. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. Res. Pract. 2010, 4, 87–100. [Google Scholar] [CrossRef]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef]
- Blinder, V.S.; Gany, F.M. Impact of Cancer on Employment. J. Clin. Oncol. 2020, 38, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Courneya, K.S.; Friedenreich, C.M. The Physical Activity and Cancer Control (PACC) framework: Update on the evidence, guidelines, and future research priorities. Br. J. Cancer 2024, 131, 957–969. [Google Scholar] [CrossRef]
- Courneya, K.S.; Vardy, J.L.; O’Callaghan, C.J.; Gill, S.; Friedenreich, C.M.; Wong, R.K.S.; Dhillon, H.M.; Coyle, V.; Chua, N.S.; Jonker, D.J.; et al. Structured Exercise after Adjuvant Chemotherapy for Colon Cancer. N. Engl. J. Med. 2025, 393, 13–25. [Google Scholar] [CrossRef]
- Courneya, K.S.; McNeely, M.L.; Booth, C.M.; Friedenreich, C.M. An integrated framework for the study of exercise across the postdiagnosis cancer continuum. Front. Oncol. 2024, 14, 1432899. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Thomsen, S.N.; Lahart, I.M.; Thomsen, L.M.; Fridh, M.K.; Larsen, A.; Mau-Sørensen, M.; Bolam, K.A.; Fairman, C.M.; Christensen, J.F.; Simonsen, C. Harms of exercise training in patients with cancer undergoing systemic treatment: A systematic review and meta-analysis of published and unpublished controlled trials. eClinicalMedicine 2023, 59, 101937. [Google Scholar] [CrossRef]
- Ramsey, I.; Chan, A.; Charalambous, A.; Cheung, Y.T.; Darling, H.S.; Eng, L.; Grech, L.; Hart, N.H.; Kirk, D.; Mitchell, S.A.; et al. Exercise counselling and referral in cancer care: An international scoping survey of health care practitioners’ knowledge, practices, barriers, and facilitators. Support. Care Cancer 2022, 30, 9379–9391. [Google Scholar] [CrossRef]
- Luo, H.; Schumacher, O.; Galvao, D.A.; Newton, R.U.; Taaffe, D.R. Adverse Events Reporting of Clinical Trials in Exercise Oncology Research (ADVANCE): Protocol for a Scoping Review. Front. Oncol. 2022, 12, 841266. [Google Scholar] [CrossRef] [PubMed]
- Clifford, B.K.; Mizrahi, D.; Sandler, C.X.; Barry, B.K.; Simar, D.; Wakefield, C.E.; Goldstein, D. Barriers and facilitators of exercise experienced by cancer survivors: A mixed methods systematic review. Support. Care Cancer 2018, 26, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Nadler, M.; Bainbridge, D.; Tomasone, J.; Cheifetz, O.; Juergens, R.A.; Sussman, J. Oncology care provider perspectives on exercise promotion in people with cancer: An examination of knowledge, practices, barriers, and facilitators. Support. Care Cancer 2017, 25, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Brown, J.C.; Schwartz, A.L.; Marshall, T.F.; Campbell, A.M.; Nekhlyudov, L.; Zucker, D.S.; Basen-Engquist, K.M.; Campbell, G.; Meyerhardt, J.; et al. An exercise oncology clinical pathway: Screening and referral for personalized interventions. Cancer 2020, 126, 2750–2758. [Google Scholar] [CrossRef]
- Tanaka, S.; Imataki, O.; Kitaoka, A.; Fujioka, S.; Hanabusa, E.; Ohbayashi, Y.; Uemura, M.; Arima, N.; Yamamoto, T. Clinical impact of sarcopenia and relevance of nutritional intake in patients before and after allogeneic hematopoietic stem cell transplantation. J. Cancer Res. Clin. Oncol. 2017, 143, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Caperchione, C.M.; English, M.; Sharp, P.; Agar, M.R.; Phillips, J.L.; Liauw, W.; Harris, C.A.; McCullough, S.; Lilian, R. Exploring the practicality and acceptability a brief exercise communication and clinician referral pathway in cancer care: A feasibility study. BMC Health Serv. Res. 2023, 23, 1023. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Exercise Management for Persons with Chronic Diseases and Disabilities, 4th ed.; Human Kinetics: Champagne, IL, USA, 2016. [Google Scholar]
- Jones, L.W. Evidence-based risk assessment and recommendations for physical activity clearance: Cancer. Appl. Physiol. Nutr. Metab. 2011, 36 (Suppl. 1), S101–S112. [Google Scholar] [CrossRef]
- McNeely, M.L.; Sellar, C.; Williamson, T.; Shea-Budgell, M.; Joy, A.A.; Lau, H.Y.; Easaw, J.C.; Murtha, A.D.; Vallance, J.; Courneya, K.; et al. Community-based exercise for health promotion and secondary cancer prevention in Canada: Protocol for a hybrid effectiveness-implementation study. BMJ Open 2019, 9, e029975. [Google Scholar] [CrossRef]
- McNeely, M.L.; Shallwani, S.M.; Williamson, T.; Sellar, C.; Gobeil, E.; Joy, A.A.; Lau, H.; Easaw, J.; Sexsmith, J.; Courneya, K.S.; et al. Baseline Characteristics of Participants in the Alberta Cancer Exercise Hybrid Effectiveness-Implementation Study: A Wake-Up Call for Action. Cancers 2025, 17, 772. [Google Scholar] [CrossRef]
- Maxwell-Smith, C.; Hagger, M.S.; Kane, R.; Cohen, P.A.; Tan, J.; Platell, C.; Makin, G.B.; Saunders, C.; Nightingale, S.; Lynch, C.; et al. Psychological correlates of physical activity and exercise preferences in metropolitan and nonmetropolitan cancer survivors. Psychooncology 2021, 30, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Suderman, K.; Dolgoy, N.; Yurick, J.; Sellar, C.; Nishimura, K.; Culos-Reed, S.N.; Joy, A.A.; McNeely, M.L. A Practical Approach to Using Integrated Knowledge Translation to Inform a Community-Based Exercise Study. Int. J. Environ. Res. Public Health 2020, 17, 3911. [Google Scholar] [CrossRef] [PubMed]
- Suderman, K.; Culos-Reed, N.; Pituskin, E.; McNeely, M. Integrated Knowledge Translation to Inform Implementation of Exercise Counselling and Referral of Cancer Survivors: 1978 Board #3 May 28 3:45 PM–5:45 PM. Med. Sci. Sports Exerc. 2020, 52, 522–523. [Google Scholar] [CrossRef]
- Jones, L.W.; Eves, N.D.; Peppercorn, J. Pre-exercise screening and prescription guidelines for cancer patients. Lancet Oncol. 2010, 11, 914–916. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2017. [Google Scholar]
- Warburton, D.; Jamnik, V.; Bredin, S.; Shephard, R.; Gledhill, N. The 2023 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fit. J. Can. 2023, 16, 46–49. [Google Scholar] [CrossRef]
- Canadian Society for Exercise Physiology. CSEP Certified Exercise Physiologist. Available online: https://csep.ca/csep-certification/csep-professional-standards-program-cep/ (accessed on 5 June 2025).
- Burr, J.F.; Shephard, R.J.; Jones, L.W. Physical activity for cancer patients: Clinical risk assessment for exercise clearance and prescription. Can. Fam. Physician 2012, 58, 970–973. [Google Scholar]
- McAllister, L.S.; Palombaro, K.M. Modified 30-Second Sit-to-Stand Test: Reliability and Validity in Older Adults Unable to Complete Traditional Sit-to-Stand Testing. J. Geriatr. Phys. Ther. 2019, 43, 153–158. [Google Scholar] [CrossRef]
- Kolber, M.J.; Hanney, W.J. The Reliability and Concurrent Validity of Shoulder Mobility Measurements Using a Digital Inclinometer and Goniometer: A Technical Report. Int. J. Sports Phys. Ther. 2012, 7, 306–313. [Google Scholar]
- Franchignoni, F.; Tesio, L.; Martino, M.T.; Ricupero, C. Reliability of four simple, quantitative tests of balance and mobility in healthy elderly females. Aging 1998, 10, 26–31. [Google Scholar] [CrossRef]
- Bellace, J.V.; Healy, D.; Besser, M.P.; Byron, T.; Hohman, L. Validity of the Dexter Evaluation System’s Jamar dynamometer attachment for assessment of hand grip strength in a normal population. J. Hand Ther. 2000, 13, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.F.; McDonald, C.; Chenier, T.C. Measurement of grip strength: Validity and reliability of the sphygmomanometer and jamar grip dynamometer. J. Orthop. Sports Phys. Ther. 1992, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Massy-Westropp, N.; Evans, A.M. Reliability and validity of indices of hand-grip strength and endurance. Aust. Occup. Ther. J. 2011, 58, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Vogt, L.; Thiel, C.; Jager, E.; Banzer, W. Validity of the six-minute walk test in cancer patients. Int. J. Sports Med. 2013, 34, 631–636. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R.H. Two-Minute Step Test of Exercise Capacity: Systematic Review of Procedures, Performance, and Clinimetric Properties. J. Geriatr. Phys. Ther. 2019, 42, 105–112. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Guan, Z.; Stephan, B.C.M.; Donini, L.M.; Prado, C.M.; Sim, M.; Siervo, M. Exploring the association between sarcopenic obesity and cardiovascular risk: A summary of findings from longitudinal studies and potential mechanisms. Proc. Nutr. Soc. 2024, 18, 1–9. [Google Scholar] [CrossRef]
- Whitfield, G.P.; Riebe, D.; Magal, M.; Liguori, G. Applying the ACSM Preparticipation Screening Algorithm to U.S. Adults: National Health and Nutrition Examination Survey 2001–2004. Med. Sci. Sports Exerc. 2017, 49, 2056–2063. [Google Scholar] [CrossRef]
- Brawner, C.A.; Lazar, M.H. Cardiopulmonary exercise testing criteria for advanced therapies in patients with heart failure. Heart Fail. Rev. 2023, 28, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, A.J.; Williams, A.D.; Askew, C.D.; Levinger, I.; Coombes, J.; Vicenzino, B.; Davison, K.; Smart, N.A.; Selig, S.E. Exercise Professionals with Advanced Clinical Training Should be Afforded Greater Responsibility in Pre-Participation Exercise Screening: A New Collaborative Model between Exercise Professionals and Physicians. Sports Med. 2018, 48, 1293–1302. [Google Scholar] [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.R.; Loblaw, A.; Petrella, T.; Exercise for People with Cancer Guideline Development Group. Exercise for people with cancer: A clinical practice guideline. Curr. Oncol. 2017, 24, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Maher, C.G.; Briffa, T.; Sherrington, C.; Bennell, K.; Alison, J.; Singh, M.F.; Glasziou, P.P. Prescribing exercise interventions for patients with chronic conditions. CMAJ 2016, 188, 510–518. [Google Scholar] [CrossRef]
- Santa Mina, D.; Sabiston, C.M.; Au, D.; Fong, A.J.; Capozzi, L.C.; Langelier, D.; Chasen, M.; Chiarotto, J.; Tomasone, J.R.; Jones, J.M.; et al. Connecting people with cancer to physical activity and exercise programs: A pathway to create accessibility and engagement. Curr. Oncol. 2018, 25, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Covington, K.R.; Marshall, T.; Campbell, G.; Williams, G.R.; Fu, J.B.; Kendig, T.D.; Howe, N.; Alfano, C.M.; Pergolotti, M. Development of the Exercise in Cancer Evaluation and Decision Support (EXCEEDS) algorithm. Support. Care Cancer 2021, 29, 6469–6480. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Daun, J.T.; Francis, G.J.; de Guzman Wilding, M.; Urgoiti, G.R.; Langelier, D.; Culos-Reed, N. Feasibility and Implementation of an Oncology Rehabilitation Triage Clinic: Assessing Rehabilitation, Exercise Need, and Triage Pathways within the Alberta Cancer Exercise-Neuro-Oncology Study. Curr. Oncol. 2023, 30, 6220–6245. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [PubMed]
- Pastore, O.; McFadden, T.; Fortier, M. Investigating the Impact of Physical Activity Counselling on Self-Compassion and Physical Activity. Curr. Psychol. 2023, 42, 10951–10963. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Vallance, J.K.; McNeely, M.L.; Culos-Reed, S.N.; Matthews, C.E.; Bell, G.J.; Mackey, J.R.; Kopciuk, K.A.; Dickau, L.; Wang, Q.; et al. The Alberta moving beyond breast cancer (AMBER) cohort study: Baseline description of the full cohort. Cancer Causes Control 2022, 33, 441–453. [Google Scholar] [CrossRef]
- Leach, H.J.; Danyluk, J.M.; Nishimura, K.C.; Culos-Reed, S.N. Evaluation of a Community-Based Exercise Program for Breast Cancer Patients Undergoing Treatment. Cancer Nurs. 2015, 38, 417–425. [Google Scholar] [CrossRef]
- An, K.Y.; Morielli, A.R.; Kang, D.W.; Friedenreich, C.M.; McKenzie, D.C.; Gelmon, K.; Mackey, J.R.; Reid, R.D.; Courneya, K.S. Effects of exercise dose and type during breast cancer chemotherapy on longer-term patient-reported outcomes and health-related fitness: A randomized controlled trial. Int. J. Cancer 2020, 146, 150–160. [Google Scholar] [CrossRef]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.; Lane, K.; et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef]
- Thraen-Borowski, K.M.; Gennuso, K.P.; Cadmus-Bertram, L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS ONE 2017, 12, e0182554. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, A.; Yu, M.; Moser, R.P.; Phillips, S.M.; Alfano, C.; Perna, F.M. Population Estimates of Meeting Strength Training and Aerobic Guidelines, by Gender and Cancer Survivorship Status: Findings From the Health Information National Trends Survey (HINTS). J. Phys. Act. Health 2015, 12, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, S.J.; Hancox, J.; Hattar, A.; Maxwell-Smith, C.; Thogersen-Ntoumani, C.; Hagger, M.S. Motivating the unmotivated: How can health behavior be changed in those unwilling to change? Front. Psychol. 2015, 6, 835. [Google Scholar] [CrossRef]
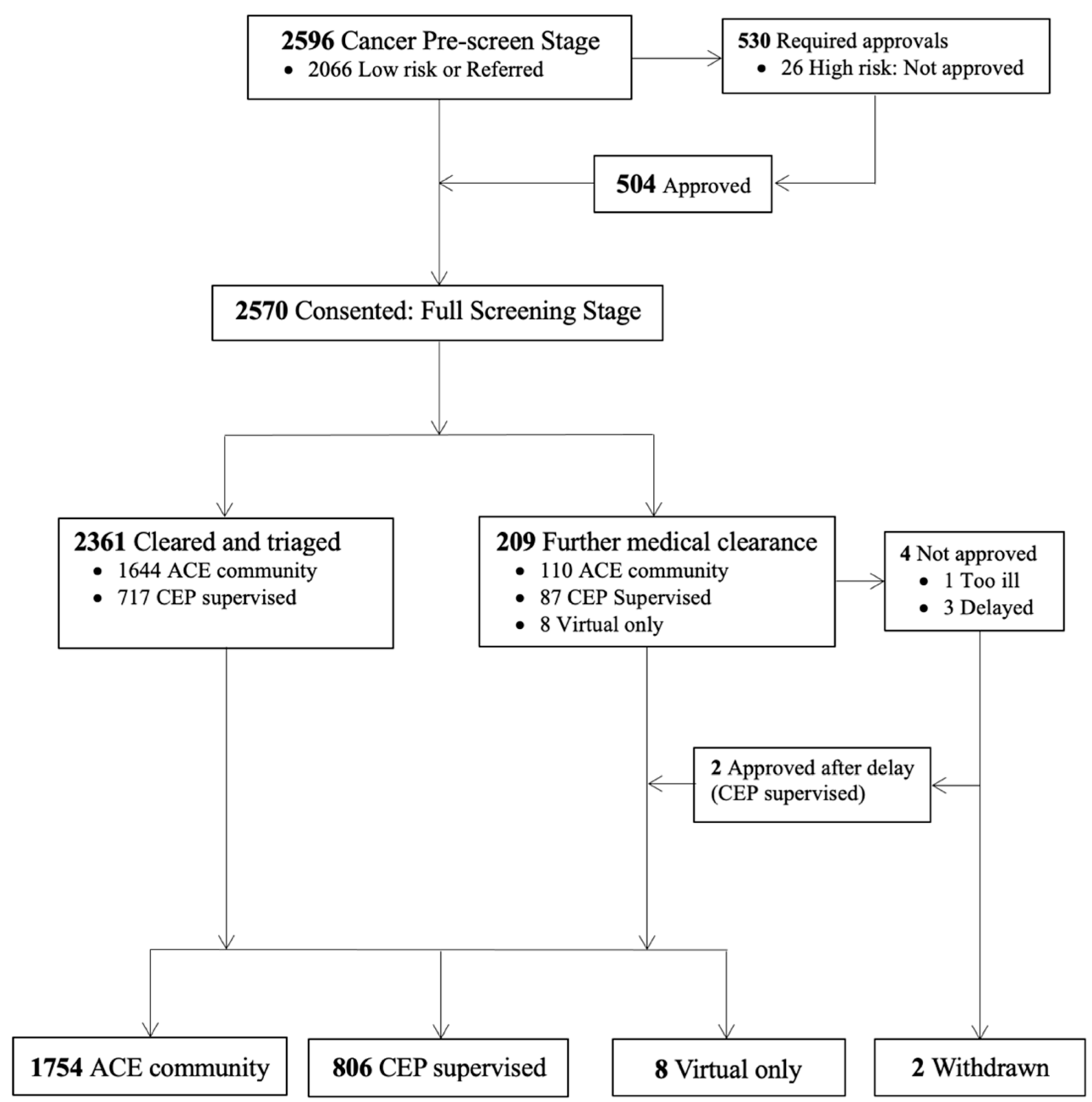
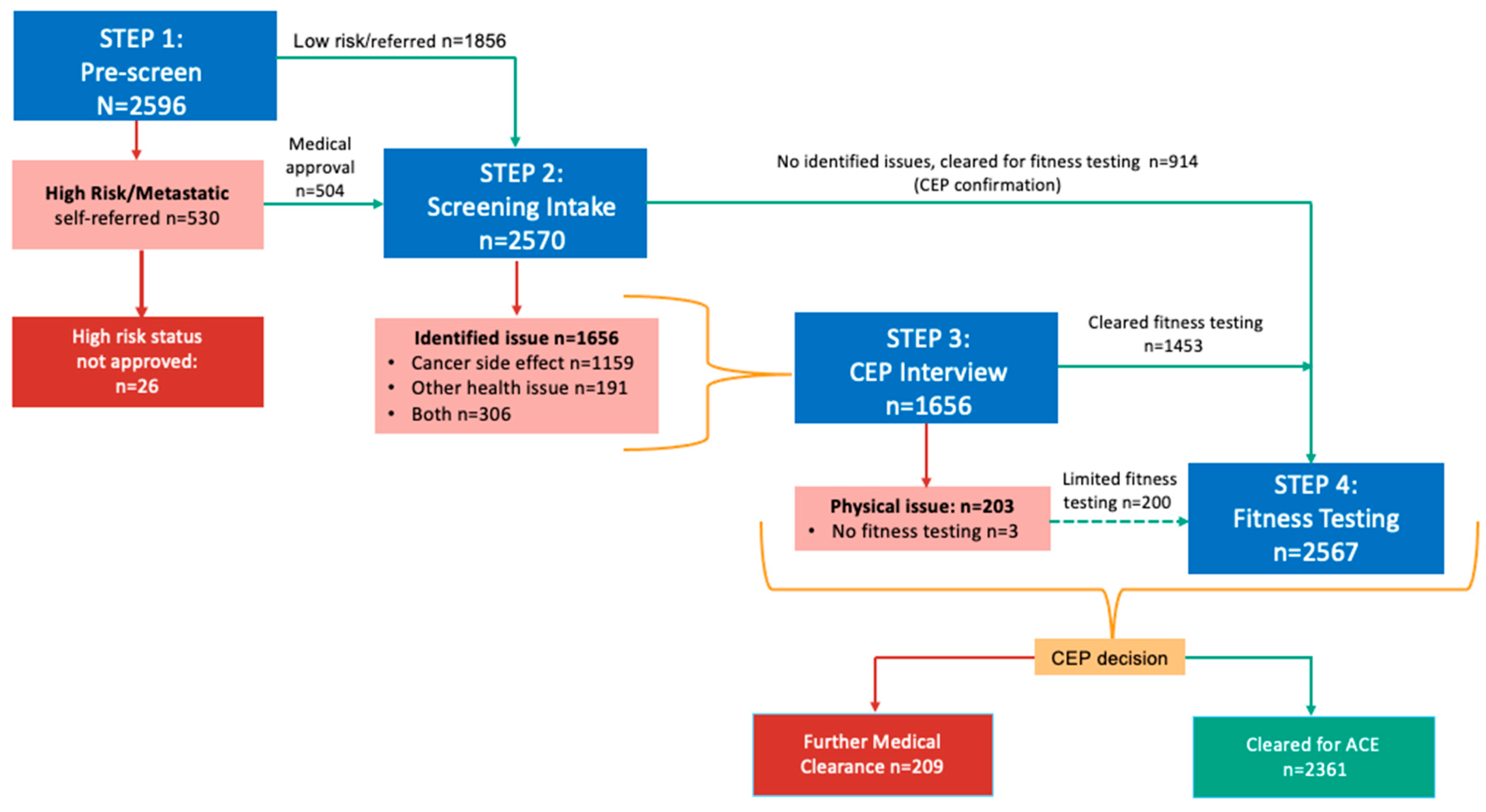
| Steps in Screening | Screening Considerations | Action Taken if Risk Identified | Comments |
|---|---|---|---|
| Step 1: Pre-screen for high-risk cancers | Identify individuals reporting a high-risk cancer type (i.e., head and neck cancer, neurological, lung, pancreatic or multiple myeloma) or reporting local or distant metastatic disease | HCP * guidance on appropriateness for program and recommended level of exercise supervision | This step was instituted after the first year of ACE programming to avoid delays in baseline fitness assessment due to concerns over high-risk status |
| Step 2: Screening intake | CEP review of cancer intake and PAR-Q+ questionnaires to identify cancer-related and/or current conditions respectively | Further HCP * guidance/clearance as needed before conducting baseline fitness assessment | Further questions were added to expand the intake for cancer category types and sub-types; and for those reporting >1 cancer diagnosis |
| Step 3: CEP-led interview with participant | In-person/virtual or phone interview with CEP to review screening findings with the participant to determine status, and the need for further medical clearance | HCP * guidance if further cancer-related issues identified on interview Primary care provider or specialist guidance on non-cancer related issues | Informed level of supervision, need for tailored or personalized exercise prescription (e.g., monitoring of shortness of breath; modification to exercise due to arthritis in knee) |
| Step 4: Physical fitness assessment | Evaluate physical function, mobility and fitness. Ensure there are no underlying conditions (e.g., hypertension, tachycardia, poor mobility) that may be made worse by engaging in exercise | HCP * or primary care provider (if discharged from cancer care) contacted if identified issues requiring further medical guidance or investigation | Identify need for medical follow-up or monitoring of vitals; inform tailoring of exercise based on results |
| Demographic Characteristic | Total Cohort (N = 2570) | Self-Referral (n = 1693) | Medical Referral (n = 877) | |||
|---|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | |
| Age in years, mean, SD | 57.8 | 12.0 | 57.9 | 12.1 | 57.7 | 11.8 |
| 17 to 39 years (AYA) | 190 | 7.4% | 127 | 7.5% | 63 | 7.2% |
| 40 to 64 years | 1582 | 61.6% | 1030 | 60.8% | 552 | 62.9% |
| 65+ years | 798 | 31.1% | 536 | 31.7% | 262 | 29.9% |
| Biological Sex | ||||||
| Female | 1832 | 71.3% | 1179 | 69.6% | 653 | 74.5% † |
| Male | 738 | 28.7% | 514 | 30.4% | 224 | 25.5% |
| Population Group | ||||||
| European descent | 1859 | 72.3% | 1212 | 71.6% | 647 | 73.8% |
| Other | 711 | 27.7% | 481 | 28.4% | 230 | 26.2% |
| Urban Site | ||||||
| Calgary | 1149 | 44.7% | 714 | 42.2% | 435 | 49.6% † |
| Edmonton | 1122 | 43.7% | 764 | 45.1% | 358 | 40.8% † |
| South urban sites | 122 | 4.7% | 85 | 5.0% | 37 | 4.2% |
| North urban sites | 177 | 6.9% | 130 | 7.7% | 47 | 5.4% † |
| Marital status | ||||||
| Never married | 261 | 10.2% | 162 | 9.6% | 99 | 11.3% |
| Married/common-law | 1858 | 72.3% | 1233 | 72.9% | 624 | 71.2% |
| Divorced/separated | 339 | 13.2% | 214 | 12.6% | 125 | 14.3% |
| Widowed | 112 | 4.4% | 83 | 4.9% | 29 | 3.3% |
| Highest level of education | ||||||
| High School * or less | 823 | 32.0% | 579 | 34.2% | 244 | 27.8% |
| University/college | 1334 | 51.9% | 868 | 51.3% | 466 | 53.1% |
| Graduate school | 412 | 16.0% | 245 | 14.5% | 167 | 19.0% † |
| Missing/not reported | 1 | 0% | 1 | 0% | - | - |
| Income | ||||||
| <$60,000 | 767 | 43.6% | 518 | 30.6% | 249 | 28.4% |
| $60,000 to $99,999 | 716 | 27.9% | 458 | 27.1% | 258 | 29.4% |
| ≥$100,000 | 810 | 31.5% | 533 | 31.5% | 277 | 31.6% |
| Missing/not reported | 277 | 10.8% | 184 | 10.9% | 93 | 10.6% |
| Cancer and Treatment Characteristics | Total Cohort (N = 2570) | Self-Referral (n = 1693) | Medical Referral (n = 877) | |||
|---|---|---|---|---|---|---|
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | |
| PRE-SCREEN (self-report) | ||||||
| High-risk cancer type * | 455 | 17.7% | 312 | 18.4% | 143 | 16.3% |
| Metastatic disease | 450 | 17.5% | 306 | 18.1% | 144 | 16.4% |
| High-risk and metastatic | 91 | 3.5% | 57 | 3.4% | 34 | 3.9% |
| CANCER INTAKE FORM | ||||||
| Single primary cancer | 2392 | 93.1% | 1567 | 92.6% | 823 | 93.8% |
| Two/more primary cancers | 178 | 7.0% | 126 | 7.4% | 54 | 6.2% |
| Primary Cancer Type | ||||||
| Breast | 1167 | 45.4% | 738 | 43.6% | 429 | 48.9% † |
| Hematologic | 355 | 13.8% | 221 | 13.1% | 134 | 15.3% |
| Genitourinary | 241 | 9.4% | 163 | 9.6% | 78 | 8.9% |
| Digestive | 231 | 9.0% | 169 | 10.0% † | 62 | 7.1% |
| Head and Neck | 159 | 6.2% | 110 | 6.5% | 49 | 5.6% |
| Gynecologic | 152 | 5.9% | 97 | 5.7% | 55 | 6.3% |
| Neurological | 93 | 3.6% | 74 | 4.4% † | 19 | 2.2% |
| Lung | 90 | 3.5% | 62 | 3.7% | 28 | 3.2% |
| Other | 82 | 3.2% | 59 | 3.5% | 23 | 2.6% |
| Cancer Treatment Status | ||||||
| On treatment | 1254 | 48.8% | 823 | 48.6% | 431 | 49.1% |
| Off treatment | 1316 | 51.2% | 870 | 51.4% | 446 | 50.9% |
| Current cancer treatment—type | ||||||
| Chemotherapy Treatment | 477 | 18.6% | 324 | 19.1% | 153 | 17.4% |
| Radiation Therapy | 134 | 5.2% | 84 | 5.0% | 50 | 5.7% |
| Hormonal Therapy | 570 | 22.2% | 364 | 21.5% | 206 | 23.5% |
| Targeted/Biological Therapy | 229 | 8.9% | 152 | 9.0% | 77 | 8.8% |
| Other treatment | 42 | 1.6% | 26 | 1.5% | 16 | 1.8% |
| Treatment-related effects potentially interfering with exercise | ||||||
| Number reporting ≥1 effects | 1465 | 57.0% | 988 | 58.4% | 477 | 54.4% |
| Fatigue | 984 | 38.3% | 662 | 39.1% | 322 | 36.7% |
| Muscle or joint issues | 799 | 31.1% | 539 | 31.8% | 260 | 29.6% |
| Peripheral Neuropathy | 590 | 23.0% | 391 | 23.1% | 199 | 22.7% |
| Pain | 503 | 19.6% | 337 | 19.9% | 166 | 18.9% |
| Weight-related issues | 416 | 16.2% | 278 | 16.4% | 138 | 15.7% |
| Cognitive challenges | 413 | 16.1% | 287 | 17.0% | 126 | 14.4% |
| Lymphedema | 249 | 9.7% | 185 | 10.9% | 64 | 7.3% |
| Bladder/bowel issues | 219 | 8.5% | 154 | 9.1% | 65 | 7.4% |
| Shortness of breath | 189 | 7.4% | 126 | 7.4% | 63 | 7.2% |
| Osteoporosis | 88 | 3.4% | 62 | 3.7% | 26 | 3.0% |
| Communication issues | 69 | 2.7% | 42 | 2.5% | 27 | 3.1% |
| Cardiac issues | 55 | 2.1% | 35 | 2.1% | 20 | 2.3% |
| Ostomy issues | 33 | 1.3% | 19 | 1.1% | 14 | 1.6% |
| Other | 182 | 7.1% | 122 | 7.2% | 60 | 6.8% |
| Lifestyle Characteristics | Total Cohort (N = 2570) Mean/n (SD/%) | Self-Referral (n = 1693) Mean/n (SD/%) | Medical Referral (n = 877) Mean/n (SD/%) | |||
|---|---|---|---|---|---|---|
| Number with Other Identified Health Issues on PAR-Q+ | ||||||
| One health issue PAR-Q+ | 886 | 34.5% | 603 | 35.6% | 283 | 32.3% |
| ≥Two health issues PAR-Q+ | 584 | 22.7% | 615 | 36.3% | 334 | 38.1% |
| One uncontrolled health issue | 365 | 14.2% | 265 | 15.7% † | 100 | 11.4% |
| ≥Two uncontrolled health issues | 132 | 5.1% | 81 | 4.8% | 51 | 5.8% |
| PAR-Q+ Responses | ||||||
| Arthritis | 1168 | 45.5% | 769 | 45.4% | 399 | 45.5% |
| Difficulty controlling | 127 | 4.9% | 78 | 4.6% | 49 | 5.6% |
| Pain/recent fracture | 300 | 11.7% | 192 | 11.3% | 108 | 12.3% |
| Heart Disease | 659 | 25.6% | 452 | 26.7% | 207 | 23.6% |
| Difficulty controlling | 61 | 2.4% | 42 | 2.5% | 19 | 2.2% |
| Mental Health Issue | 388 | 15.1% | 247 | 14.6% | 141 | 16.1% |
| Difficulty controlling | 60 | 2.3% | 41 | 2.4% | 19 | 2.2% |
| Respiratory Disease | 304 | 11.8% | 202 | 11.9% | 102 | 11.6% |
| Difficulty controlling | 31 | 1.2% | 23 | 1.4% | 8 | 0.9% |
| Low oxygen levels | 12 | 0.5% | 8 | 0.7% | 4 | 0.5% |
| Metabolic Disease | 250 | 9.7% | 167 | 9.9% | 83 | 9.5% |
| Difficulty controlling | 45 | 1.8% | 32 | 1.9% | 13 | 1.5% |
| Diabetic neuropathy | 48 | 1.9% | 37 | 2.2% | 11 | 1.3% |
| Prior Stroke | 65 | 2.5% | 44 | 2.6% | 21 | 2.4% |
| Difficulty controlling | 4 | 0.2% | 2 | 0.1% | 2 | 0.2% |
| Difficulty walking | 8 | 0.3% | 6 | 0.4% | 2 | 0.2% |
| Prior Spinal Cord Injury | 21 | 0.8% | 13 | 0.8% | 8 | 0.9% |
| Fainting/loss consciousness | 146 | 5.7% | 105 | 6.2% | 41 | 4.7% |
| Other Health Condition | 405 | 16.6% | 251 | 14.8% | 154 | 17.6% |
| Physical Activity category | ||||||
| Insufficiently Active | 796 | 31.0% | 405 | 31.3% | 391 | 30.8% |
| Sedentary | 1195 | 46.6% | 616 | 47.7% | 579 | 45.6% |
| Health-Related Fitness Measures |
Total Cohort
(N = 2570) |
Self-Referral
(n = 1693) |
Medical Referral
(n = 877) | |||
|---|---|---|---|---|---|---|
| Mean/No. | SD/% | Mean/No. | SD/% |
Mean/
No. | SD/% | |
| Underweight: BMI < 18.5 | 40 | 1.6% | 25 | 1.5% | 15 | 1.7% |
| Obese Class III or higher: BMI ≥ 40 | 165 | 6.4% | 107 | 6.3% | 58 | 6.6% |
| ≥1 Vital sign outside recommended level | 712 | 27.7% | 497 | 29.4% † | 215 | 24.5% |
| High resting heart rate | 571 | 22.2% | 395 | 23.3% | 176 | 20.1% |
| Resting systolic BP > 160 mmHg | 30 | 1.2% | 24 | 1.4% | 6 | 0.7% |
| Resting diastolic BP > 100 mmHg | 33 | 1.3% | 23 | 1.4% | 10 | 1.1% |
| Resting systolic BP < 90 mmHg | 453 | 19.0% | 315 | 20.1% † | 138 | 16.7% |
| Resting diastolic BP < 60 mmHg | 40 | 1.9% | 28 | 2.0% | 12 | 1.7% |
| Resting oxygen saturation < 90 | 8 | 0.3% | 5 | 0.3% | 3 | 0.3% |
| Profile of Participants | Overall N = 209 * No., (%) | Early-Stage Disease n = 18 No., (%) | High-Risk Status n = 93 No., (%) | Metastatic Disease n = 96 No., (%) | Palliative Stage-Withdrawn n = 2 No., (%) |
|---|---|---|---|---|---|
| Cancer Type | Breast: 36 Lung: 17 Digestive: 27 Hematologic: 40 Gynecologic: 6 Genitourinary: 25 Neurologic: 48 Other: 10 | Breast: 1 Digestive: 4 Hematologic: 6 Gynecologic: 2 Genitourinary: 2 Other: 3 | Lung: 10 Pancreatic: 2 Multiple Myeloma: 33 Neurologic: 48 | Breast: 34 Lung: 7 Digestive: 20 Hematologic: 1 Gynecologic: 4 Genitourinary: 23 Other: 7 | Breast: 1 Digestive: 1 |
| Self-referred | 149 (71.3%) | 10 (55.8%) | 68 (73.1%) | 69 (71.2%) | 2 (100%) |
| On treatment | 152 (72.7%) | 9 (50.0%) | 59 (63.4%) | 82 (85.4%) | 2 (100%) |
| Symptoms affecting exercise | 172 (82.3%) | 11 (61.1%) | 76 (81.7%) | 83 (86.5%) | 2 (100%) |
| Physical activity level: inactive/ sedentary | 177 (85.1%) | 17 (94.4%) | 78 (83.9%) | 80 (84.2%) | 2 (100%) |
| ≥1 uncontrolled health conditions | 43 (20.6%) | 4 (22.2%) | 13 (14.0%) | 26 (27.1%) | 0 (0%) |
| BMI > 40 | 12 (5.7%) | 1 (5.6%) | 3 (3.2%) | 8 (8.3%) | - |
| BMI < 18.5 | 3 (1.4%) | - | 1 (1.1%) | 2 (2.1%) | 2 (100%) |
| ≥1 Vital sign outside of recommended level | 103 (49.3%) | 11 (61.1%) | 36 (38.7%) | 55 (57.3%) | 1 (50.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McNeely, M.L.; Williamson, T.; Shallwani, S.M.; Ternes, L.; Sellar, C.; Joy, A.A.; Lau, H.; Easaw, J.; Brown, A.; Courneya, K.S.; et al. Role of Qualified Exercise Professionals in Medical Clearance for Exercise: Alberta Cancer Exercise Hybrid Effectiveness-Implementation Study. Cancers 2025, 17, 2873. https://doi.org/10.3390/cancers17172873
McNeely ML, Williamson T, Shallwani SM, Ternes L, Sellar C, Joy AA, Lau H, Easaw J, Brown A, Courneya KS, et al. Role of Qualified Exercise Professionals in Medical Clearance for Exercise: Alberta Cancer Exercise Hybrid Effectiveness-Implementation Study. Cancers. 2025; 17(17):2873. https://doi.org/10.3390/cancers17172873
Chicago/Turabian StyleMcNeely, Margaret L., Tanya Williamson, Shirin M. Shallwani, Leslie Ternes, Christopher Sellar, Anil Abraham Joy, Harold Lau, Jacob Easaw, Adam Brown, Kerry S. Courneya, and et al. 2025. "Role of Qualified Exercise Professionals in Medical Clearance for Exercise: Alberta Cancer Exercise Hybrid Effectiveness-Implementation Study" Cancers 17, no. 17: 2873. https://doi.org/10.3390/cancers17172873
APA StyleMcNeely, M. L., Williamson, T., Shallwani, S. M., Ternes, L., Sellar, C., Joy, A. A., Lau, H., Easaw, J., Brown, A., Courneya, K. S., & Culos-Reed, S. N. (2025). Role of Qualified Exercise Professionals in Medical Clearance for Exercise: Alberta Cancer Exercise Hybrid Effectiveness-Implementation Study. Cancers, 17(17), 2873. https://doi.org/10.3390/cancers17172873









