Anticancer Effect of Nature-Inspired Indolizine-Based Pentathiepines in 2D and 3D Cellular Model
Simple Summary
Abstract
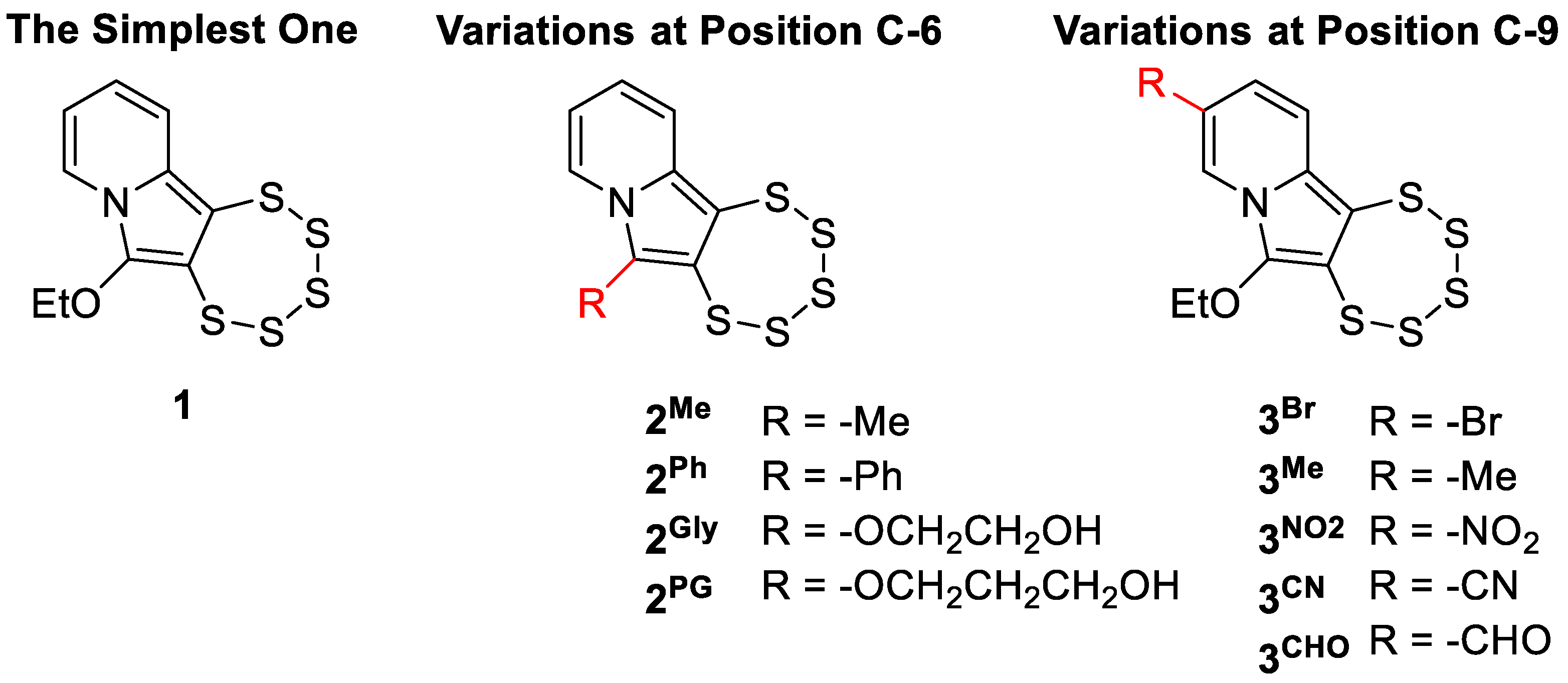
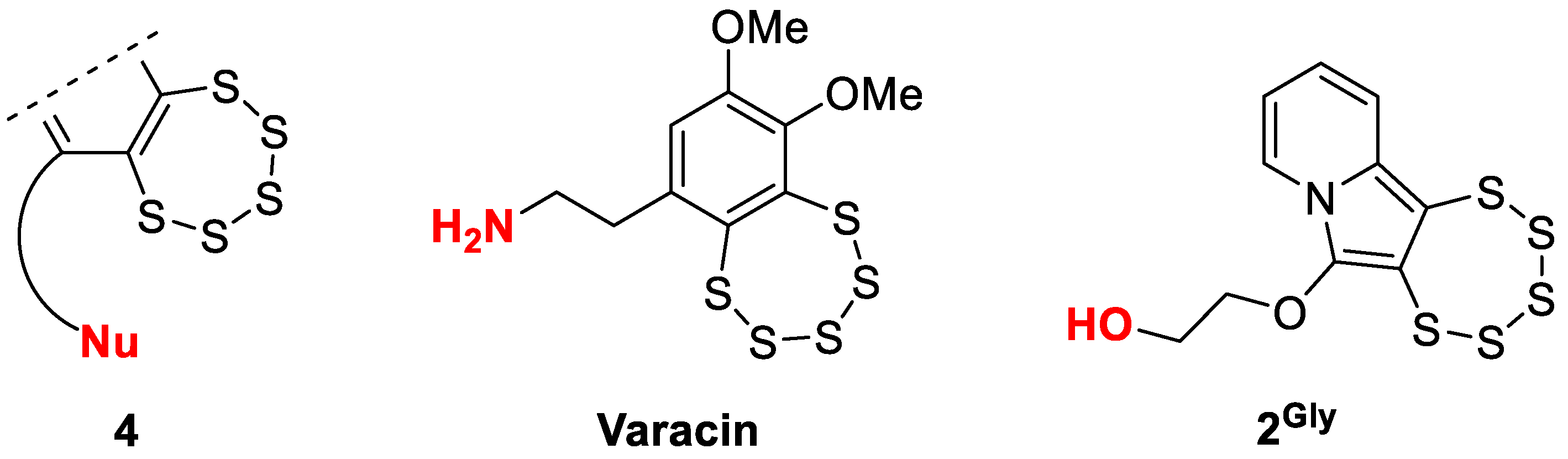
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Pentathiepine Synthesis and Characterization
2.2.1. General Experimental Procedures
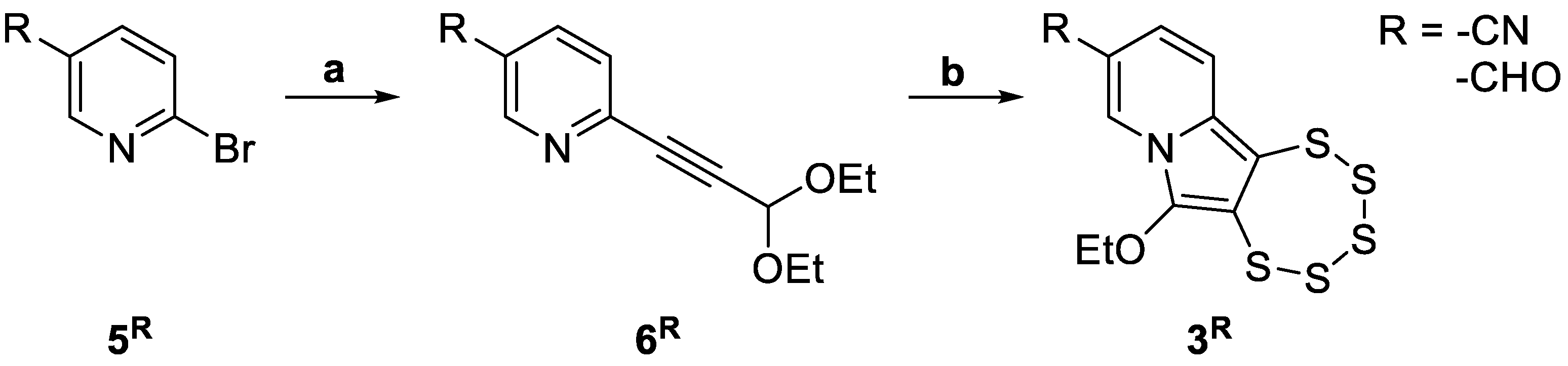
2.2.2. Sonogashira Cross-Coupling Reactions
2.2.3. Pentathiepine-Pyrrole Double Ring Closing Reactions
2.2.4. Synthesis and Solid-State Characterization of Dansyl Azide
2.2.5. Single-Crystal X-Ray Diffraction
2.3. In Vitro Biological Studies
2.3.1. Cell Lines and Culture Conditions
2.3.2. Cell Viability Assay
2.3.3. Flow Cytometric Analysis
2.3.4. Cleavage Assay
2.3.5. Scratch Wound Healing Assay
2.3.6. Western Blot Analysis
2.3.7. Statistical Analysis
3. Results
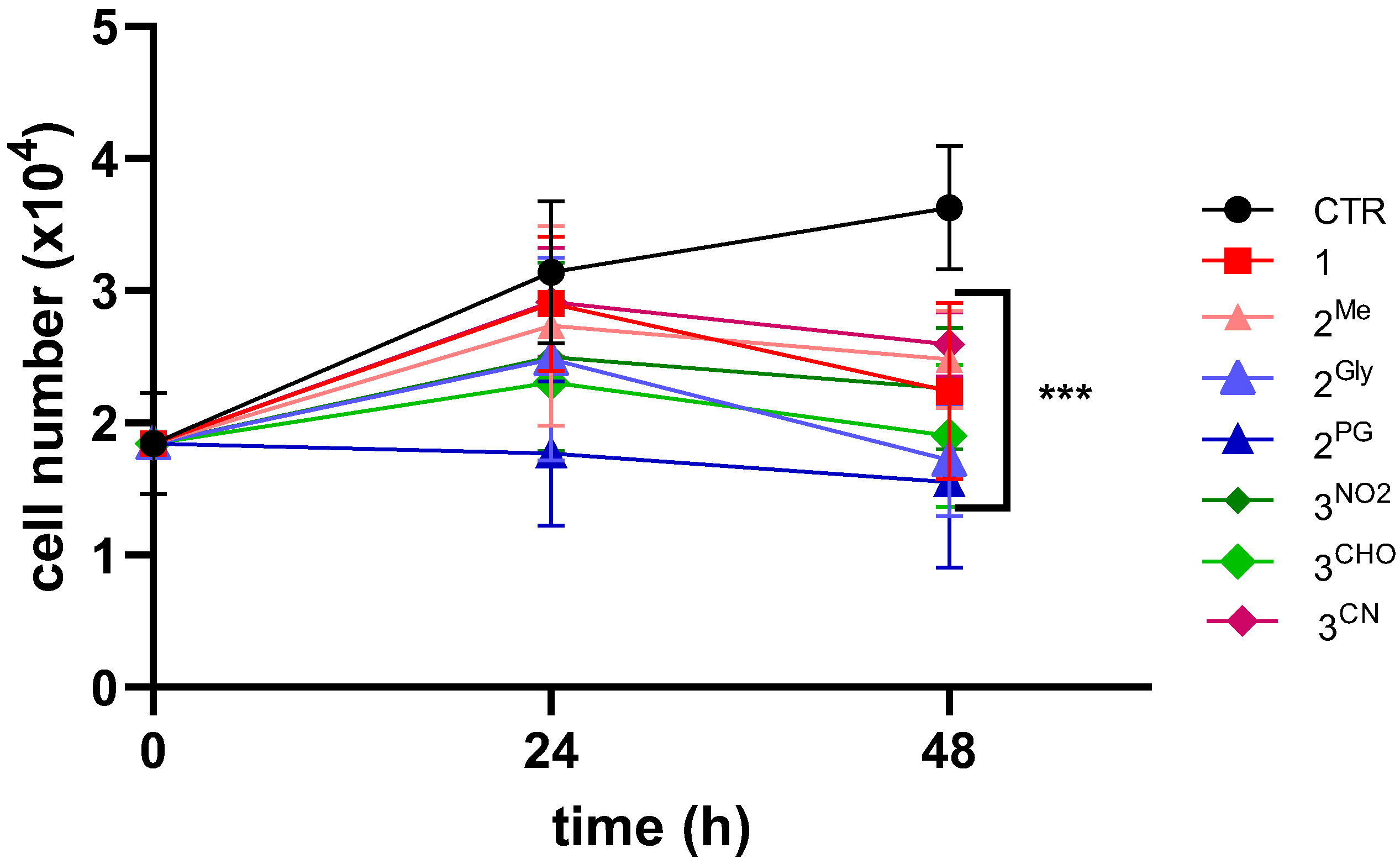
3.1. Effects on Cell Viability
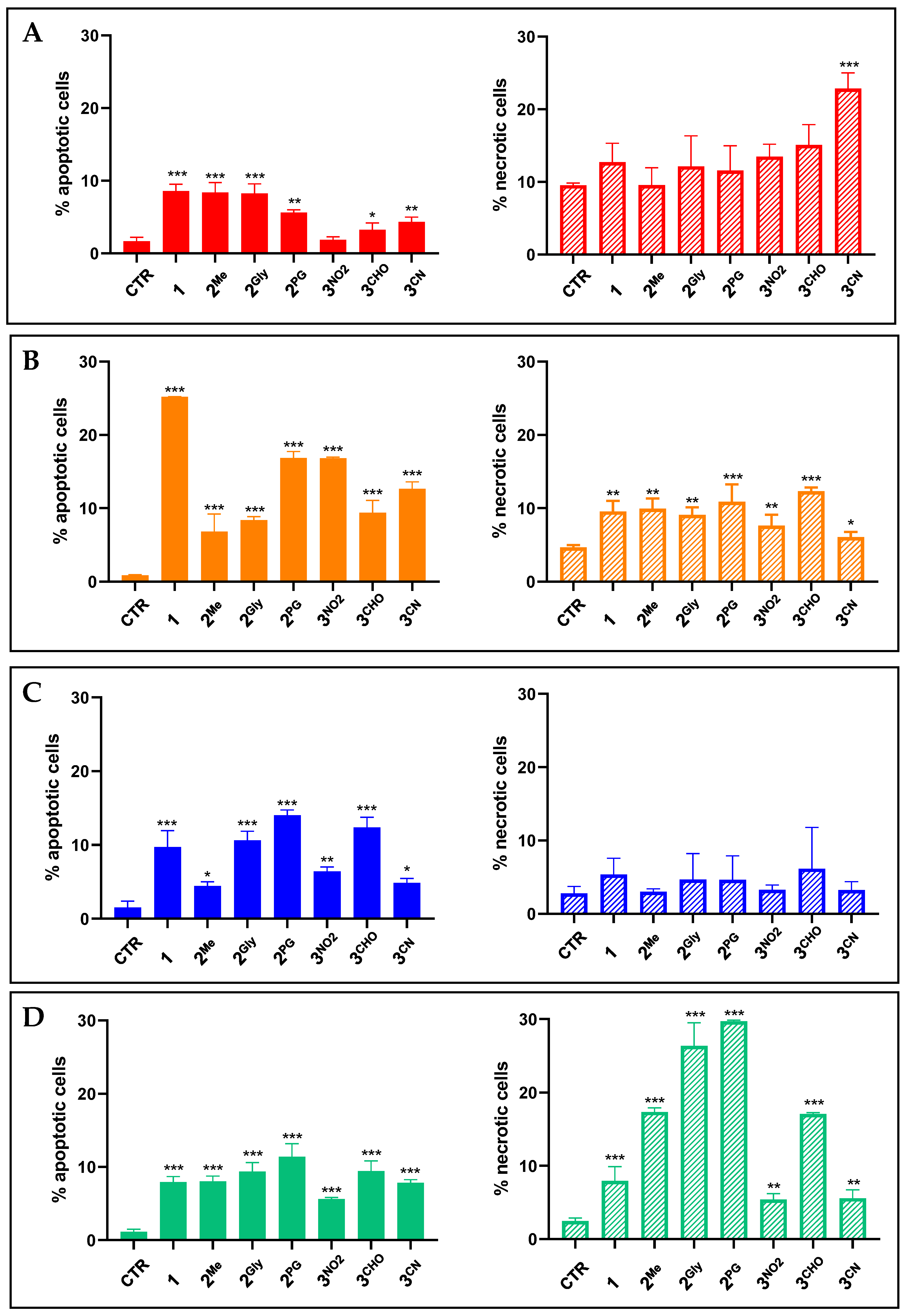
3.2. Cell Death Induction
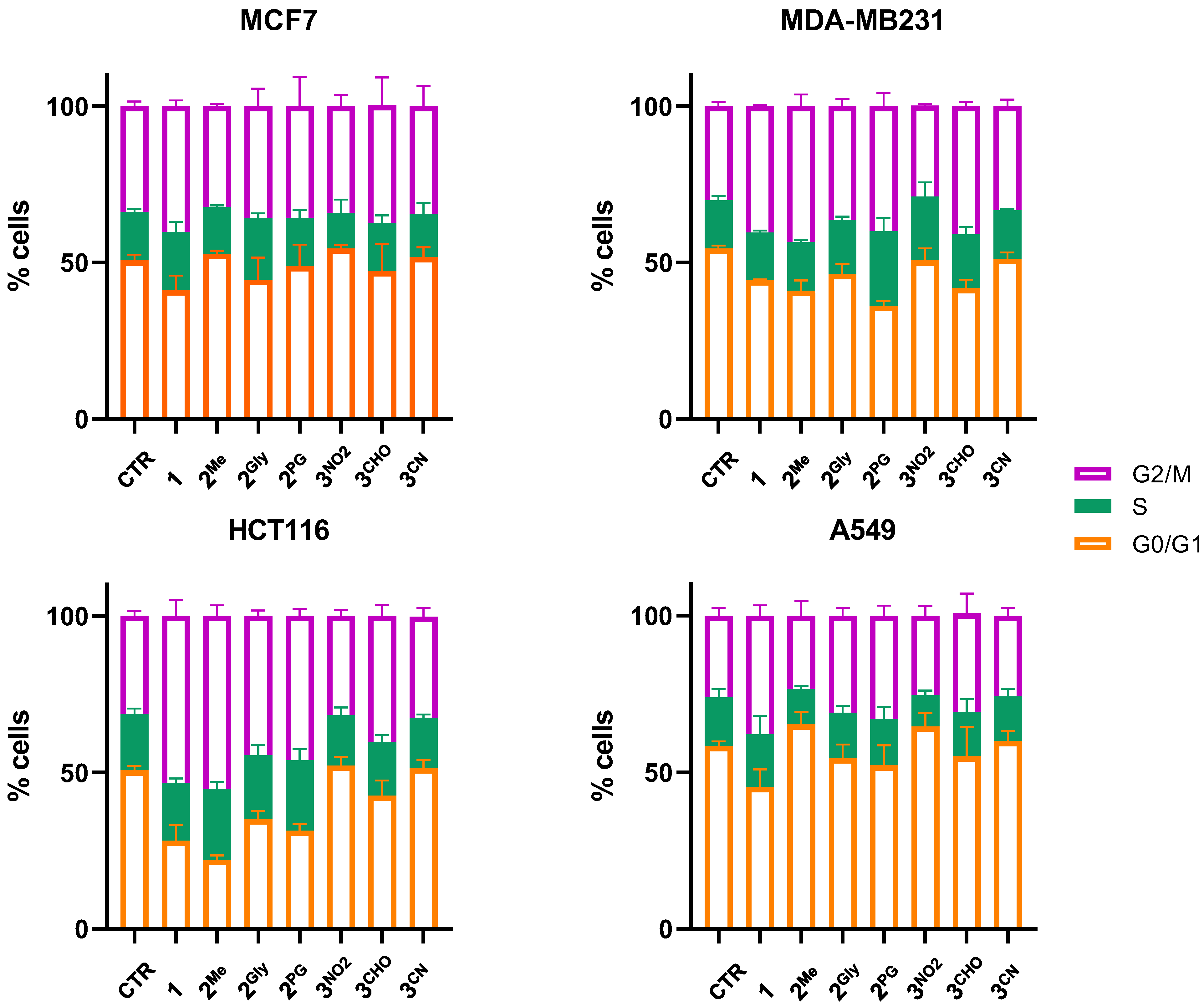
3.3. Cell Cycle Alterations
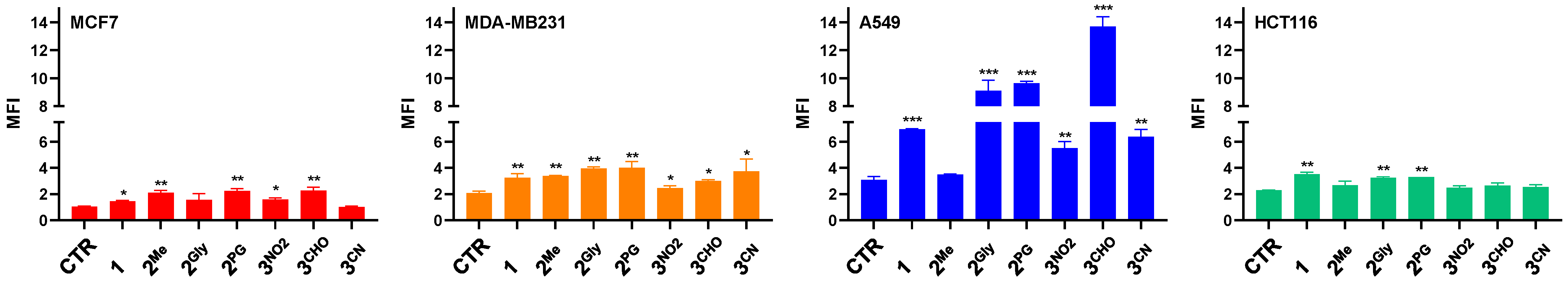
3.4. ROS Production
3.5. Hydrosulfide Production
3.6. DNA Cleavage
3.7. Cell Migration and Matrix Metalloproteinase Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Muinao, T.; Boruah, H.P.D.; Mahindroo, N. Current Advances in Prognostic and Diagnostic Biomarkers for Solid Cancers: Detection Techniques and Future Challenges. Biomed. Pharmacother. 2022, 146, 112488. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.M.; Tallarita, R.; Bandaru, S.S.M.; Schulzke, C. Synthesis of Pharmacologically Significant Pentathiepins: A Journey from Harsh to Mild Conditions. In Non-Conventional Synthesis: Bioactive Heterocycles; De Gruyter Brill: Berlin, Germany, 2023. [Google Scholar]
- Pozzetti, L.; Asquith, C.R.M. Pentathiepins Are an Understudied Molecular Prism of Biological Activities. Arch. Pharm. 2024, 357, e2400646. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.S.; Molinski, T.F.; Barrows, L.R.; Ireland, C.M. Varacin: A Novel Benzopentathiepin from Lissoclinum vareau That Is Cytotoxic Toward a Human Colon Tumor. J. Am. Chem. Soc. 1991, 113, 4709–4710. [Google Scholar] [CrossRef]
- Zhou, J.; Li, W.L.; Wang, Z.X.; Chen, N.Y.; Tang, Y.; Hu, X.X.; Deng, J.H.; Lu, Y.; Lu, G. Dong varacin-1, a Novel Analog of Varacin C, Induces P53-Independent Apoptosis in Cancer Cells through ROS-Mediated Reduction of XIAP. Acta Pharmacol. Sin. 2019, 40, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, L.S.; Oleg, A. Rakitin Pentathiepins. Chem. Rev. 2004, 104, 2617–2630. [Google Scholar] [CrossRef] [PubMed]
- Wolff, L.; Bandaru, S.S.M.; Eger, E.; Lam, H.N.; Napierkowski, M.; Baecker, D.; Schulzke, C.; Bednarski, P.J. Comprehensive Evaluation of Biological Effects of Pentathiepins on Various Human Cancer Cell Lines and Insights into Their Mode of Action. Int. J. Mol. Sci. 2021, 22, 7631. [Google Scholar] [CrossRef] [PubMed]
- Baguley, T.D.; Nairn, A.C.; Lombroso, P.J.; Ellman, J.A. Synthesis of Benzopentathiepin Analogs and Their Evaluation as Inhibitors of the Phosphatase STEP. Bioorg. Med. Chem. Lett. 2015, 25, 1044–1046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Kurup, P.; Xu, J.; Carty, N.; Fernandez, S.M.; Nygaard, H.B.; Pittenger, C.; Greengard, P.; Strittmatter, S.M.; Nairn, A.C.; et al. Genetic Reduction of Striatal-Enriched Tyrosine Phosphatase (STEP) Reverses Cognitive Andcellular Deficits in an Alzheimer’s Disease Mouse Model. Proc. Natl. Acad. Sci. USA 2010, 107, 19014–19019. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chatterjee, M.; Baguley, T.D.; Brouillette, J.; Kurup, P.; Ghosh, D.; Kanyo, J.; Zhang, Y.; Seyb, K.; Ononenyi, C.; et al. Inhibitor of the Tyrosine Phosphatase Step Reverses Cognitive Deficits in a Mouse Model of Alzheimer’s Disease. PLoS Biol. 2014, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ghosh, A.C.; Schulzke, C. The Unexpected and Facile Molybdenum Mediated Formation of Tri- and Tetracyclic Pentathiepins from Pyrazine-Alkynes and Sulfur. Chem. Commun. 2013, 49, 4343–4345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Behnisch-Cornwell, S.; Bandaru, S.S.M.; Napierkowski, M.; Wolff, L.; Zubair, M.; Urbainsky, C.; Lillig, C.; Schulzke, C.; Bednarski, P.J. Pentathiepins: A Novel Class of Glutathione Peroxidase 1 Inhibitors That Induce Oxidative Stress, Loss of Mitochondrial Membrane Potential and Apoptosis in Human Cancer Cells. ChemMedChem 2020, 15, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Napierkowski, M.; Schöne, T.; Bandaru, S.S.M.; Judernatz, J.; Schulig, L.; Schmidt, L.; Schulzke, C.; Bednarski, P.J. Structure-Activity-Relationships of the Stability of Six Pentathiepins Towards Glutathione: Possible Correlations with Biological Activities. ChemMedChem 2025, 20, e202400727. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Roh, J.L.; Lee, S.M.; Park, Y.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Overexpression of Glutathione Peroxidase 1 Predicts Poor Prognosis in Oral Squamous Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, E.; Jablonowski, Z.; Tomasik, B.; Gromadzinska, J.; Jablonska, E.; Konecki, T.; Fendler, W.; Sosnowski, M.; Wasowicz, W.; Reszka, E. Different Gene Expression and Activity Pattern of Antioxidant Enzymes in Bladder Cancer. Anticancer. Res. 2017, 37, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.J.; Mitros, F.A.; Oberley, L.W. Expression of Antioxidant Enzymes in Diseases of the Human Pancreas: Another Link between Chronic Pancreatitis and Pancreatic Cancer. Pancreas 2003, 26, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Shen, Z.; Wu, D.; Xie, X.; Xu, X.; Lv, L.; Dai, H.; Chen, J.; Gan, X. Glutathione Peroxidase 1 Promotes NSCLC Resistance to Cisplatin via ROS-Induced Activation of PI3K/AKT Pathway. BioMed Res. Int. 2019, 2019, 7640547. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The Role of Glutathione Peroxidase-1 in Health and Disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Qiu, H.; Xu, J.; Mo, J.; Liu, Y.; Gui, Y.; Huang, G.; Zhang, S.; Yao, H.; Huang, X.; et al. Expression and Prognostic Potential of GPX1 in Human Cancers Based on Data Mining. Ann. Transl. Med. 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Emmrich, T.; Lemmerhirt, H.; Leffler, U.; Sydow, K.; Hirt, C.; Kiefer, T.; Link, A.; Bednarski, P.J. Identification of a Glutathione Peroxidase Inhibitor That Reverses Resistance to Anticancer Drugs in Human B-Cell Lymphoma Cell Lines. Bioorg. Med. Chem. Lett. 2012, 22, 6712–6715. [Google Scholar] [CrossRef] [PubMed]
- Napierkowski, M.; Janke, U.; Rong, A.; Delcea, M.; Bandaru, S.S.M.; Schulzke, C.; Bednarski, P.J. Liposomal Formulation of Model Pentathiepin Improves Solubility and Stability toward Glutathione While Preserving Anticancer Activity. Arch. Pharm. 2023, 356, e2300087. [Google Scholar] [CrossRef] [PubMed]
- Tallarita, R.; Jacobsen, L.M.; Elvers, B.J.; Richter, S.; Bandaru, S.S.M.; Correia, J.V.; Schulzke, C. Synthesis of Seven Indolizine-Derived Pentathiepines: Strong Electronic Structure Response to Nitro Substitution in Position C-9. Molecules 2024, 29, 216. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew. Chem. 2011, 123, 9846–9849. [Google Scholar] [CrossRef]
- Tallarita, R.; Jacobsen, L.M.; Bandaru, S.S.M.; Elvers, B.J.; Schulzke, C. The Role of –OEt Substituents in Molybdenum-Assisted Pentathiepine Formation—Access to Diversely Functionalized Azines. Molecules 2024, 29, 3806. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A. Infrared and Raman Characteristic Group Frequencies. Tables and Charts George Socrates John Wiley and Sons, Ltd., Chichester, Third Edition, 2001. Price £135. J. Raman Spectrosc. 2004, 35, 905. [Google Scholar] [CrossRef]
- Cantor, S.E. Nonmigrating Antioxidants via Sulfonyl Azide Intermediates. In Stabilization and Degradation of Polymers; American Chemical Society: Washington, DC, USA, 1978; Volume 169, pp. 253–260. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Alexeyenko, A.; Alkasalias, T.; Pavlova, T.; Szekely, L.; Kashuba, V.; Rundqvist, H.; Wiklund, P.; Egevad, L.; Csermely, P.; Korcsmaros, T.; et al. Confrontation of Fibroblasts with Cancer Cells In Vitro: Gene Network Analysis of Transcriptome Changes and Differential Capacity to Inhibit Tumor Growth. J. Exp. Clin. Cancer Res. 2015, 34, 62. [Google Scholar] [CrossRef] [PubMed]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In Vitro Wound Healing Assays—State of the Art. In BioNanoMaterials; De Gruyter Brill: Berlin, Germany, 2016; Volume 17. [Google Scholar]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef] [PubMed]
- Gampe, C.; Verma, V.A. Curse or Cure? A Perspective on the Developability of Aldehydes as Active Pharmaceutical Ingredients. J. Med. Chem. 2020, 63, 14357–14381. [Google Scholar] [CrossRef] [PubMed]
- Brzostowska, E.M.; Greer, A. The Role of Amine in the Mechanism of Pentathiepin (Polysulfur) Antitumor Agents. J. Am. Chem. Soc. 2003, 125, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Ohyama, T.; Ogawa, S. Efficient Synthesis and Biological Properties of New Benzopentathiepins. Heterocycles 1995, 41, 893. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic in Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.Q.X.; Lim, H.H.; Surana, U. DNA Damage Checkpoint Execution and the Rules of Its Disengagement. Front. Cell Dev. Biol. 2022, 10, 1020643. [Google Scholar] [CrossRef] [PubMed]
- Barnum, K.J.; O’Connell, M.J. Cell Cycle Regulation by Checkpoints. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; pp. 29–40. [Google Scholar] [CrossRef]
- Mueller, E.G. Trafficking in Persulfides: Delivering Sulfur in Biosynthetic Pathways. Nat. Chem. Biol. 2006, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Sawa, T.; Motohashi, H.; Akaike, T. Persulfide Synthases That Are Functionally Coupled with Translation Mediate Sulfur Respiration in Mammalian Cells. Br. J. Pharmacol. 2019, 176, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive Cysteine Persulfides and S-Polythiolation Regulate Oxidative Stress and Redox Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-TRNA Synthetase Governs Cysteine Polysulfidation and Mitochondrial Bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Asquith, C.R.M.; Laitinen, T.; Konstantinova, L.S.; Tizzard, G.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R.; Hilton, S.T. Investigation of the Pentathiepin Functionality as an Inhibitor of Feline Immunodeficiency Virus (FIV) via a Potential Zinc Ejection Mechanism, as a Model for HIV Infection. ChemMedChem 2019, 14, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Moos, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of Class Names of Organic Compounds and Reactivity Intermediates Based on Structure. Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Lindahl, S.; Xian, M. Recent Development of Polysulfides: Chemistry and Biological Applications. Curr. Opin. Chem. Biol. 2023, 75, 102325. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Yao, W.; Shi, D.; Shao, X.; Lu, Z.; Chai, Y.; Song, J.; Tang, W.; Wang, X. Mechanism Insights and Therapeutic Intervention of Tumor Metastasis: Latest Developments and Perspectives. Signal Transduct. Target. Ther. 2024, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-Cell Invasion and Migration: Diversity and Escape Mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Jiang, J.; Chen, B.J.; Wang, K.; Tang, Y.L.; Liang, X. Hua plasticity of Cancer Cell Invasion: Patterns and Mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef] [PubMed]
- Ashja Ardalan, A.; Khalili-Tanha, G.; Shoari, A. Shaping the Landscape of Lung Cancer: The Role and Therapeutic Potential of Matrix Metalloproteinases. Int. J. Transl. Med. 2024, 4, 661–679. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, M.; Zielińska, W.; Krajewski, A.; Hałas-Wiśniewska, M.; Mikołajczyk, K.; Gagat, M.; Grzanka, A. Downregulation of Mmp-9 Enhances the Anti-Migratory Effect of Cyclophosphamide in Mda-Mb-231 and Mcf-7 Breast Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 12783. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Motherwell, W.S.; Purkis, L.H.; Smith, B.R.; Taylor, R.; Cooper, R.I.; Harris, S.E.; et al. Retrieval of crystallographically-derived molecular geometry information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
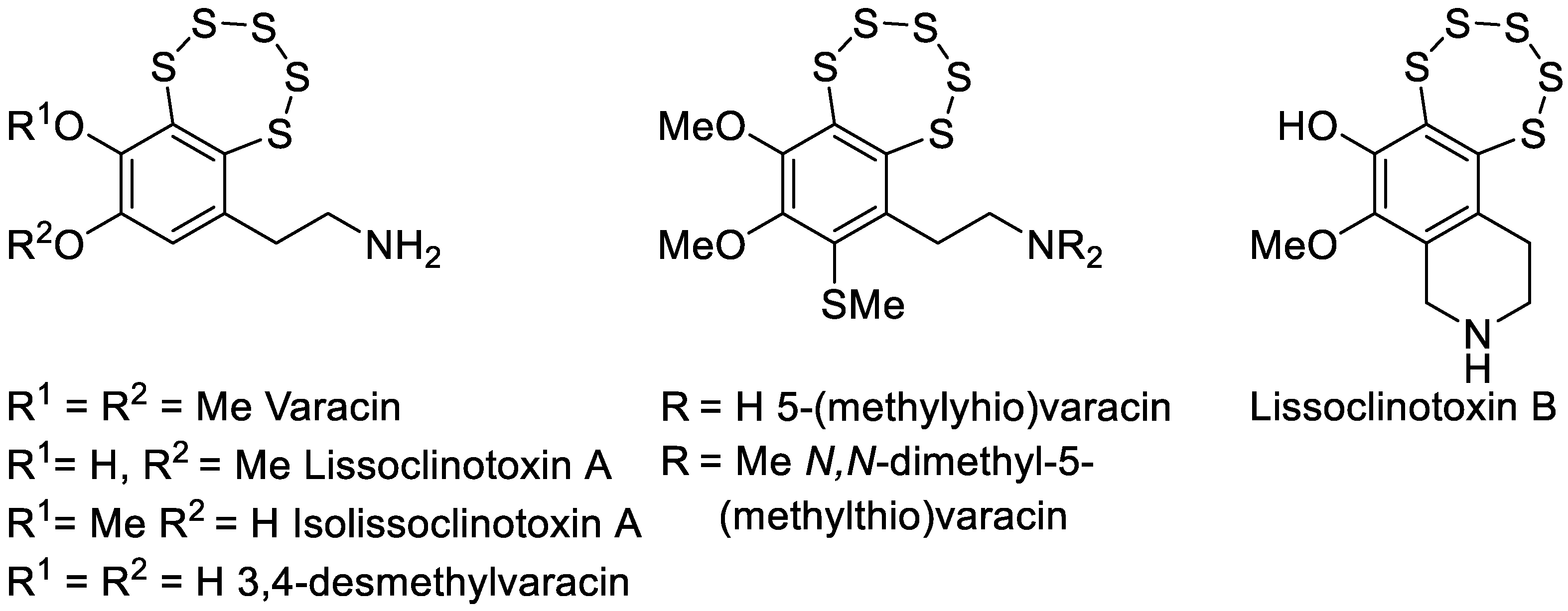


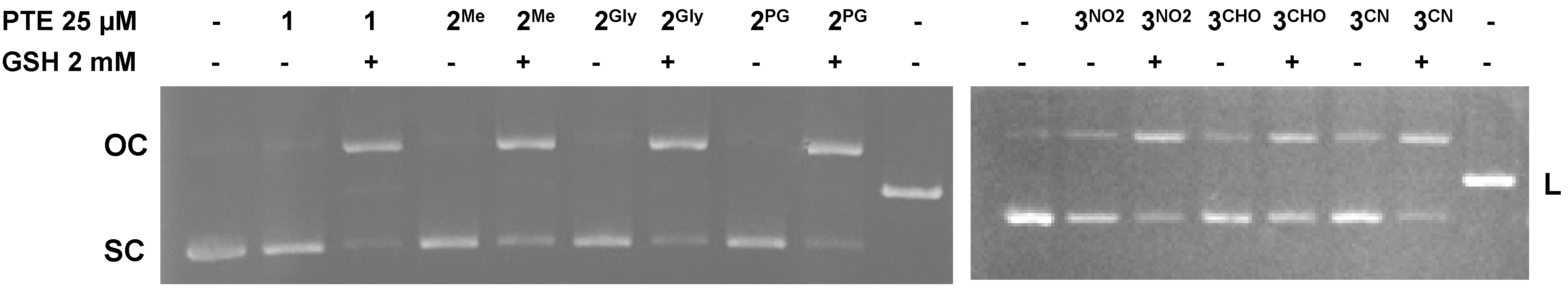
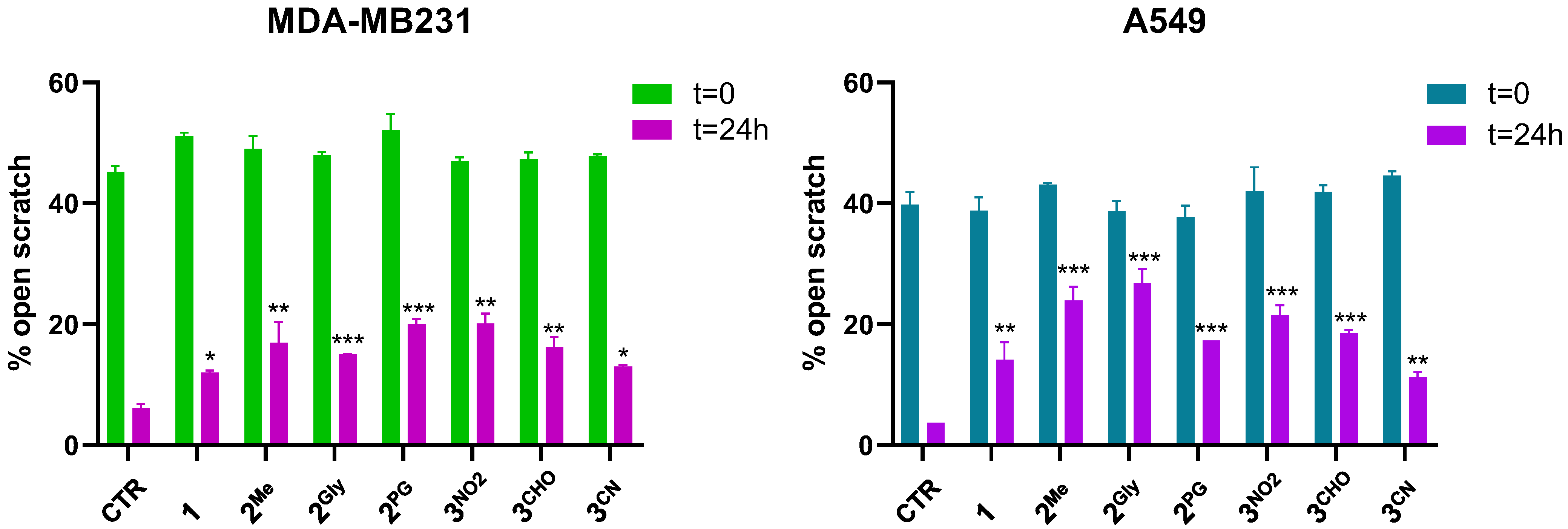
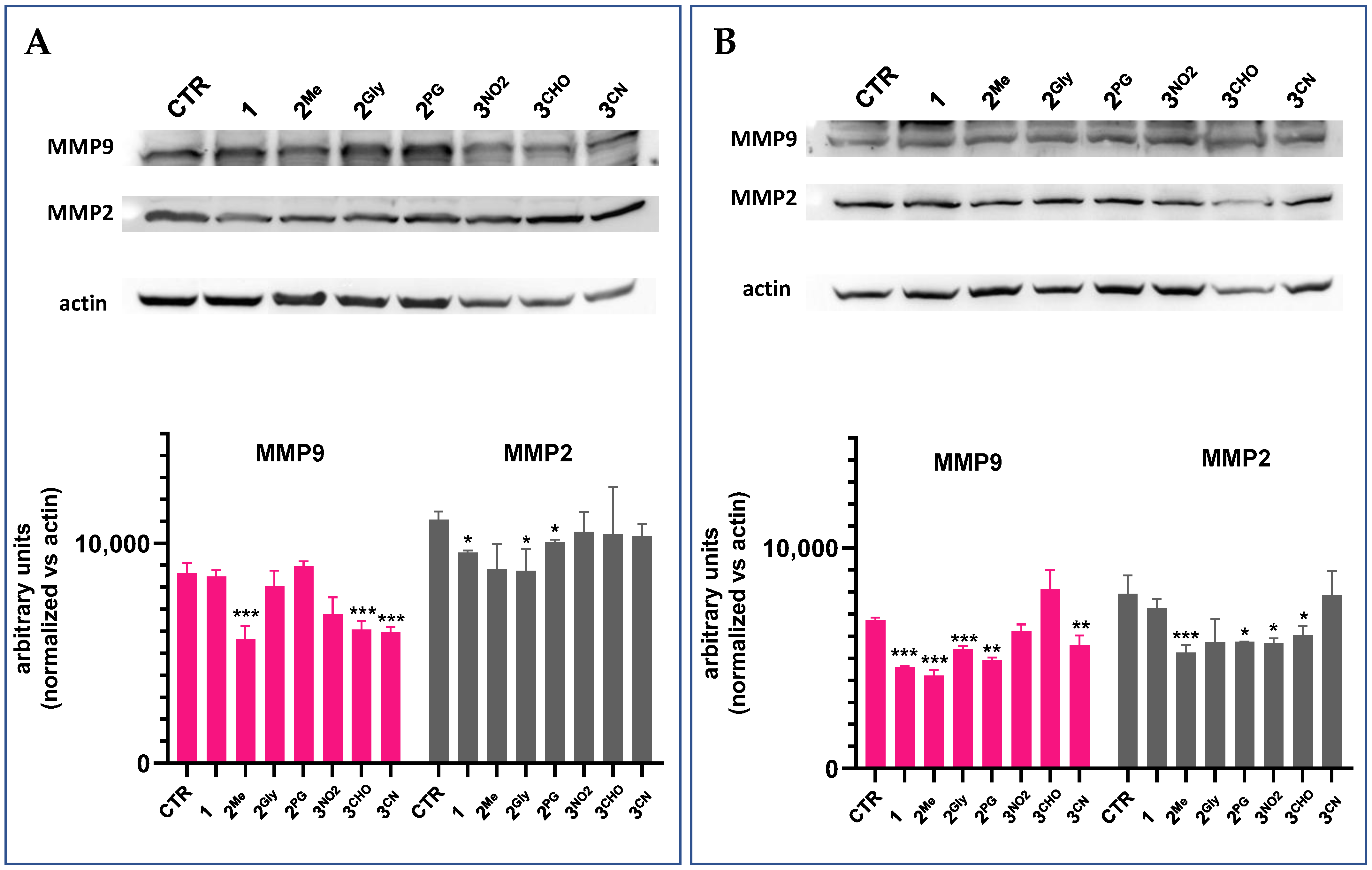
| IC50 (µM) | MCF7 | MDA-MB231 | A549 | HCT116 | CR9 |
|---|---|---|---|---|---|
| 1 | 2.68 ± 0.31 | 0.63 ± 0.04 | 1.537 ± 0.08 | 0.83 ± 0.04 | 7.32 ± 0.15 # |
| 2Me | 4.77 ± 0.14 | 1.45 ± 0.04 | 2.54 ± 0.27 | 2.27 ± 0.34 | 7.91 ± 0.32 # |
| 2Gly | 1.13 ± 0.02 | 0.30 ± 0.03 | 0.79 ± 0.06 | 0.603 ± 0.04 | 1.61 ± 0.13 @ |
| 2Ph | 14.93 ± 1.74 *** | 4.41 ± 0.22 *** | 12.22 ± 0.66 *** | 4.79 ± 0.45 *** | 26.88 ± 0.78 # |
| 2PG | 0.90 ± 0.07 | 0.35 ± 0.06 | 0.72 ± 0.08 | 0.50 ± 0.01 | 2.04 ± 0.12 # |
| 3Me | 13.66 ± 1.51 *** | 2.80 ± 0.12 *** | 4.50 ± 0.69 *** | 3.28 ± 0.22 *** | 75.92 ± 0.16 # |
| 3Br | 8.35 ± 0.35 *** | 3.62 ± 0.21 *** | 6.42 ± 0.31 *** | 5.83 ± 0.07 *** | 10.9 ± 0.89 @ |
| 3NO2 | 3.21 ± 0.13 | 0.63 ± 0.75 | 3.22 ± 0.39 | 1.59 ± 0.15 | 2.87 ± 0.14 |
| 3CHO | 1.70 ± 0.03 | 0.39 ± 0.04 | 1.03 ± 0.04 | 0.74 ± 0.06 | 1.9 ± 0.12 @ |
| doxo | 4.5 ± 0.51 | 17.8 ± 1.96 | 1.5 ± 0.21 | 3.5 ± 0.60 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallarita, R.; Randisi, F.; Jacobsen, L.M.; Marras, E.; Riva, M.; Modoni, G.; Fimmen, J.; Bandaru, S.S.M.; Schulzke, C.; Gariboldi, M.B. Anticancer Effect of Nature-Inspired Indolizine-Based Pentathiepines in 2D and 3D Cellular Model. Cancers 2025, 17, 2393. https://doi.org/10.3390/cancers17142393
Tallarita R, Randisi F, Jacobsen LM, Marras E, Riva M, Modoni G, Fimmen J, Bandaru SSM, Schulzke C, Gariboldi MB. Anticancer Effect of Nature-Inspired Indolizine-Based Pentathiepines in 2D and 3D Cellular Model. Cancers. 2025; 17(14):2393. https://doi.org/10.3390/cancers17142393
Chicago/Turabian StyleTallarita, Roberto, Federica Randisi, Lukas Manuel Jacobsen, Emanuela Marras, Mattia Riva, Giulia Modoni, Johannes Fimmen, Siva Sankar Murthy Bandaru, Carola Schulzke, and Marzia Bruna Gariboldi. 2025. "Anticancer Effect of Nature-Inspired Indolizine-Based Pentathiepines in 2D and 3D Cellular Model" Cancers 17, no. 14: 2393. https://doi.org/10.3390/cancers17142393
APA StyleTallarita, R., Randisi, F., Jacobsen, L. M., Marras, E., Riva, M., Modoni, G., Fimmen, J., Bandaru, S. S. M., Schulzke, C., & Gariboldi, M. B. (2025). Anticancer Effect of Nature-Inspired Indolizine-Based Pentathiepines in 2D and 3D Cellular Model. Cancers, 17(14), 2393. https://doi.org/10.3390/cancers17142393







