FOLFIRINOX: The Best Adjuvant Treatment for Ampullary Adenocarcinoma? A Multicenter Study by the Turkish Oncology Group (TOG)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Patients and Disease Features
2.3. Adjuvant Treatments
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Tumor Characteristics
3.2. Effect of Clinicopathologic, Inflammatory, and Treatment-Related Parameters on Disease-Free Survival
3.3. Effect of Clinicopathologic, Inflammatory, and Treatment-Related Parameters on Overall Survival
3.4. Toxicity
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, D.H.; Bekaii-Saab, T. Ampullary Cancer: An Overview. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Futakawa, N.; Yamagata, S.; Wada, Y.; Kuroda, A.; Muto, T.; Esaki, Y. Different Clinicopathologic Findings in Two Histologic Types of Carcinoma of Papilla of Vater. Jpn. J. Cancer Res. 1994, 85, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Kohler, I.; Jacob, D.; Budzies, J.; Lehmann, A.; Weichert, W.; Schulz, S.; Neuhaus, P.; Röcken, C. Phenotypic and Genotypic Characterization of Carcinomas of the Papilla of Vater Has Prognostic and Putative Therapeutic Implications. Am. J. Clin. Pathol. 2011, 135, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.; Rodrigo, A.; Vázquez, S.; Carrizo, V.; Vilardell, F.; Mira, M. Adjuvant treatment in ampullary adenocarcinoma: A systematic review. Clin. Transl. Oncol. 2020, 22, 1407–1413. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, J.H.; Ryoo, B.Y.; Kim, K.P.; Chang, H.M.; Oh, D.; Song, T.J.; Lee, S.S.; Seo, D.W.; Lee, S.K.; et al. Adjuvant Chemotherapy for Resected Ampulla of Vater Carcinoma: Retrospective Analysis of 646 Patients. Cancer Res. Treat. 2021, 53, 424–435. [Google Scholar] [CrossRef]
- Vining, C.C.; Schuitevoerder, D.; Turaga, K.K. Ampullary adenocarcinoma: The current state of adjuvant therapies. Hepatobiliary Surg. Nutr. 2020, 9, 477–489. [Google Scholar] [CrossRef]
- Tsagkalidis, V.; Langan, R.C.; Ecker, B.L. Ampullary Adenocarcinoma: A Review of the Mutational Landscape and Implications for Treatment. Cancers 2023, 15, 5772. [Google Scholar] [CrossRef]
- Westgaard, A.; Tafjord, S.; Farstad, I.N.; Cvancarova, M.; Eide, T.J.; Mathisen, O.; Clausen, O.P.F.; Gladhaug, I.P. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer 2008, 8, 170. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Del Chiaro, M.; Tempero, M.A.; Malafa, M.P.; Benson, A.B.; Cardin, D.B.; Christensen, J.A.; Chung, V.; Czito, B.; Dillhoff, M.; et al. Ampullary Adenocarcinoma, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 753–782. [Google Scholar] [CrossRef]
- Siebenhüner, A.R.; Seifert, H.; Bachmann, H.; Seifert, B.; Winder, T.; Feilchenfeldt, J.; Breitenstein, S.; Clavien, P.-A.; Stupp, R.; Knuth, A.; et al. Adjuvant treatment of resectable biliary tract cancer with cisplatin plus gemcitabine: A prospective single center phase II study. BMC Cancer 2018, 18, 72. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. ADAPTA Trial: Adjuvant CAPOX vs mFOLFIRINOX in Ampullary Adenocarcinoma by Histology. NCT06068023. Available online: https://clinicaltrials.gov/study/NCT06068023 (accessed on 20 August 2025).
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Heinrich, K.; Tougeron, D.; Modest, D.P.; Schwaner, I.; Eucker, J.; Pihusch, R.; Stauch, M.; Kaiser, F.; Kahl, C.; et al. FOLFOXIRI Plus Cetuximab or Bevacizumab as First-Line Treatment of BRAFV600E-Mutant Metastatic Colorectal Cancer: The Randomized Phase II FIRE-4.5 (AIO KRK0116) Study. J. Clin. Oncol. 2023, 41, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Ecker, B.L.; Vollmer, C.M.; Behrman, S.W.; Allegrini, V.; Aversa, J.; Ball, C.G.; Barrows, C.E.; Berger, A.C.; Cagigas, M.N.; Christein, J.D.; et al. Role of Adjuvant Multimodality Therapy After Curative-Intent Resection of Ampullary Carcinoma. JAMA Surg. 2019, 154, 706–714. [Google Scholar] [CrossRef]
- Vo, N.P.; Nguyen, H.S.; Loh, E.W.; Tam, K.W. Efficacy and safety of adjuvant therapy after curative surgery for ampullary carcinoma: A systematic review and meta-analysis. Surgery 2021, 170, 1205–1214. [Google Scholar] [CrossRef]
- Falconi, M.; Crippa, S.; Domínguez, I.; Barugola, G.; Capelli, P.; Marcucci, S.; Beghelli, S.; Scarpa, A.; Bassi, C.; Pederzoli, P. Prognostic Relevance of Lymph Node Ratio and Number of Resected Nodes after Curative Resection of Ampulla of Vater Carcinoma. Ann. Surg. Oncol. 2008, 15, 3178–3186. [Google Scholar] [CrossRef]
- Smith, R.A.; Ghaneh, P.; Sutton, R.; Raraty, M.; Campbell, F.; Neoptolemos, J.P. Prognosis of Resected Ampullary Adenocarcinoma by Preoperative Serum CA19-9 Levels and Platelet-Lymphocyte Ratio. J. Gastrointest. Surg. 2008, 12, 1422–1428. [Google Scholar] [CrossRef]
- Klinkenbijl, J.H.; Jeekel, J.; Sahmoud, T.; van Pel, R.; Couvreur, M.L.; Veenhof, C.H.; Arnaud, J.P.; Gonzalez, D.G.; de Wit, L.T.; Hennipman, A.; et al. Adjuvant Radiotherapy and 5-Fluorouracil After Curative Resection of Cancer of the Pancreas and Periampullary Region. Ann. Surg. 1999, 230, 776. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, B.; Zhao, A.; Tian, Q.; Zhang, L.; Wang, L.; Zhao, X.; Yang, J.; Dong, D. The role of radiotherapy in patients with resected ampullary carcinoma: Findings based on the SEER database. HPB 2019, 21, 1535–1540. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of Adjuvant Chemotherapy with Fluorouracil Plus Folinic Acid or Gemcitabine vs Observation on Survival in Patients with Resected Periampullary Adenocarcinoma. JAMA 2012, 308, 147–156. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Varadhachary, G.R.; Kopetz, S.; Adinin, R.; Lin, E.; Morris, J.S.; Eng, C.; Abbruzzese, J.L.; Wolff, R.A. Phase II Study of Capecitabine and Oxaliplatin for Advanced Adenocarcinoma of the Small Bowel and Ampulla of Vater. J. Clin. Oncol. 2009, 27, 2598–2603. [Google Scholar] [CrossRef]
- Adjuvant chemotherapy with oxaliplatin, in combination with fluorouracil plus leucovorin prolongs disease-free survival, but causes more adverse events in people with stage II or III colon cancer. Abstracted from: André T, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators. Cancer Treat Rev. 2004, 30, 711–713. [CrossRef]
- Haller, D.G.; Tabernero, J.; Maroun, J.; de Braud, F.; Price, T.; Van Cutsem, E.; Hill, M.; Gilberg, F.; Rittweger, K.; Schmoll, H.-J. Capecitabine Plus Oxaliplatin Compared with Fluorouracil and Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer. J. Clin. Oncol. 2011, 29, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Markar, S.R.; Sodergren, M.H.; Malietzis, G.; Darzi, A.; Athanasiou, T.; Khan, A.Z. Meta-analysis of adjuvant therapy following curative surgery for periampullary adenocarcinoma. Br. J. Surg. 2017, 104, 814–822. [Google Scholar] [CrossRef]
- Keane, F.; O’Connor, C.; Moss, D.; Chou, J.F.; Perry, M.A.; Crowley, F.; Saxena, P.; Chan, A.; Schoenfeld, J.D.; Singhal, A.; et al. Adjuvant modified FOLFIRINOX for resected pancreatic adenocarcinoma: Clinical insights and genomic features from a large contemporary cohort. J. Natl. Cancer Inst. 2025, 117, 496–506. [Google Scholar] [CrossRef]
| mFOLFIRINOX N (%) | The Other Treatments N (%) | p Value | |
|---|---|---|---|
| Age, years | |||
| (median; range) | |||
| <65 | 33 (9.4.3) | 106 (60.2) | <0.001 |
| ≥65 | 2 (5.7) | 70 (39.8) | |
| Gender | |||
| Female | 19 (54.3) | 104 (59.1) | 0.60 |
| Male | 16 (45.7) | 72 (40.9) | |
| Comorbidity | |||
| Present | 17 (48.6) | 85 (48.3) | 0.97 |
| Absent | 18 (51.4) | 91 (51.7) | |
| ECOG PS | |||
| 0 | 15 (42.9) | 74 (42.0) | 0.96 |
| 1 | 18 (51.4) | 91 (51.7) | |
| 2 | 2 (5.7) | 11 (6.2) | |
| BMI | |||
| <18.5, underweight | 1 (3.1) | 5 (3.8) | 0.98 |
| 18.5–24.9, normal | 17 (53.1) | 73 (56.2) | |
| 25–29.9, overweight | 11 (34.4) | 39 (30.0) | |
| ≥30, obese | 3 (9.4) | 13 (10.0) | |
| Smoking | 14 (40) | 76 (43.2) | 0.73 |
| None | 21 (60) | 100 (56.8) | |
| Alcohol use | 3 (8.6) | 16 (9.1) | 0.92 |
| None | 32 (91.4) | 160 (90.9) | |
| Histological subtype | |||
| Intestinal | 7 (21.2) | 37 (24.3) | 0.70 |
| Pancreaticobiliary/mix | 26 (78.8) | 115 (75.7) | |
| pT stage | |||
| T1 | 3 (8.6) | 21 (11.9) | 0.66 |
| T2 | 11 (31.4) | 49 (27.8) | |
| T3 | 20 (57.1) | 92 (52.3) | |
| T4 | 1 (2.9) | 14 (8.0) | |
| pN stage | |||
| N0 | 13 (38.2) | 65 (37.1) | 0.77 |
| N1 | 16 (47.19) | 91 (52.0) | |
| N2 | 5 (14.7) | 19 (10.9) | |
| LNR | |||
| 0 | 14 (41.2) | 61 (41.9) | 0.84 |
| <0.2 | 10 (29.4) | 47 (29.4) | |
| 0.2–0.4 | 5 (14.7) | 30 (18.8) | |
| ≥0.4 | 5 (14.7) | 16 (10.0) | |
| THLN | |||
| ≥16 | 18 (52.9) | 75 (48.1) | 0.61 |
| <16 | 16 (47.1) | 81 (51.9) | |
| Surgical margin | |||
| R0 | 33 (97.1) | 160 (92.0) | 0.29 |
| R1 | 1 (2.9) | 14 (8.0) | |
| Pancreatic invasion | |||
| Present | 21 (61.8) | 98 (61.6) | 0.98 |
| Absent | 13 (38.2) | 61 (38.4) | |
| LVI | |||
| Present | 23 (67.6) | 121 (70.8) | 0.71 |
| Absent | 11 (32.4) | 50 (29.2) | |
| PNI | |||
| Present | 19 (55.9) | 87 (50.3) | 0.55 |
| Absent | 15 (44.1) | 86 (49.7) | |
| Biliary obstruction | |||
| Present | 16 (45.7) | 82 (47.4) | 0.86 |
| Absent | 19 854.3) | 91 (52.6) | |
| CA 19-9 U/mL | |||
| <150 | 30 (85.7) | 129 (81.6) | 0.57 |
| ≥150 (if bilirubin is high: 300) | 5 (14.3) | 29 (18.4) | |
| Albumi, g/dL | |||
| <3.5 | 7 (20) | 44 (27.3) | 0.37 |
| ≥3.5 | 28 (80) | 117 (72.7) | |
| Hemoglobin, g/dL | |||
| <12 for women; 13 for men | 15 (44.1) | 83 (50.6) | 0.49 |
| ≥3.5 | 19 (55.9) | 81 (49.4) | |
| Neutrophils, ×109/L | |||
| ≥7000 | 28 (84.8) | 137 (82.0) | 0.69 |
| <7000 | 5 (15.2) | 30 (18.0) | |
| Lymphocyte, ×109/L | |||
| <1500 | 8 (24.2) | 57 (34.3) | 0.26 |
| ≥1500 | 25 (75.8) | 109 (65.7) | |
| Platelets, ×109/L | |||
| <500 | 34 (97.1) | 156 (91.2) | 0.23 |
| ≥500 | 1 (2.9) | 15 (8.8) | |
| Radiotherapy | |||
| Received | 5 (14.3) | 76 (43.2) | 0.001 |
| None | 30 (85.7) | 100 (56.8) |
| Univariate Analysis, Mo | p Value | Multivariate Analysis | p Value | |
|---|---|---|---|---|
| (95%CI) | HR (95%CI) | |||
| Age, years | ||||
| (median; range) | ||||
| <65 | 31.0 (24.1–37.9) | 0.58 | ||
| ≥65 | 24.0 (8.8–39.2) | |||
| Gender | ||||
| Female | 31.0 (27.1–34.9) | 0.56 | ||
| Male | 23.0 (19.8–26.1) | |||
| Comorbidity | ||||
| Present | 24.0 (14.2–33.8) | 0.90 | ||
| Absent | 31.0 (22.5–39.4) | |||
| ECOG PS | ||||
| 0 | 33 (28.7–37.3) | 0.088 | 1 (ref) | 0.91 |
| 1 | 24 (15.1–32.9) | 0.9 (0.6–1.5) | ||
| 2 | 11.0 (8.2–13.7) | 1.1 (0.5–2.7) | ||
| BMI | ||||
| <18.5, underweight | 10.0 (3.0–24.0) | 0.82 | ||
| 18.5–24.9, normal | 31.0 (22.1–39.9) | |||
| 25–29.9, overweight | 24.0 (13.5–34.6) | |||
| ≥30, obese | 21.0 (16.1–25.6) | |||
| Smoking | 27.0 (16.9–37.0) | 0.72 | ||
| None | 31.0 (22.6–39.1) | |||
| Alcohol use | 30 (23.9–36.0) | 0.64 | ||
| None | 14 (3.0–31.47) | |||
| Histological subtype | ||||
| Pancreaticobiliary/mix | 27.0 (20.8–33.1) | 0.28 | ||
| Intestinal | 34.0 (26.8–41.2) | |||
| pT stage | - | |||
| T1 | 34.0 (29.3–38.7) | 0.059 | ||
| T2 | 37.0 (22.9–51.0) | |||
| T3 | 23.0 (19.0–26.6) | |||
| T4 | 10.0 (8.2–11.7) | |||
| pN stage | - | |||
| N0 | 43.0 (9.1–76.9) | <0.001 | ||
| N1 | 23.0 (18.4–27.6) | |||
| N2 | 14.0 (4.8–23.2) | |||
| Stage | ||||
| I (T1-2N0) | 72 (Non-reached) | 1 (ref) | ||
| II (T3N0) | 33.0 (28.3–37.7) | <0.001 | 1.1 (0.5–2.7) | 0.26 |
| IIIA (T 1-3N1) | 24.0 (17.2–30.8) | 1.5 (0.7–3.2) | ||
| IIIB (T4 or N2) | 13.0 (3.8–22.1) | 2.0 (0.9–4.7) | ||
| LNR | ||||
| 0 | 43.0 (15.5–70.5) | 0.007 | - | |
| <0.2 | 21.0 (14.6–27.3) | |||
| 0.2–0.4 | 33.0 (7.7–58.2) | |||
| ≥0.4 | 17.0 (9.0–24.9) | |||
| THLN | ||||
| ≥16 | 24.0 (15.4–32.5) | 0.49 | ||
| <16 | 30.0 (20.1–39.9) | |||
| Surgical margin | ||||
| R0 | 32.0 (25.1–38.8) | 0.001 | 2.1 (1.1–4.2) | 0.035 |
| R1 | 14.0 (6.4–21.6) | |||
| Pancreatic invasion | ||||
| Present | 19.0 (12.6–25.3) | <0.001 | 1.62 (0.91–2.8) | 0.073 |
| Absent | 51.0 (14.6–87.4) | |||
| LVI | ||||
| Present | 23.0 (17.5–28.4) | <0.001 | 2.1 (1.2–3.8) | 0.010 |
| Absent | NR | |||
| PNI | ||||
| Present | 21.0 (16.6–25.4) | 0.010 | 0.94 (0.58–1.50) | 0.80 |
| Absent | 34.0 (22.6–45.4) | |||
| Biliary obstruction | ||||
| Present | 23.0 (13.9–32.1) | 0.017 | ||
| Absent | 33.0 (18.8–47.1) | |||
| CA 19-9 U/mL | ||||
| <150 | 31.0 (23.9–38.0) | 0.003 | 1.70 (0.9–3.1) | 0.076 |
| ≥150 (if bilirubin is high: 300) | 13.0 (8.3–17.7) | |||
| Albumin, g/dL | ||||
| <3.5 | 24.0 (7.4–40.6) | 0.20 | ||
| ≥3.5 | 30.0 (24.4–35.5) | |||
| Hemoglobin, g/dL | ||||
| <12 for women; 13 for men | 24.0 (17.9–30.0) | 0.15 | ||
| ≥12–13 | 32.0 (15.4–48.6) | |||
| Neutrophils, ×109/L | ||||
| ≥7000 | 24.0 (16.9–31.1) | 0.15 | ||
| <7000 | 32.0 (22.9–41.0) | |||
| Lymphocyte, ×109/L | ||||
| <1500 | 27.0 (20.0–33.9) | 0.68 | ||
| ≥1500 | 31.0 (17.9–44.1) | |||
| Platelets, ×109/L | ||||
| <500 | 15.0 (2.0–31.6) | 0.27 | ||
| ≥500 | 30.0 (23.9–36.1) | |||
| Radiotherapy | ||||
| Received | 29.0 (21.5–36.5) | 0.45 | ||
| None | 31.0 (21.2–40.8) | |||
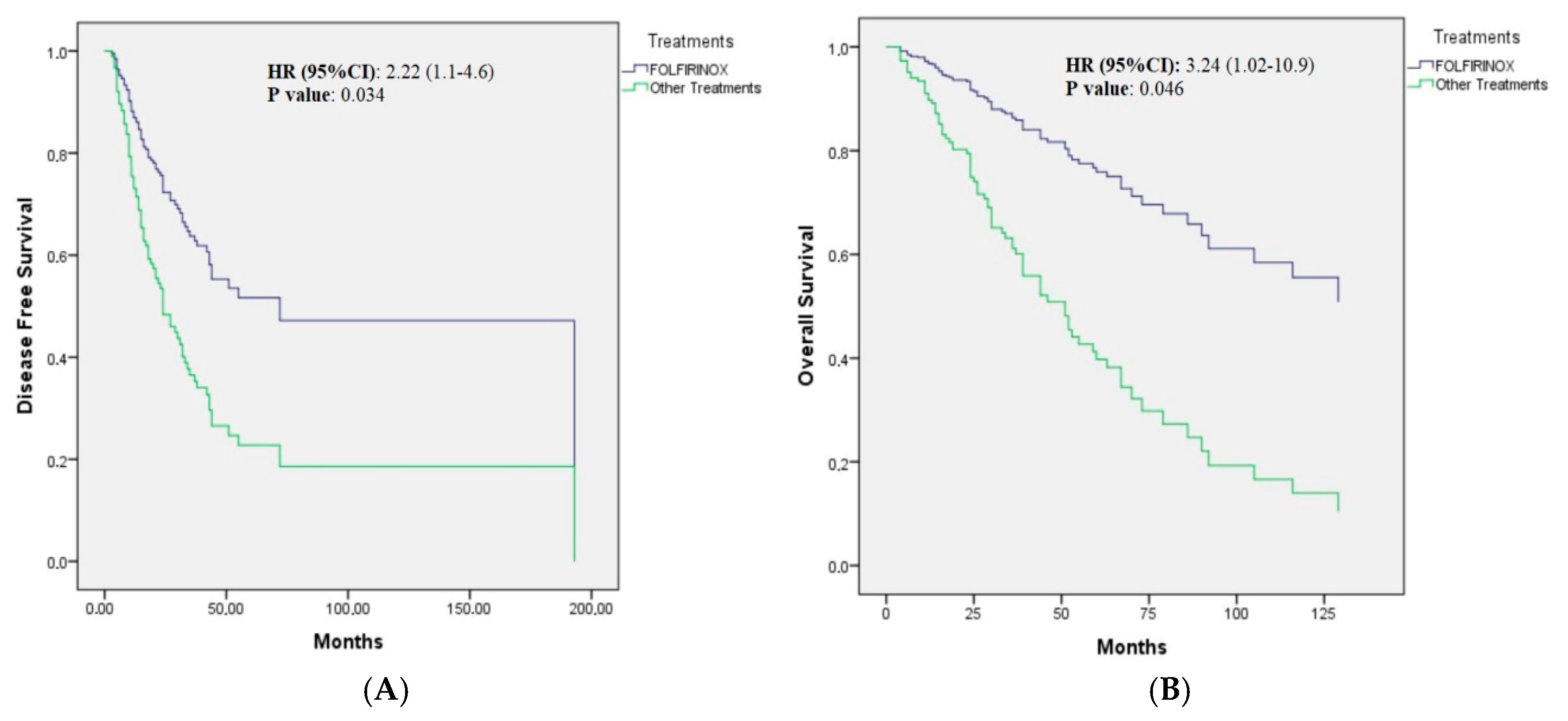
| mFOLFIRINOX | NR | 0.034 | 2.22 (1.1–4.6) | 0.034 |
| The other treatments | 27.0 (20.7–33.3) | |||
| mFOLFIRINOX | NR | 0.064 | ||
| FU-doublet | 16.0 (1.5–30.6) | |||
| Gemcitabine doublet | 24.0 (17.1–30.9) | |||
| Gemcitabine or 5-FU | 33.0 (19.2–46.7) | |||
| CRT | 34.0 (18.8–35.7) |
| Univariate Analysis, Mo | p Value | Multivariate Analysis | p Value | |
|---|---|---|---|---|
| (95%CI) | HR (95%CI) | |||
| Age, years | ||||
| (median; range) | ||||
| <65 | 59.0 (41.0–76.9) | 0.045 | 1.1 (0.63–1.85) | 0.76 |
| ≥65 | 44.0 (28.7–59.3) | |||
| Gender | ||||
| Female | 51.0 (34.7–67.3) | 0.98 | ||
| Male | 55.0 (35.9–74.0) | |||
| Comorbidity | ||||
| Present | 44.0 (30.7–57.3) | 0.007 | 1.57 (0.94–2.6) | 0.084 |
| Absent | 70.0 (31.2–108.8) | |||
| ECOG PS | - | |||
| 0 | 52.0 (24.9–79.1) | 0.009 | 0.90 | |
| 1 | 55.0 (36.0–73.9) | |||
| 2 | 11.0 (3.0–34.8) | |||
| BMI | ||||
| <18.5, underweight | 11.0 (3.0–33.2) | 0.226 | ||
| 18.5–24.9, normal | 51.0 (39.6–62.4) | |||
| 25–29.9, overweight | 90.0 (32.9–147.1) | |||
| ≥30, obese | 42.0 (29.3–54.7) | |||
| Smoking | 53.0 (36.8–69.2) | 0.93 | ||
| None | 51.0 (34.3–67.7) | |||
| Alcohol use | 52 (38.7–65.3) | 0.78 | ||
| None | 32 (3.0–109) | |||
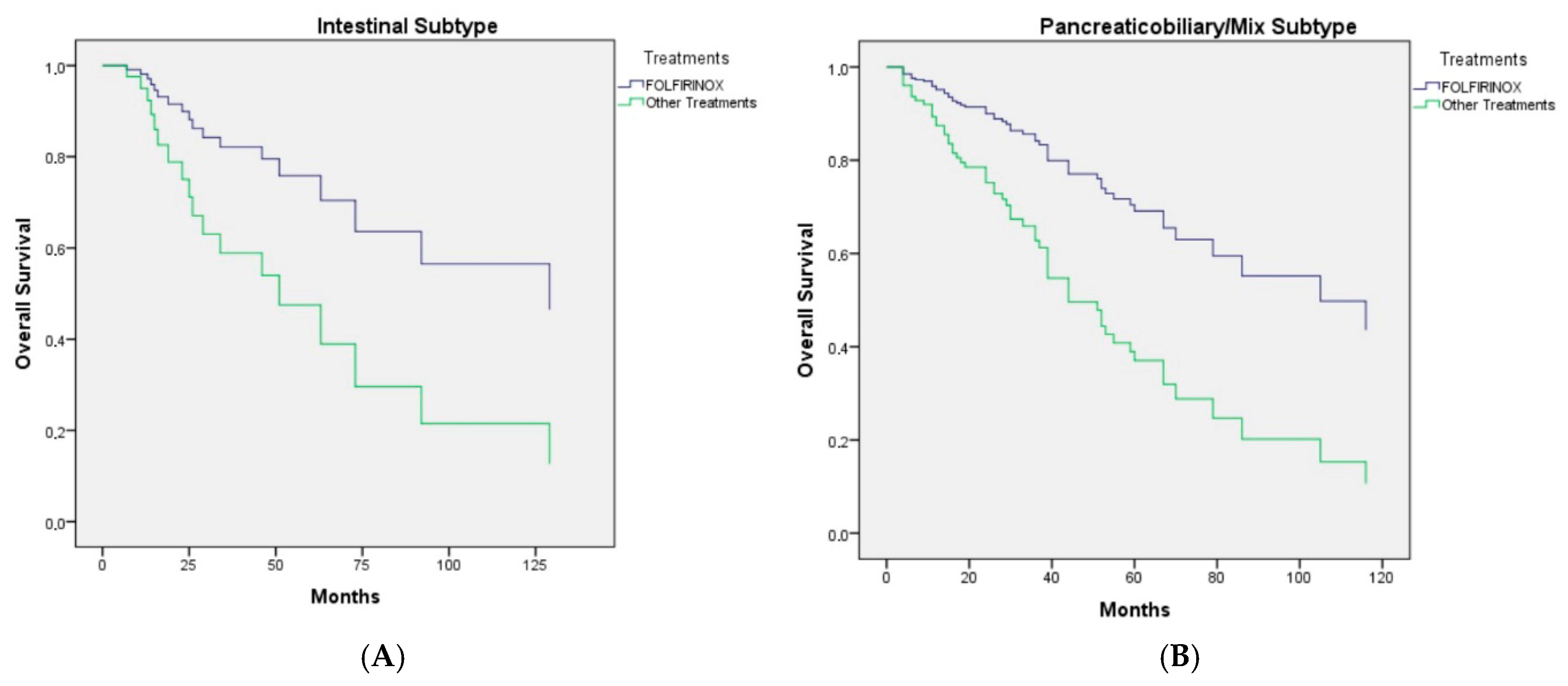
| Histological subtype | ||||
| Pancreaticobiliary/mix | 44.0 (34.0–53.9) | 0.56 | ||
| Intestinal | 63.0 (41.7–84.3) | |||
| Stage | - | |||
| I (T1-2N0) | 92 (59.2–124.7) | |||
| II (T3N0) | 44.0 (10.0–77.8) | 0.098 | 0.59 | |
| IIIA (T1-3N1) | 48.0 (33.4–61.6) | |||
| IIIB (T4 or N2) | 44.0 (25.0–56.8) | |||
| LNR | ||||
| 0 | 67.0 (52.4–81.6) | 0.42 | ||
| <0.2 | 44.0 (23.9–64.1) | |||
| 0.2–0.4 | 51.0 (43.9–58.1) | |||
| ≥0.4 | 36.0 (17.6–54.4) | |||
| THLN | ||||
| ≥16 | 51.0 (38.8–63.2) | 0.51 | ||
| <16 | 52.0 (31.4–72.6) | |||
| Surgical margin | ||||
| R0 | 55.0 (42.6–67.0) | 0.008 | 1.87 (0.79–4.3) | 0.15 |
| R1 | 30.0 (27.7–32.3) | |||
| Pancreatic invasion | ||||
| Present | 36.0 (25.1–46.8) | <0.001 | 1.61 (0.90–2.86) | 0.107 |
| Absent | 73.0 (46.1–99.9) | |||
| LVI | ||||
| Present | 44.0 (36.2–51.8) | 0.020 | 1.33 (0.8–2.6) | 0.23 |
| Absent | 92.0 (43.8–140.1) | |||
| PNI | ||||
| Present | 51.0 (37.1–64.9) | 0.016 | 1.38 (0.82–2.34) | 0.62 |
| Absent | 73.0 (42.3–103.6) | |||
| Biliary obstruction | ||||
| Present | 51.0 (37.6–64.4) | 0.61 | ||
| Absent | 63.0 (40.9–85.1) | |||
| CA 19-9 U/mL | ||||
| <150 | 55.0 (41.4–68.6) | 0.001 | 1.62 (0.83–3.2) | 0.11 |
| ≥150 (if bilirubin is high: 300) | 28.0 (20.8–35.1) | |||
| Radiotherapy | ||||
| Received | 52.0 (32.6–71.4) | 0.607 | ||
| None | 52.0 (40.3–63.7) | |||
| mFOLFIRINOX | NR | 0.071 | 3.24 (1.02–10.9) | 0.046 |
| The other treatments | 51.0 (41.6–60.4) |
| Adverse Event | FOLFIRINOX Grade 1–2 n (%) | FOLFIRINOX Grade 3–4 n (%) | Other Tx Grade 1–2 n (%) | Other Tx Grade 3–4 n (%) | p-Value |
|---|---|---|---|---|---|
| Neutropenia | 11 (31.4) | 7 (20.0) | 65 (36.9) | 25 (14.2) | 0.013 |
| Febrile neutropenia | - | 5 (14.7) | - | 6 (3.5) | 0.008 |
| Anemia | 19 (55.9) | 2 (5.9) | 92 (55.5) | 3 (1.8) | 0.164 |
| Thrombocytopenia | 10 (29.4) | 2 (5.8) | 51 (31.3) | 4 (2.4) | 0.234 |
| Fatigue | 26 (75.8) | 4 (10.3) | 109(62.1) | 4 (2.6) | 0.043 |
| Appetite loss | 21 (60.7) | 2 (7.1) | 93 (54.3) | 3 (2.0) | 0.30 |
| Diarrhea | 11 (32.2) | 0 | 27 (16.2) | 0 | 0.034 |
| Stomatitis | 9 (25.9) | 0 | 34 (20.2) | 0 | 0.69 |
| LFT abnormalities | 12 (34.3) | 0 | 36 (20.9) | 2 (1.3) | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalem, A.; Kus, T.; Sahin, T.K.; Dizdar, O.; Efil, S.C.; Sendur, M.A.N.; Aykut, T.; Araz, M.; Bolek, H.; Urun, Y.; et al. FOLFIRINOX: The Best Adjuvant Treatment for Ampullary Adenocarcinoma? A Multicenter Study by the Turkish Oncology Group (TOG). Cancers 2025, 17, 2730. https://doi.org/10.3390/cancers17172730
Kalem A, Kus T, Sahin TK, Dizdar O, Efil SC, Sendur MAN, Aykut T, Araz M, Bolek H, Urun Y, et al. FOLFIRINOX: The Best Adjuvant Treatment for Ampullary Adenocarcinoma? A Multicenter Study by the Turkish Oncology Group (TOG). Cancers. 2025; 17(17):2730. https://doi.org/10.3390/cancers17172730
Chicago/Turabian StyleKalem, Ali, Tulay Kus, Taha Koray Sahin, Omer Dizdar, Safa Can Efil, Mehmet Ali Nahit Sendur, Talat Aykut, Murat Araz, Hatice Bolek, Yuksel Urun, and et al. 2025. "FOLFIRINOX: The Best Adjuvant Treatment for Ampullary Adenocarcinoma? A Multicenter Study by the Turkish Oncology Group (TOG)" Cancers 17, no. 17: 2730. https://doi.org/10.3390/cancers17172730
APA StyleKalem, A., Kus, T., Sahin, T. K., Dizdar, O., Efil, S. C., Sendur, M. A. N., Aykut, T., Araz, M., Bolek, H., Urun, Y., Sever, N., Bayoglu, I. V., Cavdar, E., Sagıroglu, M. F., Gunes, T. K., Ozcelik, M., Demirel, N., Yıldız, B., Karabuga, B., ... Yalcın, S. (2025). FOLFIRINOX: The Best Adjuvant Treatment for Ampullary Adenocarcinoma? A Multicenter Study by the Turkish Oncology Group (TOG). Cancers, 17(17), 2730. https://doi.org/10.3390/cancers17172730






