Stereotactic Radiosurgery for Recurrent Meningioma: A Systematic Review of Risk Factors and Management Approaches
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
3. Results
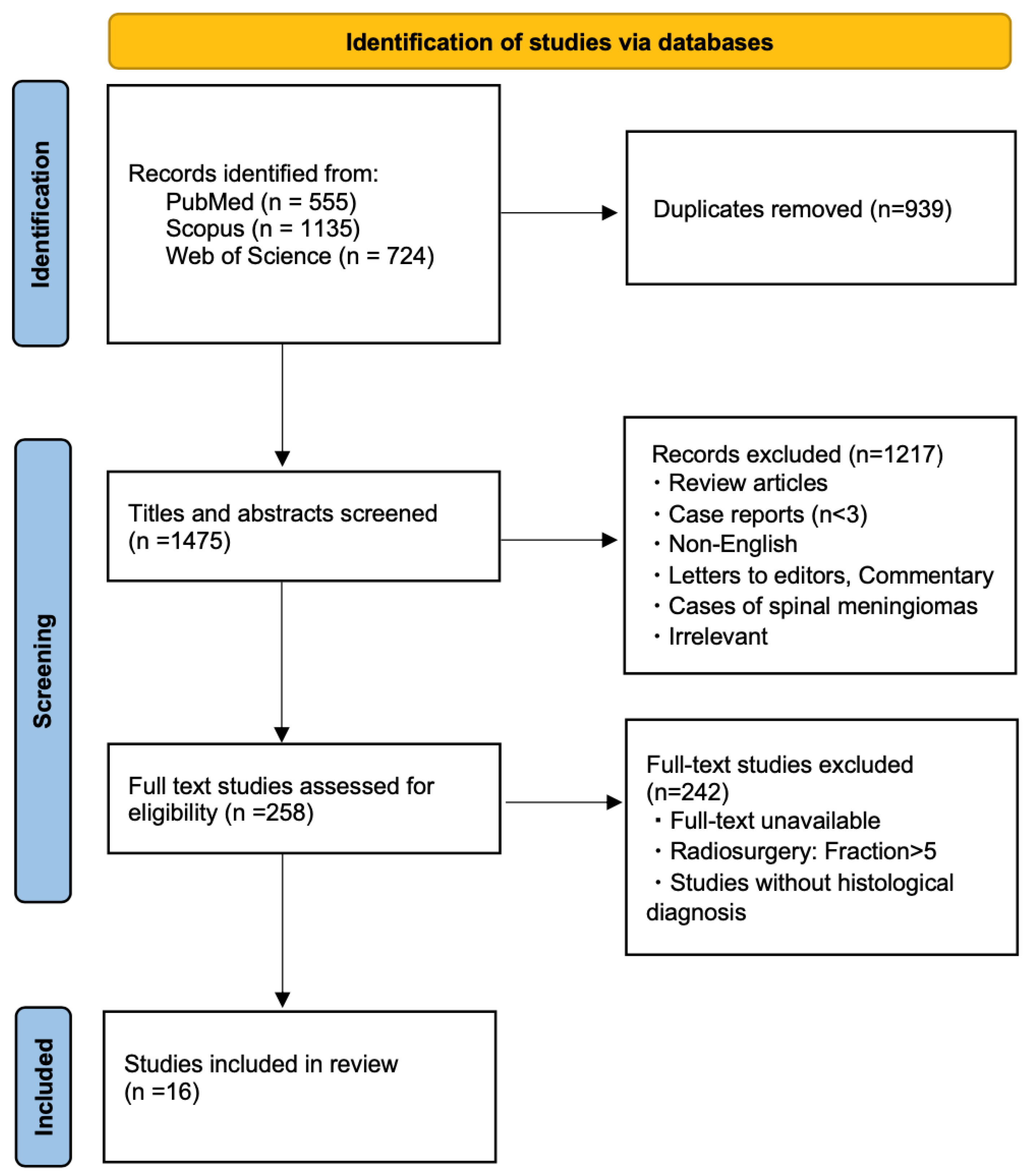
3.1. Search Results
3.2. Stereotactic Radiosurgery for Management of WHO Grade I Recurrent Meningiomas
3.3. Stereotactic Radiosurgery for Management of WHO Grade II Recurrent Meningiomas
3.4. Stereotactic Radiosurgery for Management of WHO Grade III Recurrent Meningiomas
3.5. Stereotactic Radiosurgery for Management of High-Grade (Both WHO Grade II and III)/Unknown Grade Recurrent Meningiomas
3.6. Stereotactic Radiosurgery for Recurrent Meningiomas After Surgery Alone
3.7. Stereotactic Radiosurgery for Recurrent Meningiomas After RT +/− Surgery
3.8. Treatment-Related Toxicity of Stereotactic Radiosurgery for Recurrent Meningioma
3.9. Statistical Analysis Results for Studies Including Only Salvage SRS
4. Discussion
4.1. Overall Effectiveness of SRS in Recurrent Meningiomas by Tumor Grade
4.2. Overall Effectiveness of SRS in Recurrent Meningiomas by Prior Treatment
4.3. Prognostic Factors for SRS Outcomes
4.4. Salvage Strategies After SRS Failure
4.5. Toxicity, Radiation Necrosis, and Predictive Factors
4.6. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saraf, S.; McCarthy, B.J.; Villano, J.L. Update on meningiomas. Oncologist 2011, 16, 1604–1613. [Google Scholar] [CrossRef]
- Claus, E.B.; Bondy, M.L.; Schildkraut, J.M.; Wiemels, J.L.; Wrensch, M.; Black, P.M. Epidemiology of intracranial meningioma. Neurosurgery 2005, 57, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.; Wrensch, M.; Claus, E.B. Epidemiology and etiology of meningioma. J. Neurooncol. 2010, 99, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.N.; Braun, Y.; Plate, K.H. Classification of meningiomas-advances and controversies. Chin. Clin. Oncol. 2017, 6 (Suppl. 1), S2. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef]
- Riemenschneider, M.J.; Perry, A.; Reifenberger, G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006, 5, 1045–1054, Erratum in Lancet Neurol. 2007, 6, 105. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef]
- Corniola, M.V.; Meling, T.R. Management of Recurrent Meningiomas: State of the Art and Perspectives. Cancers 2022, 14, 3995. [Google Scholar] [CrossRef]
- Lemée, J.M.; Corniola, M.V.; Meling, T.R. Benefits of re-do surgery for recurrent intracranial meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef]
- Hanakita, S.; Oya, S. Surgical Outcomes following Reoperation for Recurrent Intracranial Meningiomas. J. Clin. Med. 2024, 13, 3356. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Mathieu, D.; Lunsford, L.D.; Martin, J.J.; Madhok, R.; Niranjan, A.; Flickinger, J.C. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery 2008, 62, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021, 23, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.M.; Sarkar, A. Stereotactic radiosurgery and stereotactic radiotherapy in the treatment of skull base meningiomas. Otolaryngol. Clin. N. Am. 2009, 42, 677–688. [Google Scholar] [CrossRef]
- Przybylowski, C.J.; Raper, D.M.; Starke, R.M.; Xu, Z.; Liu, K.C.; Sheehan, J.P. Stereotactic radiosurgery of meningiomas following resection: Predictors of progression. J. Clin. Neurosci. 2015, 22, 161–165. [Google Scholar] [CrossRef]
- Huang, R.Y.; Bi, W.L.; Griffith, B.; Kaufmann, T.J.; la Fougère, C.; Schmidt, N.O.; Tonn, J.C.; Vogelbaum, M.A.; Wen, P.Y.; Aldape, K.; et al. International Consortium on Meningiomas. Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol. 2019, 21 (Suppl. 1), i44–i61. [Google Scholar] [CrossRef]
- Tsien, C.; Drzymala, R.E.; Rich, K. Imaging Advances in Stereotactic Radiosurgery. Mo. Med. 2015, 112, 373–378. [Google Scholar]
- Ehret, F.; El Baya, L.; Erridge, S.C.; Bussière, M.; Verhoeff, J.J.C.; Niyazi, M.; Preusser, M.; Minniti, G.; Shih, H.A. Radiation Therapy for Meningiomas—Where Do We Stand and What’s on the Horizon? Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 599–612. [Google Scholar] [CrossRef]
- McKay, W.H.; McTyre, E.R.; Okoukoni, C.; Alphonse-Sullivan, N.K.; Ruiz, J.; Munley, M.T.; Qasem, S.; Lo, H.W.; Xing, F.; Laxton, A.W.; et al. Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J. Neurosurg. 2017, 127, 148–156. [Google Scholar] [CrossRef]
- Kerschbaumer, J.; Demetz, M.; Krigers, A.; Nevinny-Stickel, M.; Thomé, C.; Freyschlag, C.F. Risk Factors for Radiation Necrosis in Patients Undergoing Cranial Stereotactic Radiosurgery. Cancers 2021, 13, 4736. [Google Scholar] [CrossRef]
- Withrow, D.R.; Anderson, H.; Armstrong, G.T.; Hawkins, M.; Journy, N.; Neglia, J.P.; de Vathaire, F.; Tucker, M.A.; Inskip, P.D.; Brenner, A.V.; et al. Pooled Analysis of Meningioma Risk Following Treatment for Childhood Cancer. JAMA Oncol. 2022, 8, 1756–1764, Erratum in JAMA Oncol. 2022, 8, 1856. [Google Scholar] [CrossRef]
- Sadetzki, S.; Flint-Richter, P.; Ben-Tal, T.; Nass, D. Radiation-induced meningioma: A descriptive study of 253 cases. J. Neurosurg. 2002, 97, 1078–1082. [Google Scholar] [CrossRef]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-Induced Meningiomas: An Exhaustive Review of the Literature. World Neurosurg. 2017, 97, 635–644.e8. [Google Scholar] [CrossRef] [PubMed]
- Caccese, M.; Busato, F.; Guerriero, A.; Padovan, M.; Cerretti, G.; Gardiman, M.P.; Zagonel, V.; Lombardi, G. The role of radiation therapy and systemic treatments in meningioma: The present and the future. Cancer Med. 2023, 12, 16041–16053. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Drappatz, J. Advances in the systemic therapy for recurrent meningiomas and the challenges ahead. Expert. Rev. Neurother. 2023, 23, 995–1004. [Google Scholar] [CrossRef]
- Valery, C.A.; Faillot, M.; Lamproglou, I.; Golmard, J.L.; Jenny, C.; Peyre, M.; Mokhtari, K.; Mazeron, J.J.; Cornu, P.; Kalamarides, M. Grade II meningiomas and Gamma Knife radiosurgery: Analysis of success and failure to improve treatment paradigm. J. Neurosurg. 2016, 125 (Suppl. 1), 89–96. [Google Scholar] [CrossRef]
- Chen, W.C.; Lucas, C.G.; Magill, S.T.; Rogers, C.L.; Raleigh, D.R. Radiotherapy and radiosurgery for meningiomas. Neuro-Oncol Adv. 2023, 5 (Suppl. 1), i67–i83. [Google Scholar] [CrossRef]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef]
- Spiegl-Kreinecker, S.; Lötsch, D.; Neumayer, K.; Kastler, L.; Gojo, J.; Pirker, C.; Pichler, J.; Weis, S.; Kumar, R.; Webersinke, G.; et al. TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018, 20, 1584–1593. [Google Scholar] [CrossRef]
- PPage, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021, 372, n71. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 January 2025).
- Mattozo, C.A.; De Salles, A.A.; Klement, I.A.; Gorgulho, A.; McArthur, D.; Ford, J.M.; Agazaryan, N.; Kelly, D.F.; Selch, M.T. Stereotactic radiation treatment for recurrent nonbenign meningiomas. J. Neurosurg. 2007, 106, 846–854. [Google Scholar] [CrossRef]
- Kano, H.; Takahashi, J.A.; Katsuki, T.; Araki, N.; Oya, N.; Hiraoka, M.; Hashimoto, N. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J. Neurooncol 2007, 84, 41–47. [Google Scholar] [CrossRef]
- Aboukais, R.; Zairi, F.; Lejeune, J.P.; Le Rhun, E.; Vermandel, M.; Blond, S.; Devos, P.; Reyns, N. Grade 2 meningioma and radiosurgery. J. Neurosurg. 2015, 122, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Kaprealian, T.; Raleigh, D.R.; Sneed, P.K.; Nabavizadeh, N.; Nakamura, J.L.; McDermott, M.W. Parameters influencing local control of meningiomas treated with radiosurgery. J. Neurooncol. 2016, 128, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Hara, J.; Magill, S.T.; Wu, A.; Aghi, M.K.; Theodosopoulos, P.V.; Perry, A.; McDermott, M.W.; Sneed, P.K.; Raleigh, D.R.; et al. Salvage therapy outcomes for atypical meningioma. J. Neurooncol. 2018, 138, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.C.; Lee, C.C.; Guo, W.Y.; Shiau, C.Y.; Chang, Y.C.; Pan, D.H.; Sheehan, J.P.; Chung, W.Y. Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas: Post-treatment long-term clinical outcomes, complications, and volume changes. J. Neurooncol. 2019, 143, 261–270. [Google Scholar] [CrossRef]
- Park, K.J.; Kano, H.; Iyer, A.; Liu, X.; Tonetti, D.A.; Lehocky, C.; Faramand, A.; Niranjan, A.; Flickinger, J.C.; Kondziolka, D.; et al. Gamma Knife stereotactic radiosurgery for cavernous sinus meningioma: Long-term follow-up in 200 patients. J. Neurosurg. 2018, 130, 1799–1808. [Google Scholar] [CrossRef]
- Acker, G.; Meinert, F.; Conti, A.; Kufeld, M.; Jelgersma, C.; Nguyen, P.; Kluge, A.; Lukas, M.; Loebel, F.; Pasemann, D.; et al. Image-Guided Robotic Radiosurgery for Treatment of Recurrent Grade II and III Meningiomas. A Single-Cent. Study. World Neurosurg. 2019, 131, e96–e107. [Google Scholar] [CrossRef]
- Hasegawa, H.; Vakharia, K.; Link, M.J.; Stafford, S.L.; Brown, P.D.; Parney, I.F.; Burns, T.C.; Yan, E.S.; Mahajan, A.; Laack, N.N.; et al. The role of single-fraction stereotactic radiosurgery for atypical meningiomas (WHO grade II): Treatment results based on a 25-year experience. J. Neurooncol. 2021, 155, 335–342. [Google Scholar] [CrossRef]
- Momin, A.A.; Shao, J.; Soni, P.; Almeida, J.P.; Suh, J.H.; Murphy, E.S.; Chao, S.T.; Angelov, L.; Mohammadi, A.M.; Barnett, G.H.; et al. Outcomes of salvage radiation for recurrent world health organization grade II meningiomas: A retrospective cohort study. J. Neurooncol. 2021, 152, 373–382. [Google Scholar] [CrossRef]
- Shepard, M.J.; Xu, Z.; Kearns, K.; Li, C.; Chatrath, A.; Sheehan, K.; Sheehan, D.; Faramand, A.; Niranjan, A.; Kano, H.; et al. Stereotactic Radiosurgery for Atypical (World Health Organization II) and Anaplastic (World Health Organization III) Meningiomas: Results From a Multicenter, International Cohort Study. Neurosurgery 2021, 88, 980–988. [Google Scholar] [CrossRef]
- Marchetti, M.; Pinzi, V.; Iezzoni, C.; Morlino, S.; Tramacere, I.; De Martin, E.; Cane, I.; Fariselli, L. Multisession radiosurgery for grade 2 (WHO), high risk meningiomas. A phase II clinical trial. J. Neurooncol. 2022, 157, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Schmutzer, M.; Skrap, B.; Thorsteinsdottir, J.; Fürweger, C.; Muacevic, A.; Schichor, C. Meningioma involving the superior sagittal sinus: Long-term outcome after robotic radiosurgery in primary and recurrent situation. Front. Oncol. 2023, 13, 1206059. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.B.; Weil, C.R.; Rock, C.B.; Gravbrot, N.; Burt, L.M.; DeCesaris, C.; Menacho, S.T.; Jensen, R.L.; Shrieve, D.C.; Cannon, D.M. Patterns of failure after radiosurgery for WHO grade 1 or imaging defined meningiomas: Long-term outcomes and implications for management. J. Clin. Neurosci. 2024, 120, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Gallitto, M.; Sedor, G.; Lee, A.; Pasetsky, J.; Kinslow, C.J.; Santos, G.L.; Obiri-Yeboah, D.; Kshettry, V.R.; Helis, C.A.; Chan, M.D.; et al. Salvage Stereotactic Radiosurgery for Recurrent WHO Grade 2 and 3 Meningiomas: A Multicenter Study (STORM). Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 730–737. [Google Scholar] [CrossRef]
- Kaley, T.; Barani, I.; Chamberlain, M.; McDermott, M.; Panageas, K.; Raizer, J.; Rogers, L.; Schiff, D.; Vogelbaum, M.; Weber, D.; et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: A RANO review. Neuro Oncol. 2014, 16, 829–840. [Google Scholar] [CrossRef]
- Simonetti, G.; Silvani, A.; Tramacere, I.; Farinotti, M.; Legnani, F.; Pinzi, V.; Pollo, B.; Erbetta, A.; Gaviani, P. Long term follow up in 183 high grade meningioma: A single institutional experience. Clin. Neurol. Neurosurg. 2021, 207, 106808. [Google Scholar] [CrossRef]
- Wang, J.Z.; Patil, V.; Landry, A.P.; Gui, C.; Ajisebutu, A.; Liu, J.; Saarela, O.; Pugh, S.L.; Won, M.; Patel, Z.; et al. Molecular classification to refine surgical and radiotherapeutic decision-making in meningioma. Nat. Med. 2024, 30, 3173–3183. [Google Scholar] [CrossRef]
- Patel, A.J.; Wan, Y.W.; Al-Ouran, R.; Revelli, J.P.; Cardenas, M.F.; Oneissi, M.; Xi, L.; Jalali, A.; Magnotti, J.F.; Muzny, D.M.; et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 21715–21726. [Google Scholar] [CrossRef]
- Kondziolka, D.; Patel, A.D.; Kano, H.; Flickinger, J.C.; Lunsford, L.D. Long-term Outcomes After Gamma Knife Radiosurgery for Meningiomas. Am. J. Clin. Oncol. 2016, 39, 453–457. [Google Scholar] [CrossRef]
- Sethi, R.A.; Rush, S.C.; Liu, S.; Sethi, S.A.; Parker, E.; Donahue, B.; Narayana, A.; Silverman, J.; Kondziolka, D.; Golfinos, J.G. Dose-Response Relationships for Meningioma Radiosurgery. Am. J. Clin. Oncol. 2015, 38, 600–604. [Google Scholar] [CrossRef]
- Wang, W.H.; Lee, C.C.; Yang, H.C.; Liu, K.D.; Wu, H.M.; Shiau, C.Y.; Guo, W.Y.; Pan, D.H.; Chung, W.Y.; Chen, M.T. Gamma Knife Radiosurgery for Atypical and Anaplastic Meningiomas. World Neurosurg. 2016, 87, 557–564. [Google Scholar] [CrossRef]
- Ferraro, D.J.; Funk, R.K.; Blackett, J.W.; Ju, M.R.; DeWees, T.A.; Chicoine, M.R.; Dowling, J.L.; Rich, K.M.; Drzymala, R.E.; Zoberi, I.; et al. A retrospective analysis of survival and prognostic factors after stereotactic radiosurgery for aggressive meningiomas. Radiat. Oncol. 2014, 9, 38. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Chen, W.C.; Lucas, C.G.; Bayley, J.C.; Harmanci, A.S.; Maas, S.L.N.; Santagata, S.; Klisch, T.; Perry, A.; Bi, W.L.; et al. Hypermitotic meningiomas harbor DNA methylation subgroups with distinct biological and clinical features. Neuro Oncol. 2023, 25, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef]
- Korytko, T.; Radivoyevitch, T.; Colussi, V.; Wessels, B.W.; Pillai, K.; Maciunas, R.J.; Einstein, D.B. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 419–424. [Google Scholar] [CrossRef]
- Parikh, S.; Alluri, U.; Heyes, G.; Evison, F.; Meade, S.; Benghiat, H.; Hartley, A.; Hickman, M.; Sawlani, V.; Chavda, S.; et al. Clinical Outcomes and Relevance of Composite V12 Gy in Patients With Four or More Brain Metastases Treated With Single Fraction Stereotactic Radiosurgery. Clin. Oncol. (R Coll Radiol) 2025, 37, 103663. [Google Scholar] [CrossRef]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef]
- Doré, M.; Martin, S.; Delpon, G.; Clément, K.; Campion, L.; Thillays, F. Stereotactic radiotherapy following surgery for brain metastasis: Predictive factors for local control and radionecrosis. Cancer Radiother. 2017, 21, 4–9. [Google Scholar] [CrossRef]
- Huang, J.; Gao, F.; Shimony, J.; Johanns, T.M.; Mantica, M.; Gershon, T.R. The interim result of a phase I/II study of nivolumab with or without ipilimumab in combination with multi-fraction stereotactic radiosurgery for recurrent, high-grade, radiation-relapsed meningioma. J. Clin. Oncol. 2022, 40 (Suppl. 16), 2068. [Google Scholar] [CrossRef]
| Study | #Patients/ #Tumors | Prior Treatment | Median GTV/PTV (cm3) | Salvage Treatment | Median Marginal Dose (Gy) | PFS (%) | Local Control (%) | Local Control Definition |
|---|---|---|---|---|---|---|---|---|
| Rock, 2024 [45] | 22/- | Surgery: 100% RT: 0% | - | LINAC | Range: 13–20 | 5-yr PFS: 91 | 100 | No tumor progression (>20% tumor volume increase) |
| Schmutzer, 2023 [44] | 71/71 | Surgery: 100% RT: 0% | Mean: 7.0/- | CyberKnife | Mean: 15.4 | - | 91.2 | Tumor shrinkage and/or no size change |
| Park, 2019 [38] | 34/- | Surgery: 100% RT: 6% | 5.9/- | Gamma Knife | 13 | 5 yr PFS: 85 | 71 | No tumor progression (>25% tumor volume increase) |
| Kaprealian, 2016 [35] | -/96 | Surgery: 80% RT: 20% | - | Gamma Knife | - | - | Prior surgery: 87 Prior RT: 62 (5-year) | Freedom from progression (>20% tumor volume increase) |
| Mattozo, 2007 [32] | -/3 | Surgery: 100% RT: - | - | LINAC | - | 3 yr PFS: 100 | 100 | No increase in lesion size |
| Study | #Patients/ #Tumors | Prior Treatment | Median GTV/PTV (cm3) | Salvage Treatment | Median Marginal Dose (Gy) | PFS (%) | Local Control (%) | Local Control Definition |
|---|---|---|---|---|---|---|---|---|
| Mattozo, 2007 [32] | -/19 | Surgery: 100% RT: - | - | LINAC | - | 3-yr PFS: 100 | 89 | No increase in lesion size |
| Aboukais, 2015 [34] | 27/27 | Surgery: 100% RT: 30% | -/5.4 | Gamma Knife | Mean: 15.2 (Prior surgery alone: Mean 14.9, Prior RT: Mean 15.9) | - | 75, 52, 40 (1-, 2-, 3-year) | Size reduction or stability |
| Kaprealian, 2016 [35] | -/48 | Surgery: 44% RT: 56% | - | Gamma Knife | - | - | Prior surgery: 79 Prior RT: 36 (5-year) | Freedom from progression (>20% tumor volume increase) |
| Valery, 2016 [26] | 18/58 | Surgery: 100% RT: 39% | 2.5/- | Gamma Knife | Range: 14–16 | 3-yr PFS: 23 | 89, 71 (1-, 3-year) | No tumor progression (>20% tumor volume increase) |
| Chen, 2018 [36] | 24/- | Surgery: 100% RT: 29% | - | - | 15 | - | 90, 66, 44 (1-, 2-, 3-year) | No local recurrence |
| Acker, 2019 [39] | 27/105 | Surgery: 100% RT: 48% | -/1.55 | CyberKnife | Mean: 23.1 | 73, 59 (3-, 5-year) | 84 | No lesion progression |
| Hasegawa, 2021 [40] | 17: Early salvage (7–18 months); 33: Late salvage (>18 months)/- | Surgery: 100% RT: - | - | Gamma Knife | 16 | Early salvage: 33, 0 (3-, 5-year) Late salvage: 61, 48 (3-, 5-year) | 46 (3-year) | No tumor progression (>20% tumor volume increase) |
| Momin, 2021 [41] | -/51 | Surgery: 100% RT: 67% | Mean: 2.2/- | Gamma Knife | Prior surgery: 16, Prior RT: 15 | Prior surgery alone: 60.7, 40.4 (3-, 5-year), Prior RT: 41.0 (3-year) | - | - |
| Marchetti, 2022 [43] | 16/- | Surgery: 100% RT: - | - | CyberKnife | 28 Gy in 4 fractions or 24 Gy in 4 fractions | - | 75 | No tumor progression |
| Gallitto, 2024 [46] | 102/102 | - | - | Gamma Knife or LINAC | 16 | 3-yr PFS: 64 | - | - |
| Study | #Patients/ #Tumors | Prior Treatment | Median GTV/PTV (cm3) | Salvage Treatment | Median Marginal Dose (Gy) | PFS (%) | Local Control (%) | Local Control Definition |
|---|---|---|---|---|---|---|---|---|
| Mattozo, 2007 [32] | -/5 | Surgery: 100% RT: - | - | LINAC | - | 1-yr PFS: 0 | 0 | No increase in lesion size |
| Kaprealian, 2016 [35] | -/76 | Surgery: 16% RT: 84% | - | Gamma Knife | - | - | Prior surgery: 92 Prior RT: 31 (5-year) | Freedom from progression (>20% tumor volume increase) |
| Acker, 2019 [39] | 8/22 | Surgery: 100% RT: 50% | 2.38/- | CyberKnife | Mean: 19.3 | 2-yr PFS: 46 | 79 | No lesion progression |
| Study | #Patients/ #Tumors | WHO Grade | Prior Treatment | Median GTV/PTV (cm3) | Salvage Treatment | Median Marginal Dose (Gy) | PFS (%) | Local Control (%) | Local Control Definition |
|---|---|---|---|---|---|---|---|---|---|
| Kano, 2007 [33] | 12/30 | G2 (n = 10); G3 (n = 2) | Surgery: 100% RT: 33% | -/2.87 | LINAC | 19 | 3-, 5-yr PFS: 48.3 [5-yr PFS: 29.4 (<20 Gy), 63.1 (20 Gy)] | 57 | No in-field recurrence |
| Shepard, 2021 [42] | 141/- | G2, G3 | Surgery: 100% RT: - | - | Gamma Knife | 14.8 | 66.6, 33.6 (2-, 5-year) | - | - |
| Gallitto, 2024 [46] | 34/- | G2 (n = 102); G3 (n = 6) | Surgery: 98% RT: 19% | 2.80/- | Gamma Knife or LINAC | 16 | 3-yr PFS: 57 | - | - |
| Hung, 2019 [37] | 37/- | - (CSM) | Surgery: 100% RT: 0% | - | Gamma Knife | 12 | - | 86 | No tumor progression (defined as volume >110% of original) |
| Prior Resection Patients (%) | Prior RT Patients (%) | Study | WHO Grade | Median Marginal Dose (Gy) | PFS (%) | Local Control (%) | Local Control Definition |
|---|---|---|---|---|---|---|---|
| 100 | 0 | Schmutzer, 2023 [44] | G1 | Mean: 15.4 | - | 91.2 | tumor shrinkage and/or no size change |
| Rock, 2024 [45] | G1 | Range: 13–20 | 5-yr PFS: 91 | 100 | No tumor progression (>20% tumor volume increase) | ||
| Hung, 2019 [37] | - (CSM) | 12 (11–21) | - | 86 | No tumor progression (defined as volume >110% of original) | ||
| Momin, 2021 [41] | G2 | 16 (12–18) | 60.7, 40.4 (3-, 5-year) | - | - | ||
| Kaprealian, 2016 [35] | G1, G2, G3 | 15 (12–20) | 5-yr PFS: 82 | 5-year: G1 87, G2 79, G3 92 | Freedom from progression (>20% tumor volume increase) |
| Prior Resection Patients (%) | Prior RT Patients (%) | Study | WHO Grade | Median Marginal Dose (Gy) | PFS (%) | Local Control (%) | Local Control Definition |
|---|---|---|---|---|---|---|---|
| 100 | 100 | Momin, 2021 [41] | G2 | 15 (13–20) | 3-yr PFS: 41.0 | - | - |
| 0 | 100 | Kaprealian, 2016 [35] | G1, G2, G3 | 16 (12–19) | 5-yr PFS: 26 | 5-year: G1 62, G2 36, G3 31 | Freedom from progression (>20% tumor volume increase) |
| Prior Resection Patients (%) | Prior RT Patients (%) | Study | Median Marginal Dose (Gy) | SRS Treatment-Related Toxicity |
|---|---|---|---|---|
| 100 | 0 | Schmutzer, 2023 [44] | Mean: 15.4 | 3.6% (headache), 2.9% (perifocal edema), 2.2% (seizures), 2.2% (vertigo), 0.7% (radiation necrosis) |
| 100 | 0 | Momin, 2021 [41] | 16 (12–18) | 29.4% (CTCAE grade a ≥ I adverse events. Most common were alopecia, dermatitis, fatigue, and headache. No radiation necrosis.) |
| 100 | 0 | Kaprealian, 2016 [35] | 15 (12–20) | 5%, 5% (1-, 2-year probability of ARE) |
| 98 | 19 | Gallitto, 2024 [46] | 16 | 3% (radiation necrosis), 7% (cognitive disturbance), 4% (new-onset seizures) |
| 100 | 30 | Aboukais, 2015 [34] | Mean: 15.2 | 3.7% (transient hemiparesis) |
| 100 | 33 | Kano, 2007 [33] | 19 (12–20) | 17% (asymptomatic perifocal edema from radiation-induced angiopathy) |
| 100 | 39 | Valery, 2016 [26] | Range: 14–16 | 22% (2 radiation necrosis treated by corticosteroids, 1 spontaneous hemorrhage, 1 recurrent seizures) |
| 100 | 49 | Acker, 2019 [39] | 16 (15–18) | 37% (6 focal seizures, 2 mild visual deterioration, 2 dysesthesia, 1 fatigue, 1 headache, 1 fine motor skill disturbance) |
| 0 | 100 | Kaprealian, 2016 [35] | 16 (12–19) | 15%, 30% (1-, 2-year probability of ARE) |
| Study | WHO Grade | Univariable Analysis Significant (HR (95%CI)) | Univariable Analysis Not Significant (HR (95%CI)) | Multivariable Analysis Significant (HR (95%CI)) | Multivariable Analysis Not Significant (HR (95%CI)) |
|---|---|---|---|---|---|
| Kano, 2007 [33] | G2 (n = 10); G3 (n = 2) | Predictors of worse PFS: Marginal radiation dose (<20 Gy) (p < 0.05) | Sex, age, tumor location, target volume, tumor grade | - | - |
| Aboukais, 2015 [34] | G2 | Factors that may have affected LC: Age (p = 0.0496), target volume (p = 0.0445) Factors that may have affected regional control: Sex (p = 0.0333), no. of resections (p = 0.0310), postop RT (p = 0.0254) | Regarding LC: Sex, location of recurrence, Simpson grade, no. of resections, postop RT, delay between surgery and SRS, radiation dose Regarding regional control: Age, location of recurrence, Simpson grade, delay between surgery and SRS, radiation dose, target volume | - | - |
| Valery, 2016 [26] | G2 | Factors for more local relapses: treated with a minimum dose of ≤12 Gy (p = 0.04) Factors for improved marginal control: Tumor growth rate (p = 0.002) Factors for worse PFS: delay between first surgery and GKRS (p = 0.03) | Regarding LC: Tumor growth rate, tumor volume | - | - |
| Acker, 2019 [39] | G2 (n = 27); G3 (n = 8) | Risk factors in G2 meningioma for local recurrence: Age 1.133 (1.046–1.227), p = 0.002 | Gender, planning target volume, prescribed dose, minimal dose, mean dose, maximal dose, dose mean EQD2, coverage | Risk factors in G2 meningioma for local recurrence: Age 1.104 (1.038–1.175), p = 0.002 Dose mean EQD2 1.210 (1.070–1.367), p = 0.002 | Gender |
| Gallitto, 2024 [46] | G2 (n = 102); G3 (n = 6) | Predictors of worse PFS: G3 histology 11.40 (3.95–33.0), p < 0.001 Median marginal radiation dose (Gy) 1.09 (1.01–1.18), p = 0.024 History of prior RT 1.85 (1.10–3.12), p = 0.02 | Age, male gender, tumor volume, maximum point dose | Predictors of worse PFS: G3 histology 6.80 (1.61–28.6), p = 0.009 History of prior RT 2.69 (1.23–5.86), p = 0.013 Male gender 3.48 (1.47–8.26), p = 0.005 | Age, tumor volume, median marginal radiation dose, maximum point dose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizutani, Y.; Hori, Y.S.; Harary, P.M.; Lam, F.C.; Reesh, D.A.; Emrich, S.C.; Ustrzynski, L.; Tayag, A.; Park, D.J.; Chang, S.D. Stereotactic Radiosurgery for Recurrent Meningioma: A Systematic Review of Risk Factors and Management Approaches. Cancers 2025, 17, 2750. https://doi.org/10.3390/cancers17172750
Mizutani Y, Hori YS, Harary PM, Lam FC, Reesh DA, Emrich SC, Ustrzynski L, Tayag A, Park DJ, Chang SD. Stereotactic Radiosurgery for Recurrent Meningioma: A Systematic Review of Risk Factors and Management Approaches. Cancers. 2025; 17(17):2750. https://doi.org/10.3390/cancers17172750
Chicago/Turabian StyleMizutani, Yuka, Yusuke S. Hori, Paul M. Harary, Fred C. Lam, Deyaaldeen Abu Reesh, Sara C. Emrich, Louisa Ustrzynski, Armine Tayag, David J. Park, and Steven D. Chang. 2025. "Stereotactic Radiosurgery for Recurrent Meningioma: A Systematic Review of Risk Factors and Management Approaches" Cancers 17, no. 17: 2750. https://doi.org/10.3390/cancers17172750
APA StyleMizutani, Y., Hori, Y. S., Harary, P. M., Lam, F. C., Reesh, D. A., Emrich, S. C., Ustrzynski, L., Tayag, A., Park, D. J., & Chang, S. D. (2025). Stereotactic Radiosurgery for Recurrent Meningioma: A Systematic Review of Risk Factors and Management Approaches. Cancers, 17(17), 2750. https://doi.org/10.3390/cancers17172750






