Comprehensive Longitudinal Linear Mixed Modeling of CTCs Illuminates the Role of Trop2, EpCAM, and CD45 in CTC Clustering and Metastasis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. RareCyte® Sample Processing, Scanning, and Analysis
2.3. Confocal Imaging
2.4. Endpoints and Assessments
2.5. Statistical Analyses
3. Results
3.1. RareCyte Reveals Expression of Trop2 in Breast Cancer Patient CTCs and High Inter-Marker Correlation
3.2. No Individual CTC Biomarker Condition Was Significantly Associated with Receptor Status
3.3. Longitudinal Analysis Reveals Differences in CTC Clustering Between HR+ and HER2+ Cancers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTC | Circulating Tumor Cell |
| cCTC | Classical Circulating Tumor Cell |
| T2CTC | Trop2-expressing Circulating Tumor Cell |
| mBC | Metastatic Breast Cancer |
| LMM | Linear Mixed Effects Model |
| TCCP | Total Cancer Care Protocol |
| ORIEN | Oncology Research Information Exchange Network |
| CK | Cytokeratin |
| EpCAM | Epithelial Cell Adhesion Molecule |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| EGFR | Epidermal Growth Factor Receptor |
| CNS | Central Nervous System |
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global Burden and Trends in Premenopausal and Postmenopausal Breast Cancer: A Population-Based Study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Tang, Q.; Li, H.; Zhao, X.T.; Li, Z.Y.; Ma, C.X.; Zhou, S.Q.; Chen, D.D. Opportunities and Challenges in the Development of Antibody-Drug Conjugate for Triple-Negative Breast Cancer: The Diverse Choices and Changing Needs. World J. Oncol. 2024, 15, 527–542. [Google Scholar] [CrossRef]
- Garrigos, L.; Camacho, D.; Perez-Garcia, J.M.; Llombart-Cussac, A.; Cortes, J.; Antonarelli, G. Sacituzumab Govitecan for Hormone Receptor-Positive HER2-Negative Advanced Breast Cancer. Expert Rev. Anticancer Ther. 2024, 24, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Tellez-Gabriel, M.; Cartron, P.-F.; Vallette, F.M.; Heymann, M.-F.; Heymann, D. Characterization of Circulating Tumor Cells as a Reflection of the Tumor Heterogeneity: Myth or Reality? Drug Discov. Today 2019, 24, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Bardia, A.; Miyamoto, D.T.; Donaldson, M.C.; Wittner, B.S.; Spencer, J.A.; Yu, M.; Pely, A.; Engstrom, A.; Zhu, H.; et al. Circulating Tumor Cell Clusters Are Oligoclonal Precursors of Breast Cancer Metastasis. Cell 2014, 158, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Pauken, C.M.; Kenney, S.R.; Brayer, K.J.; Guo, Y.; Brown-Glaberman, U.A.; Marchetti, D. Heterogeneity of Circulating Tumor Cell Neoplastic Subpopulations Outlined by Single-Cell Transcriptomics. Cancers 2021, 13, 4885. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- LeVasseur, N.; Manna, M.; Jerzak, K.J. An Overview of Long-Acting GnRH Agonists in Premenopausal Breast Cancer Patients: Survivorship Challenges and Management. Curr. Oncol. 2024, 31, 4209–4224. [Google Scholar] [CrossRef]
- Eliassen, F.M.; Blåfjelldal, V.; Helland, T.; Hjorth, C.F.; Hølland, K.; Lode, L.; Bertelsen, B.-E.; Janssen, E.A.M.; Mellgren, G.; Kvaløy, J.T.; et al. Importance of Endocrine Treatment Adherence and Persistence in Breast Cancer Survivorship: A Systematic Review. BMC Cancer 2023, 23, 625. [Google Scholar] [CrossRef]
- Spizzo, G.; Obrist, P.; Ensinger, C.; Theurl, I.; Dünser, M.; Ramoni, A.; Gunsilius, E.; Eibl, G.; Mikuz, G.; Gastl, G. Prognostic Significance of Ep-CAM AND Her-2/Neu Overexpression in Invasive Breast Cancer. Int. J. Cancer 2002, 98, 883–888. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Wu, Y.; Wang, J.; Ma, F. Efficacy and Safety of Trastuzumab with or without a Tyrosine Kinase Inhibitor for HER2-Positive Breast Cancer: A Systematic Review and Meta-Analysis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188969. [Google Scholar] [CrossRef]
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of Distant Recurrence and Clinical Outcomes in Patients with Metastatic Triple-Negative Breast Cancer. Cancer 2008, 113, 2638–2645. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Schnell, U.; Cirulli, V.; Giepmans, B.N.G. EpCAM: Structure and Function in Health and Disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1989–2001. [Google Scholar] [CrossRef]
- Trzpis, M.; McLaughlin, P.M.J.; de Leij, L.M.F.H.; Harmsen, M.C. Epithelial Cell Adhesion Molecule: More than a Carcinoma Marker and Adhesion Molecule. Am. J. Pathol. 2007, 171, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, X.; Yang, K.; Yang, Y.; Zhou, W.; Huang, Y.; Liang, X.; Su, J.; Jiang, L.; Li, J.; et al. EpCAM-Targeting CAR-T Cell Immunotherapy Is Safe and Efficacious for Epithelial Tumors. Sci. Adv. 2023, 9, eadg9721. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, L.A.; Ebian, H.; Harb, O.; Nosery, Y.; Taha, H.; Nawar, N. Clinical Significance of Cytokeratin 19 and OCT4 as Survival Markers in Non-Metastatic and Metastatic Breast Cancer Patients. Contemp. Oncol. 2022, 26, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, E.; Moghbelinejad, S.; Mohammadi, G.; Shahbazmohammadi, H.; Abdolvahabi, Z.; Jalilvand, M.; Zare, I.; Alaei, M.; Keshavarz Shahbaz, S. Cytokeratin Expression in Breast Cancer: From Mechanisms, Progression, Diagnosis, and Prognosis to Therapeutic Implications. Mol. Cell. Oncol. 2025, 12, 2526230. [Google Scholar] [CrossRef]
- Alsharif, S.; Sharma, P.; Bursch, K.; Milliken, R.; Lam, V.; Fallatah, A.; Phan, T.; Collins, M.; Dohlman, P.; Tiufekchiev, S.; et al. Keratin 19 Maintains E-Cadherin Localization at the Cell Surface and Stabilizes Cell-Cell Adhesion of MCF7 Cells. Cell Adhes. Migr. 2021, 15, 1–17. [Google Scholar] [CrossRef]
- Meijer, S.E.; Klebanov-Akopyn, O.; Pavlov, V.; Laks, S.; Hazzan, D.; Nissan, A.; Zippel, D. Detection of Minimal Residual Disease in the Peripheral Blood of Breast Cancer Patients, with a Multi Marker (MGB-1, MGB-2, CK-19 and NY-BR-1) Assay. Breast Cancer 2021, 13, 617–624. [Google Scholar] [CrossRef]
- Parikh, R.R.; Yang, Q.; Higgins, S.A.; Haffty, B.G. Outcomes in Young Women with Breast Cancer of Triple-Negative Phenotype: The Prognostic Significance of CK19 Expression. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 35–42. [Google Scholar] [CrossRef]
- Kotsifaki, A.; Maroulaki, S.; Armakolas, A. Exploring the Immunological Profile in Breast Cancer: Recent Advances in Diagnosis and Prognosis through Circulating Tumor Cells. Int. J. Mol. Sci. 2024, 25, 4832. [Google Scholar] [CrossRef]
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The Identification and Characterization of Breast Cancer CTCs Competent for Brain Metastasis. Sci. Transl. Med. 2013, 5, 180ra48. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-Mesenchymal Transition Is Not Required for Lung Metastasis but Contributes to Chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.J.; Thomas, J.S.; Langdon, S.P.; Harrison, D.J.; Faratian, D. Quantitative Analysis of Changes in ER, PR and HER2 Expression in Primary Breast Cancer and Paired Nodal Metastases. Ann. Oncol. 2010, 21, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.J.E.; Van Dam, P.-J.; Van Den Eynden, G.G.M.; Rutten, A.; Wuyts, H.; Pouillon, L.; Peeters, M.; Pauwels, P.; Van Laere, S.J.; Van Dam, P.A.; et al. Detection and Prognostic Significance of Circulating Tumour Cells in Patients with Metastatic Breast Cancer According to Immunohistochemical Subtypes. Br. J. Cancer 2014, 110, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Wu, H.; Punyadeera, C.; Warkiani, M.E. The Use of Microfluidic Technology for Cancer Applications and Liquid Biopsy. Micromachines 2018, 9, 397. [Google Scholar] [CrossRef]
- Vishnoi, M.; Peddibhotla, S.; Yin, W.; Scamardo, A.T.; George, G.C.; Hong, D.S.; Marchetti, D. The Isolation and Characterization of CTC Subsets Related to Breast Cancer Dormancy. Sci. Rep. 2015, 5, 17533. [Google Scholar] [CrossRef]
- Lampignano, R.; Schneck, H.; Neumann, M.; Fehm, T.; Neubauer, H. Enrichment, Isolation and Molecular Characterization of EpCAM-Negative Circulating Tumor Cells. Adv. Exp. Med. Biol. 2017, 994, 181–203. [Google Scholar] [CrossRef]
- Königsberg, R.; Obermayr, E.; Bises, G.; Pfeiler, G.; Gneist, M.; Wrba, F.; de Santis, M.; Zeillinger, R.; Hudec, M.; Dittrich, C. Detection of EpCAM Positive and Negative Circulating Tumor Cells in Metastatic Breast Cancer Patients. Acta Oncol. 2011, 50, 700–710. [Google Scholar] [CrossRef]
- Fridrichova, I.; Kalinkova, L.; Ciernikova, S. Clinical Relevancy of Circulating Tumor Cells in Breast Cancer: Epithelial or Mesenchymal Characteristics, Single Cells or Clusters? Int. J. Mol. Sci. 2022, 23, 12141. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, R.; Ou, Z.; Ye, Y.; Zeng, Q.; Wang, Y.; Wang, A.; Chen, T.; Chai, C.; Guo, B. TROP2 Is Highly Expressed in Triple-Negative Breast Cancer CTCs and Is a Potential Marker for Epithelial Mesenchymal CTCs. Mol. Ther. Oncol. 2024, 32, 200762. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Lagoudaki, E.; Gounaki, S.; Hatziavraam, S.; Fotsitzoudis, C.; Michaelidou, K.; Agelaki, S.; Papadaki, M.A. Abstract P4-05-27: Comparative Analysis of TROP2 Expression in Tumor Tissues and Circulating Tumor Cells (CTCs) in the Peripheral Blood of Patients with Triple Negative Breast Cancer. Clin. Cancer Res. 2025, 31, P4-05-27. [Google Scholar] [CrossRef]
- Wu, C.-J.; Lu, M.; Feng, X.; Nakato, G.; Udey, M.C. Matriptase Cleaves EpCAM and TROP2 in Keratinocytes, Destabilizing Both Proteins and Associated Claudins. Cells 2020, 9, 1027. [Google Scholar] [CrossRef]
- Wu, C.-J.; Feng, X.; Lu, M.; Morimura, S.; Udey, M.C. Matriptase-Mediated Cleavage of EpCAM Destabilizes Claudins and Dysregulates Intestinal Epithelial Homeostasis. J. Clin. Investig. 2017, 127, 623–634. [Google Scholar] [CrossRef]
- Lenárt, S.; Lenárt, P.; Šmarda, J.; Remšík, J.; Souček, K.; Beneš, P. Trop2: Jack of All Trades, Master of None. Cancers 2020, 12, 3328. [Google Scholar] [CrossRef] [PubMed]
- Menz, A.; Lony, N.; Lennartz, M.; Dwertmann Rico, S.; Schlichter, R.; Kind, S.; Reiswich, V.; Viehweger, F.; Dum, D.; Luebke, A.M.; et al. Epithelial Cell Adhesion Molecule (EpCAM) Expression in Human Tumors: A Comparison with Pan-Cytokeratin and TROP2 in 14,832 Tumors. Diagnostics 2024, 14, 1044. [Google Scholar] [CrossRef] [PubMed]
- Aslemarz, A.; Fagotto-Kaufmann, M.; Ruppel, A.; Fagotto-Kaufmann, C.; Balland, M.; Lasko, P.; Fagotto, F. An EpCAM/Trop2 Mechanostat Differentially Regulates Collective Behaviour of Human Carcinoma Cells. EMBO J. 2025, 44, 75–106. [Google Scholar] [CrossRef]
- Szostakowska-Rodzos, M.; Fabisiewicz, A.; Wakula, M.; Tabor, S.; Szafron, L.; Jagiello-Gruszfeld, A.; Grzybowska, E.A. Longitudinal Analysis of Circulating Tumor Cell Numbers Improves Tracking Metastatic Breast Cancer Progression. Sci. Rep. 2024, 14, 12924. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, M.L.; Welte, T.; Boral, D.; Liu, H.N.; Yin, W.; Vishnoi, M.; Goswami-Sewell, D.; Li, L.; Pei, G.; Jia, P.; et al. PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling. Int. J. Mol. Sci. 2019, 20, 1916. [Google Scholar] [CrossRef]
- Duda, D.G.; Duyverman, A.M.M.J.; Kohno, M.; Snuderl, M.; Steller, E.J.A.; Fukumura, D.; Jain, R.K. Malignant Cells Facilitate Lung Metastasis by Bringing Their Own Soil. Proc. Natl. Acad. Sci. USA 2010, 107, 21677–21682. [Google Scholar] [CrossRef] [PubMed]
- Fabisiewicz, A.; Grzybowska, E. CTC Clusters in Cancer Progression and Metastasis. Med. Oncol. 2017, 34, 12. [Google Scholar] [CrossRef] [PubMed]
- Schuster, E.; Taftaf, R.; Reduzzi, C.; Albert, M.K.; Romero-Calvo, I.; Liu, H. Better Together: Circulating Tumor Cell Clustering in Metastatic Cancer. Trends Cancer 2021, 7, 1020–1032. [Google Scholar] [CrossRef]
- Tang, R.; Luo, S.; Liu, H.; Sun, Y.; Liu, M.; Li, L.; Ren, H.; Angele, M.K.; Börner, N.; Yu, K.; et al. Circulating Tumor Microenvironment in Metastasis. Cancer Res. 2025, 85, 1354–1367. [Google Scholar] [CrossRef]
- Sayed, Z.S.; Khattap, M.G.; Madkour, M.A.; Yasen, N.S.; Elbary, H.A.; Elsayed, R.A.; Abdelkawy, D.A.; Wadan, A.-H.S.; Omar, I.; Nafady, M.H. Circulating Tumor Cells Clusters and Their Role in Breast Cancer Metastasis; a Review of Literature. Discov. Oncol. 2024, 15, 94. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-Mesenchymal Transition Is Dispensable for Metastasis but Induces Chemoresistance in Pancreatic Cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Ebright, R.Y.; Lee, S.; Wittner, B.S.; Niederhoffer, K.L.; Nicholson, B.T.; Bardia, A.; Truesdell, S.; Wiley, D.F.; Wesley, B.; Li, S.; et al. Deregulation of Ribosomal Protein Expression and Translation Promotes Breast Cancer Metastasis. Science 2020, 367, 1468–1473. [Google Scholar] [CrossRef]
- Kowalik, A.; Kowalewska, M.; Góźdź, S. Current Approaches for Avoiding the Limitations of Circulating Tumor Cells Detection Methods-Implications for Diagnosis and Treatment of Patients with Solid Tumors. Transl. Res. 2017, 185, 58–84.e15. [Google Scholar] [CrossRef] [PubMed]
- Boral, D.; Vishnoi, M.; Liu, H.N.; Yin, W.; Sprouse, M.L.; Scamardo, A.; Hong, D.S.; Tan, T.Z.; Thiery, J.P.; Chang, J.C.; et al. Molecular Characterization of Breast Cancer CTCs Associated with Brain Metastasis. Nat. Commun. 2017, 8, 196. [Google Scholar] [CrossRef]
- Sutton, T.L.; Patel, R.K.; Anderson, A.N.; Bowden, S.G.; Whalen, R.; Giske, N.R.; Wong, M.H. Circulating Cells with Macrophage-like Characteristics in Cancer: The Importance of Circulating Neoplastic-Immune Hybrid Cells in Cancer. Cancers 2022, 14, 3871. [Google Scholar] [CrossRef]
- Higa, N.; Limb, A.; Hennes, V.; Rivera, A.; Nevarez, R.; Kolatkar, A.; Tweed, C.K.; Riker, A.I.; Lee, Y.; Tafra, L.; et al. Simultaneous Expression of Epithelial and Immune Cell Markers in Circulating Tumor Cells Identified in Patients with Stage 4 Breast Cancer. Commun. Med. 2025, 5, 309. [Google Scholar] [CrossRef] [PubMed]
- ORIEN: Accelerating Cancer Research and Delivering Hope Through Collaborative Learning and Partnerships. 2025. Available online: https://www.oriencancer.org/ (accessed on 20 August 2025).
- Cheng, Y.C.; Ueno, N.T. Improvement of Survival and Prospect of Cure in Patients with Metastatic Breast Cancer. Breast Cancer 2012, 19, 191–199. [Google Scholar] [CrossRef]
- Moser, E.C.; Meunier, F. Cancer Survivorship: A Positive Side-Effect of More Successful Cancer Treatment. Eur. J. Cancer Suppl. 2014, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mathias, T.J.; Chang, K.T.; Martin, S.S.; Vitolo, M.I. Gauging the Impact of Cancer Treatment Modalities on Circulating Tumor Cells (CTCs). Cancers 2020, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.A.; Anderson, R.L.; Narayan, K.; MacManus, M.P. Does the Mobilization of Circulating Tumour Cells during Cancer Therapy Cause Metastasis? Nat. Rev. Clin. Oncol. 2017, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.C.; Martin, S.S. Insights on CTC Biology and Clinical Impact Emerging from Advances in Capture Technology. Cells 2019, 8, 553. [Google Scholar] [CrossRef]
- Yan, W.-T.; Cui, X.; Chen, Q.; Li, Y.-F.; Cui, Y.-H.; Wang, Y.; Jiang, J. Circulating Tumor Cell Status Monitors the Treatment Responses in Breast Cancer Patients: A Meta-Analysis. Sci. Rep. 2017, 7, 43464. [Google Scholar] [CrossRef]
- Mercier, F.; Consalvo, N.; Frey, N.; Phipps, A.; Ribba, B. From Waterfall Plots to Spaghetti Plots in Early Oncology Clinical Development. Pharm. Stat. 2019, 18, 526–532. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating Breast Tumor Cells Exhibit Dynamic Changes in Epithelial and Mesenchymal Composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Frenel, J.-S.; Zeghondy, J.; Guérin-Charbonnel, C.; Mailliez, A.; Volant, E.; Poumeaud, F.; Patsouris, A.; Arnedos, M.; Bailleux, C.; Cabal, J.; et al. Tucatinib Combination Treatment After Trastuzumab-Deruxtecan in Patients with ERBB2-Positive Metastatic Breast Cancer. JAMA Netw. Open 2024, 7, e244435. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Muinelo-Romay, L.; Cebey-López, V.; Pereira-Veiga, T.; Martínez-Pena, I.; Abreu, M.; Abalo, A.; Lago-Lestón, R.M.; Abuín, C.; Palacios, P.; et al. Analysis of a Real-World Cohort of Metastatic Breast Cancer Patients Shows Circulating Tumor Cell Clusters (CTC-Clusters) as Predictors of Patient Outcomes. Cancers 2020, 12, 1111. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Fidler, I.J. AACR Centennial Series: The Biology of Cancer Metastasis: Historical Perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef]
- Liu, L.; Qu, X.; Wang, Z.; Ji, C.; Ling, R.; Yan, C. Leaf-Vein-Inspired Multi-Organ Microfluidic Chip for Modeling Breast Cancer CTC Organotropism. Front. Oncol. 2025, 15, 1602225. [Google Scholar] [CrossRef]
- Polioudaki, H.; Agelaki, S.; Chiotaki, R.; Politaki, E.; Mavroudis, D.; Matikas, A.; Georgoulias, V.; Theodoropoulos, P.A. Variable Expression Levels of Keratin and Vimentin Reveal Differential EMT Status of Circulating Tumor Cells and Correlation with Clinical Characteristics and Outcome of Patients with Metastatic Breast Cancer. BMC Cancer 2015, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Dianat-Moghadam, H.; Azizi, M.; Eslami-S, Z.; Cortés-Hernández, L.E.; Heidarifard, M.; Nouri, M.; Alix-Panabières, C. The Role of Circulating Tumor Cells in the Metastatic Cascade: Biology, Technical Challenges, and Clinical Relevance. Cancers 2020, 12, 867. [Google Scholar] [CrossRef] [PubMed]
- Nochi, T.; Yuki, Y.; Terahara, K.; Hino, A.; Kunisawa, J.; Kweon, M.-N.; Yamaguchi, T.; Kiyono, H. Biological Role of Ep-CAM in the Physical Interaction between Epithelial Cells and Lymphocytes in Intestinal Epithelium. Clin. Immunol. 2004, 113, 326–339. [Google Scholar] [CrossRef]
- Ju, J.-H.; Oh, S.; Lee, K.-m.; Yang, W.; Nam, K.S.; Moon, H.-G.; Noh, D.-Y.; Kim, C.G.; Park, G.; Park, J.B.; et al. Cytokeratin19 Induced by HER2/ERK Binds and Stabilizes HER2 on Cell Membranes. Cell Death Differ. 2015, 22, 665–676. [Google Scholar] [CrossRef]
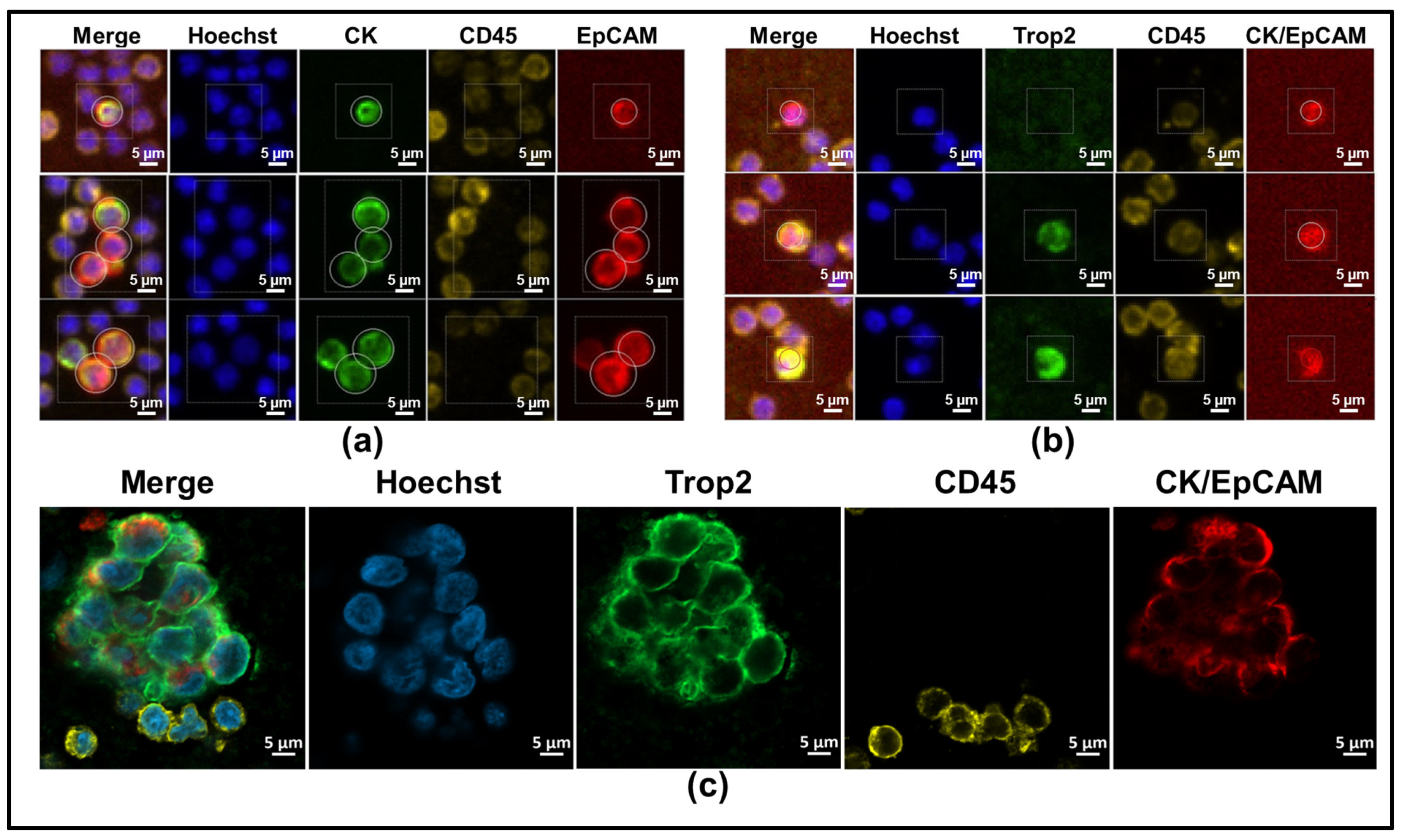
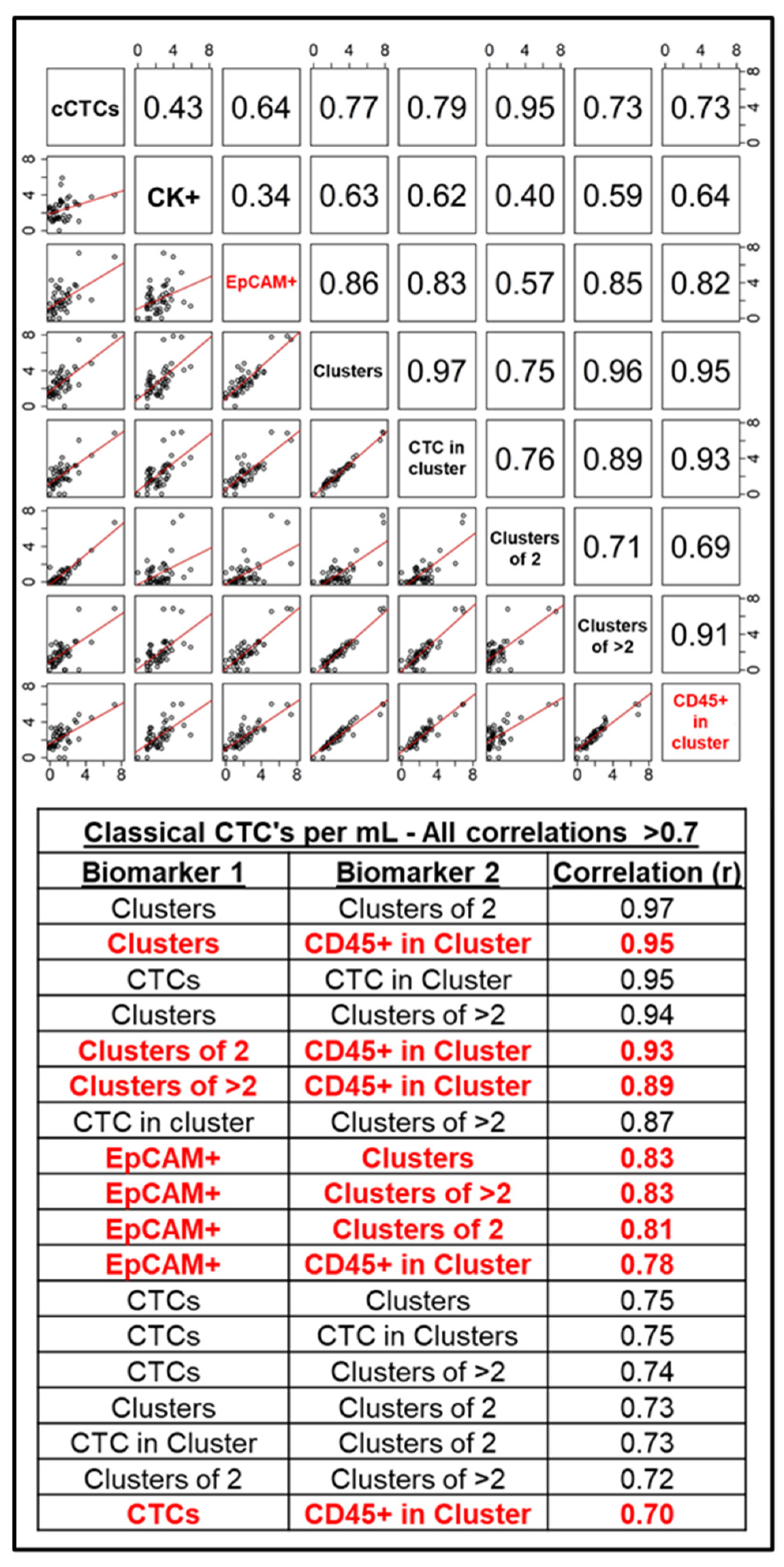
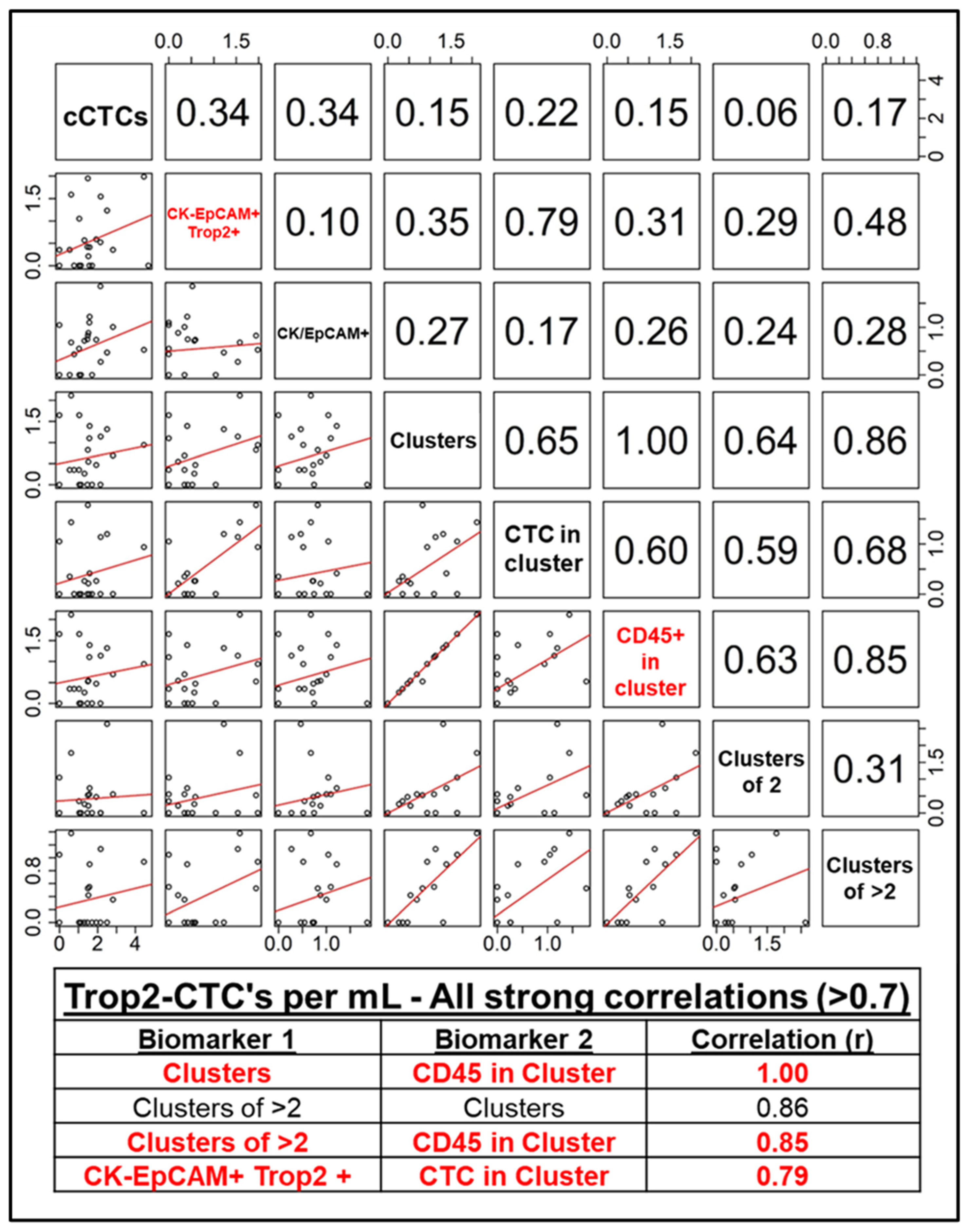
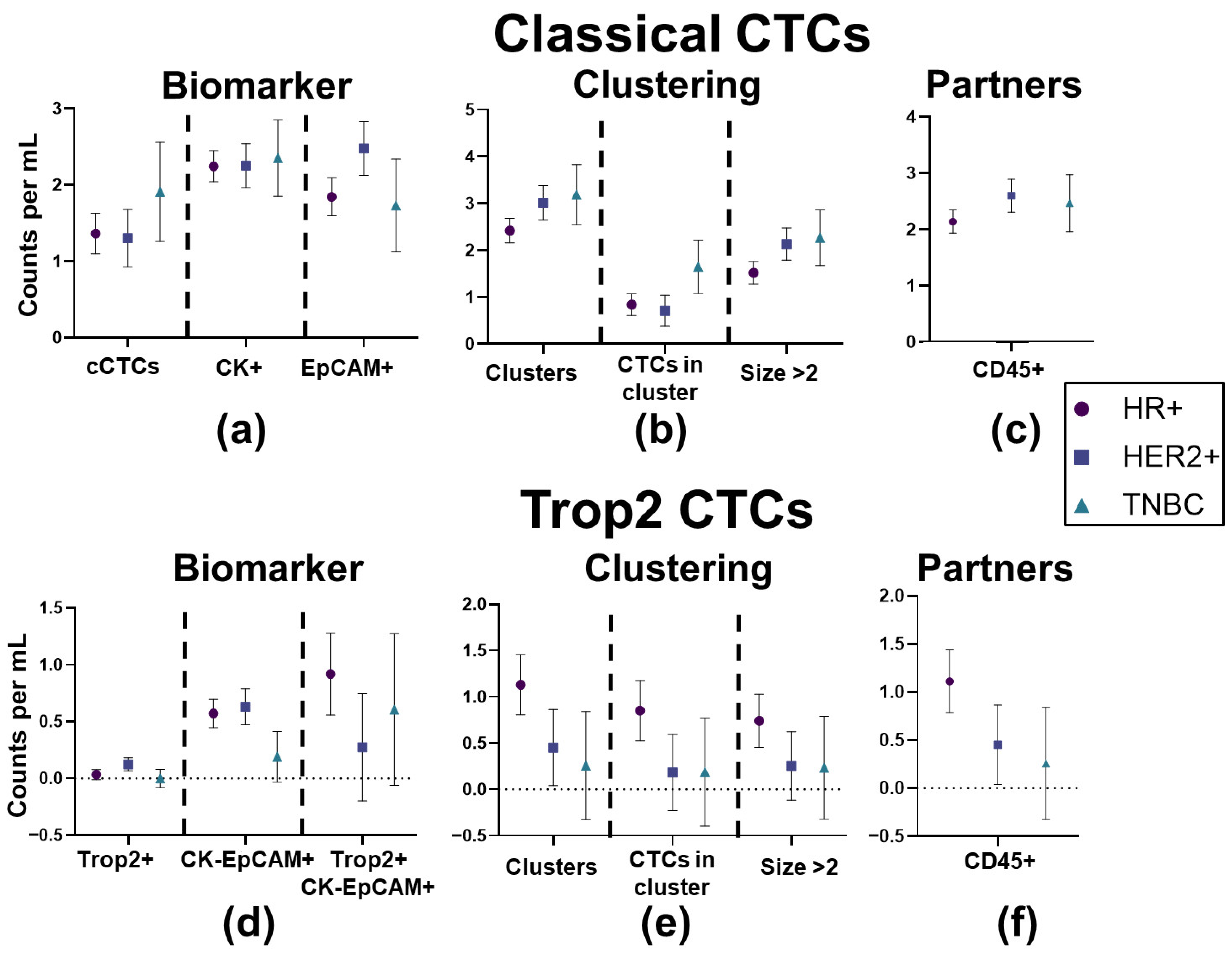
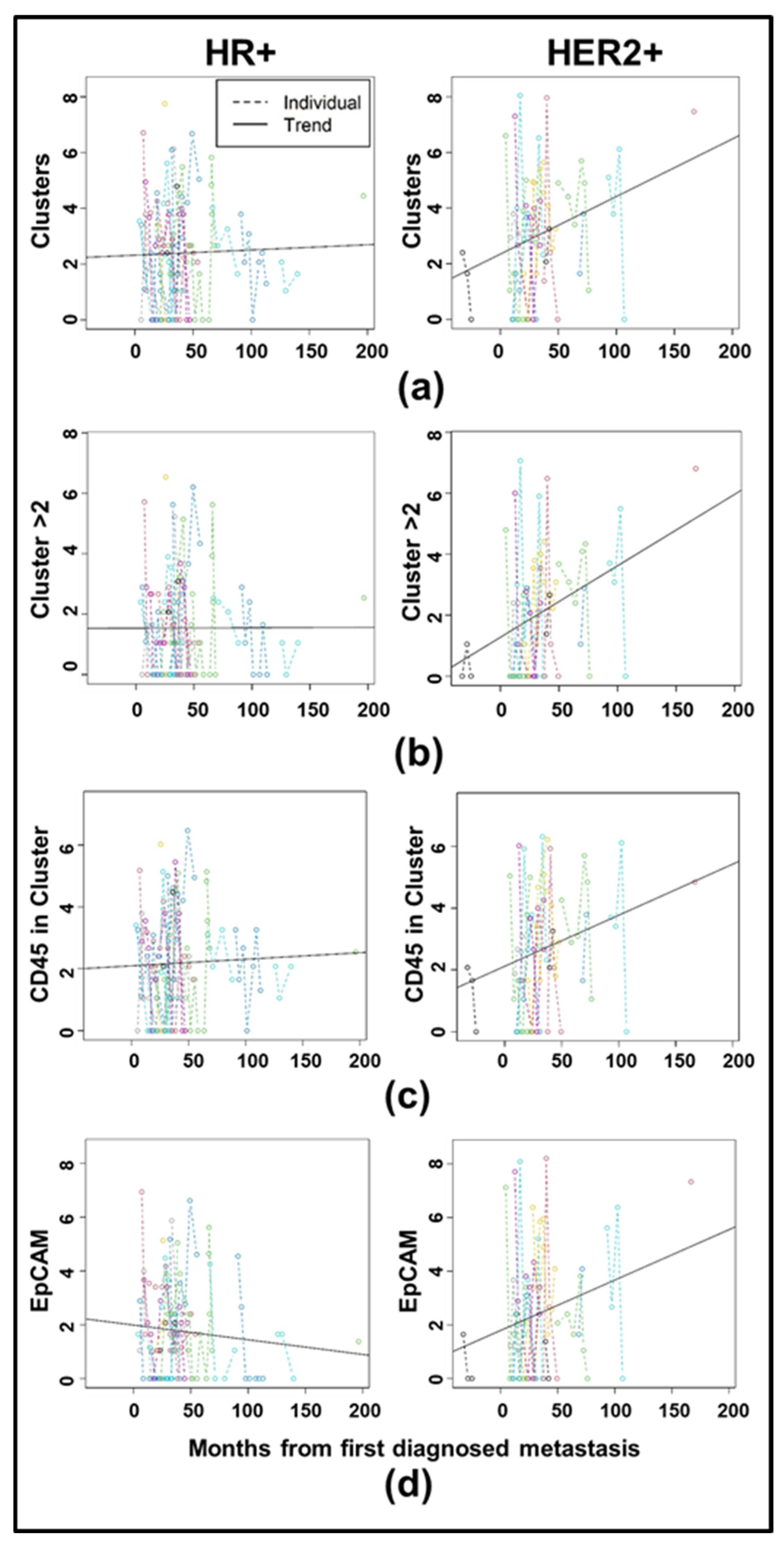
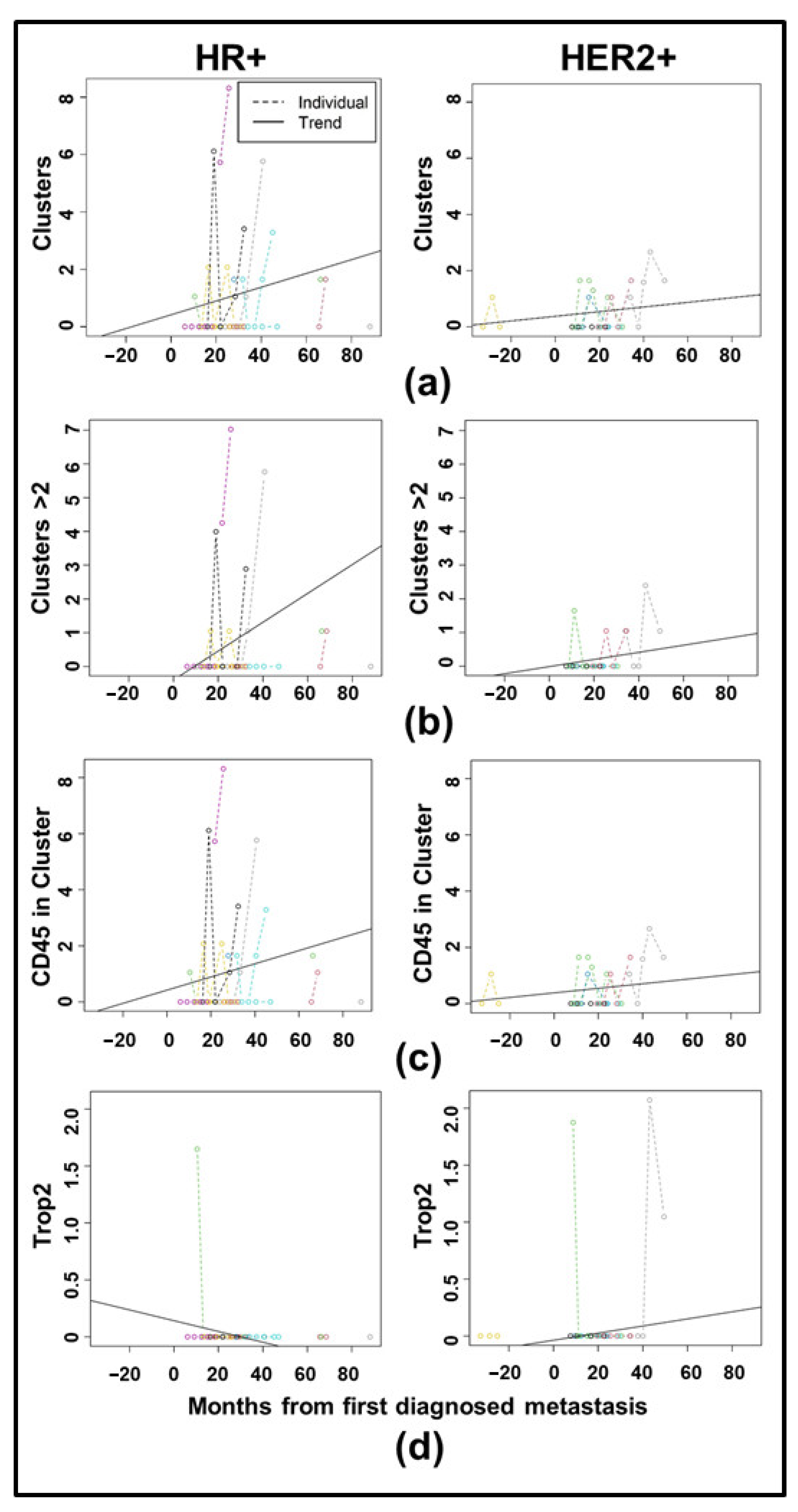
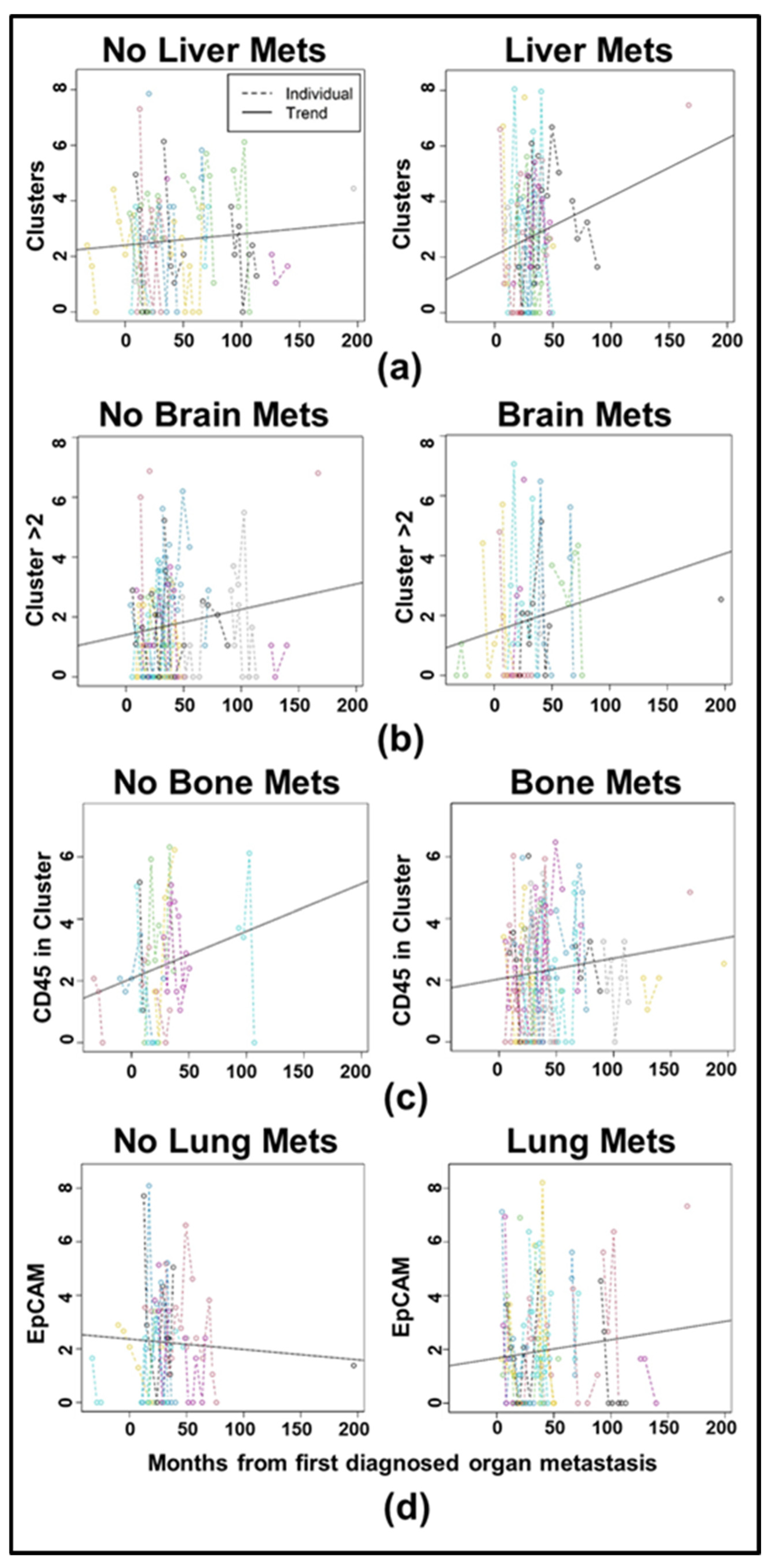
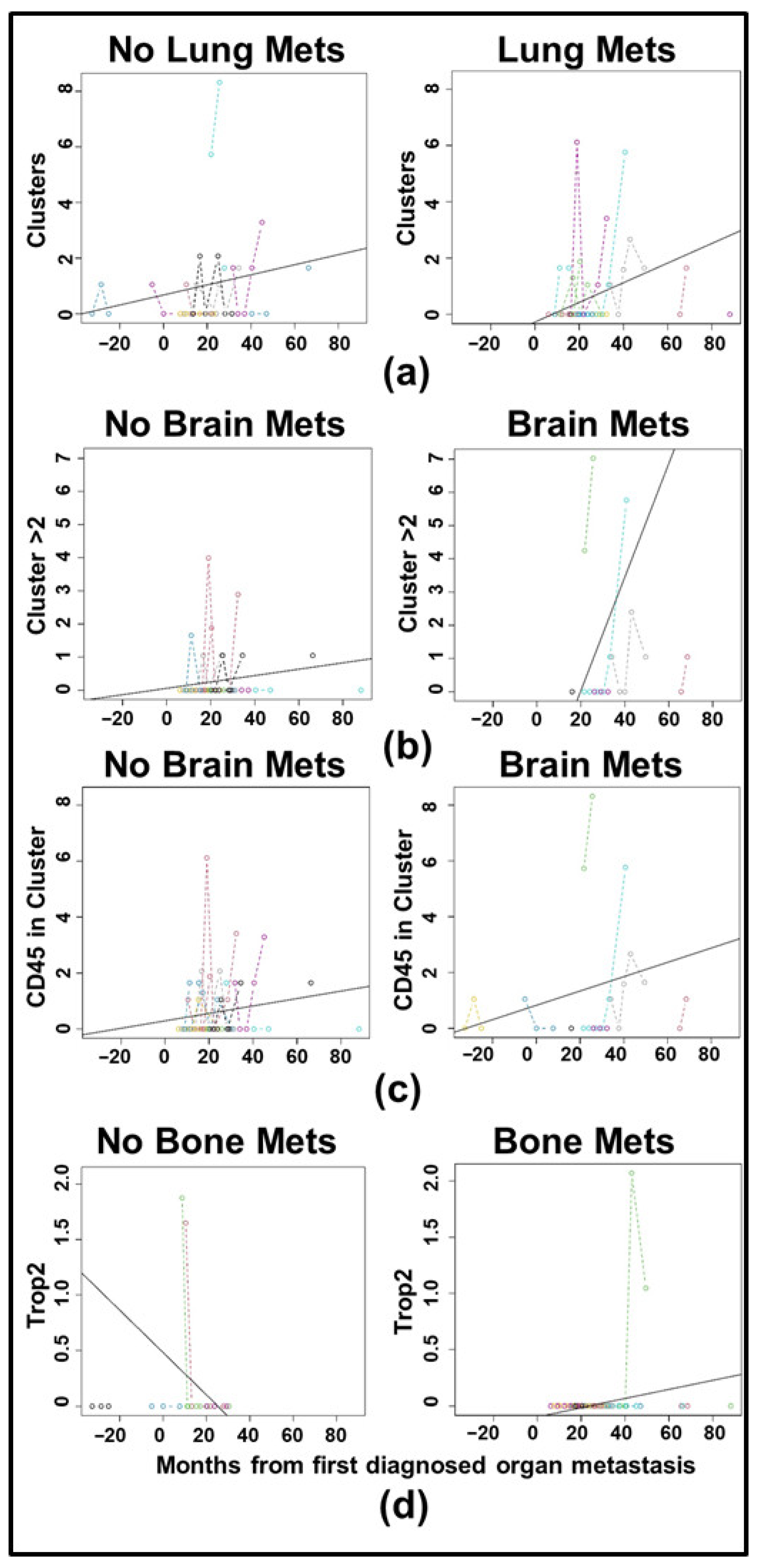
| Clinical Characteristic | Category | Full Cohort | HR+/HER2- | HER2+ | TNBC | Fisher Exact Test, p-Value |
|---|---|---|---|---|---|---|
| Age at 1st blood collection | <65 | 30 (60.0) | 17 (54.8) | 11 (68.8) | 2 (66.7) | |
| 65+ | 20 (40.0) | 14 (45.2) | 5 (31.2) | 1 (33.3) | 0.792 | |
| Total | 50 (100) | 31 (100) | 16 (100) | 3 (100) | ||
| Number of Metastatic sites | 1 | 4 (8.0) | 4 (12.9) | 0 (0.0) | 0 (0.0) | 0.417 |
| 2 | 15 (30.0) | 7 (22.6) | 7 (43.8) | 1 (33.0) | ||
| 3+ | 31 (62.0) | 20 (64.5) | 9 (56.2) | 2 (66.7) | ||
| Total | 50 (100) | 31 (100) | 16 (100) | 3 (100) | ||
| Lung metastasis | N | 22 (43.1) | 14 (43.8) | 7 (43.8) | 1 (33.0) | 1.000 |
| Y | 29 (56.9) | 18 (56.2) | 9 (56.2) | 2 (66.7) | ||
| Total | 51 (100) | 32 (100) | 16 (100) | 3 (100) | ||
| Bone metastasis | N | 14 (27.5) | 6 (18.8) | 7 (43.8) | 1 (33.0) | 0.141 |
| Y | 37 (72.5) | 26 (81.2) | 9 (56.2) | 2 (66.7) | ||
| Total | 51 (100) | 32 (100) | 16 (100) | 3 (100) | ||
| Liver metastasis | N | 25 (50.0) | 16 (51.6) | 7 (43.8) | 2 (66.7) | |
| Y | 25 (50.0) | 15 (48.4) | 9 (56.2) | 1 (33.3) | 0.816 | |
| Total | 50 (100) | 31 (100) | 16 (100) | 3 (100) | ||
| Brain metastasis | N | 34 (68.0) | 24 (77.4) | 9 (56.2) | 1 (33.3) | |
| Y | 16 (32.0) | 7 (22.6) | 7 (43.8) | 2 (66.7) | 0.105 | |
| Total | 50 (100) | 31 (100) | 16 (100) | 3 (100) |
| Clinical Characteristic | Category | Full Cohort | HR+/HER2- | HER2+ | TNBC | Fisher Exact Test, p-Value |
|---|---|---|---|---|---|---|
| Age at 1st blood collection | <65 | 16 (64.0) | 7 (50.0) | 7 (87.5) | 2 (66.7) | |
| 65+ | 9 (36.0) | 7 (50.0) | 1 (12.5) | 1 (33.3) | 0.246 | |
| Total | 25 * (100) | 14 (100) | 8 (100) | 3 (100) | ||
| Number of Metastatic sites | 1 | 2 (7.7) | 2 (13.3) | 0 (0.0) | 0 (0.0) | 0.834 |
| 2 | 9 (34.6) | 4 (26.7) | 4 (50.0) | 1 (33.0) | ||
| 3+ | 15 (57.7) | 9 (60.0) | 4 (50.0) | 2 (66.7) | ||
| Total | 26 (100) | 15 (100)) | 8 (100) | 3 (100) | ||
| Lung metastasis | N | 13 (50.0) | 8 (53.3) | 4 (50.0) | 1 (33.0) | 1.000 |
| Y | 13 (50.0) | 7 (46.7) | 4 (50.0) | 2 (66.7) | ||
| Total | 26 (100) | 15 (100) | 8 (100) | 3 (100) | ||
| Bone metastasis | N | 7 (26.9) | 2 (13.3) | 4 (50.0) | 1 (33.0) | 0.139 |
| Y | 19 (73.1) | 13 (86.7) | 4 (50.0) | 2 (66.7) | ||
| Total | 26 (100) | 15 (100) | 8 (100) | 3 (100) | ||
| Liver metastasis | N | 11 (42.3) | 6 (40.0) | 3 (37.5) | 2 (66.7) | |
| Y | 15 (57.7) | 9 (60.0) | 5 (62.5) | 1 (33.3) | 0.724 | |
| Total | 26 (100) | 15 (100) | 8 (100) | 3 (100) | ||
| Brain metastasis | N | 18 (69.2) | 11 (73.3) | 6 (75.0) | 1 (33.3) | |
| Y | 8 (30.8) | 4 (26.7) | 2 (25.0) | 2 (66.7) | 0.418 | |
| Total | 26 (100) | 15 (100) | 8 (100) | 3 (100) |
| Biomarker (per mL) | Receptor Effect | Time Effect | Interaction |
|---|---|---|---|
| cCTCs | 0.6591061 | 0.7461176 | 0.840692 |
| CK | 0.4685809 | 0.6062315 | 0.4074604 |
| EpCAM | 0.7158591 | 0.1999038 | 0.0218357 |
| Clusters | 0.9510659 | 0.028952 | 0.0674635 † |
| Clusters of 2 | 0.6826174 | 0.0805827 | 0.1121893 |
| cCTC in cluster | 0.4323639 | 0.5820074 | 0.5686966 |
| Clusters > 2 | 0.5347161 | 0.0062573 | 0.0069147 |
| CD45 in cluster | 0.9598183 | 0.0401535 | 0.1091464 |
| Biomarker (per mL) | Receptor Effect | Time Effect | Interaction |
|---|---|---|---|
| CK/EpCAM/Trop2 | 0.4802839 | 0.8340946 | 0.8919177 |
| CK/EpCAM | 0.5649867 | 0.0955775 | 0.1789835 |
| Trop2 | 0.5484958 | 0.8870074 | 0.490742 |
| Clusters | 0.966292 | 0.2493243 | 0.5708911 |
| CTC in cluster | 0.7295486 | 0.5810308 | 0.8777693 |
| CD45 in cluster | 0.9998403 | 0.2495356 | 0.5364823 |
| Clusters of 2 | 0.2829963 | 0.6461081 | 0.4631998 |
| Clusters > 2 | 0.633026 | 0.2716701 | 0.4975725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkley, S.D.; Kang, H.; Brown-Glaberman, U.; Marchetti, D. Comprehensive Longitudinal Linear Mixed Modeling of CTCs Illuminates the Role of Trop2, EpCAM, and CD45 in CTC Clustering and Metastasis. Cancers 2025, 17, 2717. https://doi.org/10.3390/cancers17162717
Merkley SD, Kang H, Brown-Glaberman U, Marchetti D. Comprehensive Longitudinal Linear Mixed Modeling of CTCs Illuminates the Role of Trop2, EpCAM, and CD45 in CTC Clustering and Metastasis. Cancers. 2025; 17(16):2717. https://doi.org/10.3390/cancers17162717
Chicago/Turabian StyleMerkley, Seth D., Huining Kang, Ursa Brown-Glaberman, and Dario Marchetti. 2025. "Comprehensive Longitudinal Linear Mixed Modeling of CTCs Illuminates the Role of Trop2, EpCAM, and CD45 in CTC Clustering and Metastasis" Cancers 17, no. 16: 2717. https://doi.org/10.3390/cancers17162717
APA StyleMerkley, S. D., Kang, H., Brown-Glaberman, U., & Marchetti, D. (2025). Comprehensive Longitudinal Linear Mixed Modeling of CTCs Illuminates the Role of Trop2, EpCAM, and CD45 in CTC Clustering and Metastasis. Cancers, 17(16), 2717. https://doi.org/10.3390/cancers17162717







