Tumor-Specific EphA2 Receptor Tyrosine Kinase Inhibits Anti-Tumor Immunity by Recruiting Suppressive Myeloid Populations in Murine Models of Non-Small Cell Lung Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blotting
2.3. Cell Viability Assays
2.4. Animal Models
2.5. Tumor Models
2.6. Flow Cytometry
2.7. NanoString nCounter Assay
2.8. RT-PCR
2.9. Human Gene Expression Correlation Analysis
2.10. Statistical Analysis
3. Results
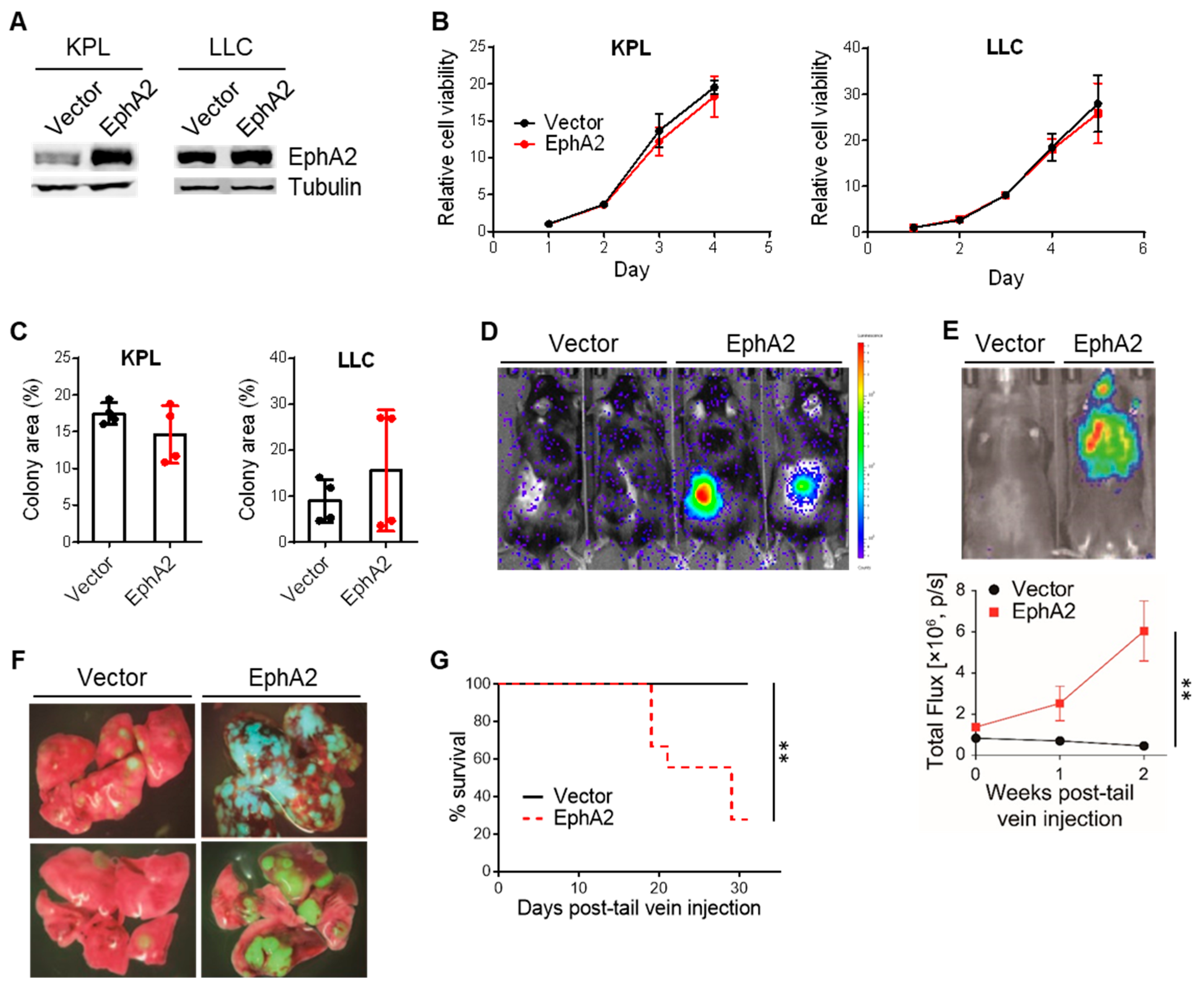
3.1. EphA2 Confers Growth Advantage to NSCLC in Vivo but Not in Vitro
3.2. EphA2 Overexpression in NSCLC Does Not Significantly Impact Tumor Burden or Immune Infiltration in Nude Mice
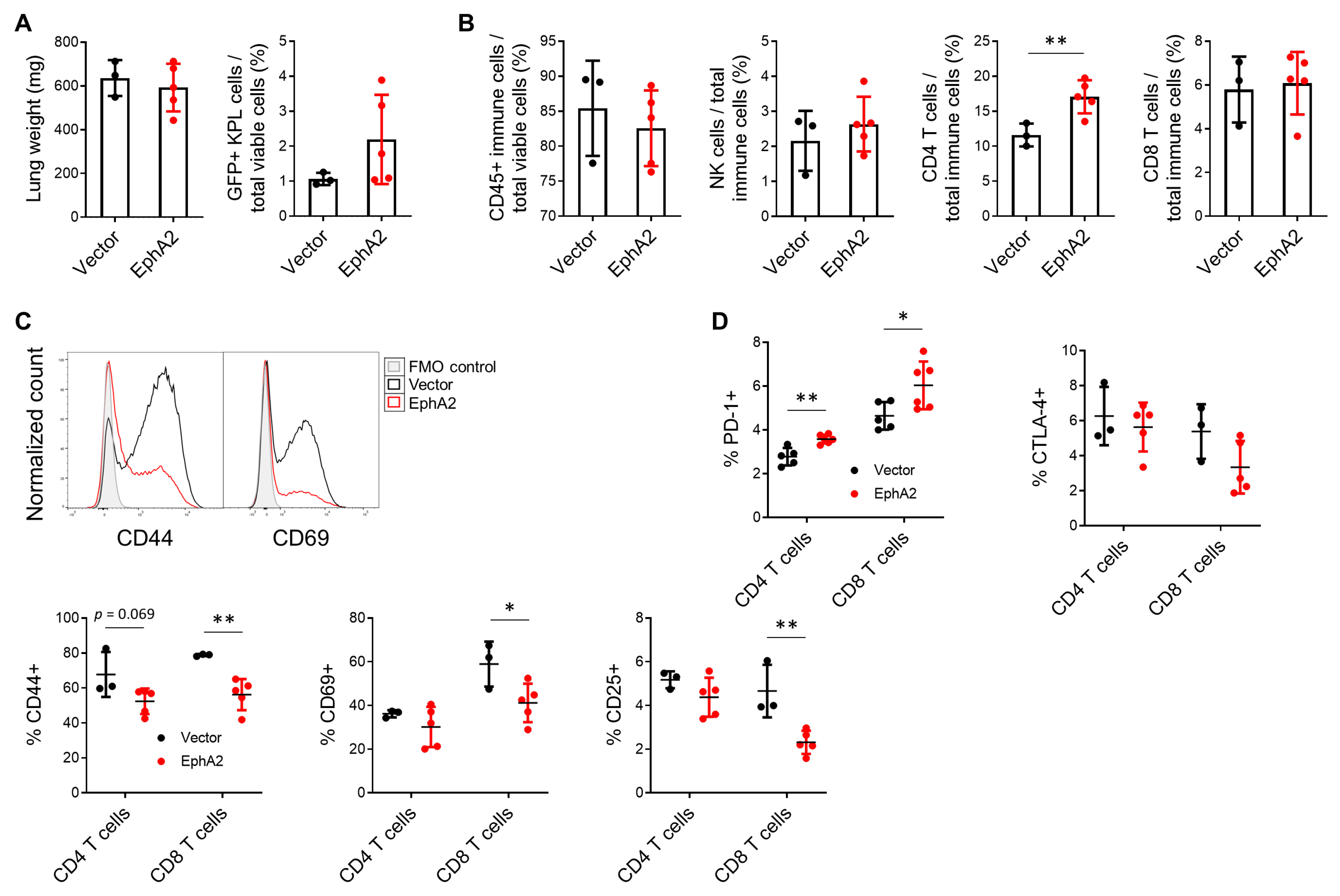
3.3. EphA2 Overexpression in NSCLC Decreases Lymphocytic and Increases Myeloid Infiltrate in Tumor-Bearing Lungs
3.4. EphA2 Overexpression in NSCLC Suppresses Tumor-Infiltrating T Cells
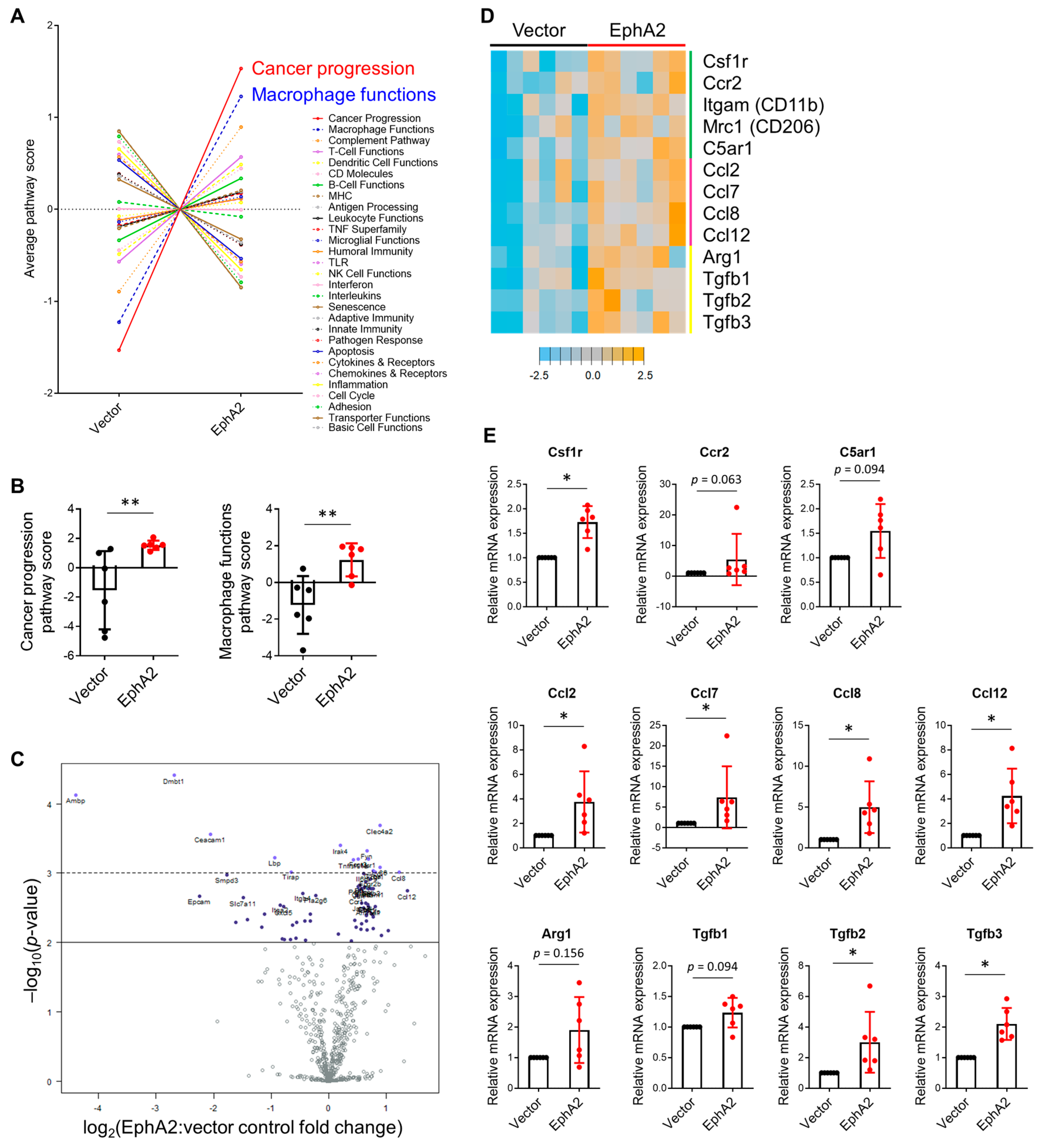
3.5. Gene Expression Profiling Reveals Higher Expression of Myeloid Markers and Chemoattractants in EphA2-Overexpressing Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergholz, J.S.; Wang, Q.; Kabraji, S.; Zhao, J.J. Integrating Immunotherapy and Targeted Therapy in Cancer Treatment: Mechanistic Insights and Clinical Implications. Clin. Cancer Res. 2021, 26, 5557–5566. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Pauken, K.E.; Wang, L.; Sharma, P. Next-Generation Combination Approaches for Immune Checkpoint Therapy. Nat. Immunol. 2024, 25, 2186–2199. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.L.; Fitzgerald, B.G.; Paz-Ares, L.; Cappuzzo, F.; Jänne, P.A.; Peters, S.; Hirsch, F.R. New Promises and Challenges in the Treatment of Advanced Non-Small-Cell Lung Cancer. Lancet 2024, 404, 803–822. [Google Scholar] [CrossRef]
- Toracchio, L.; Carrabotta, M.; Mancarella, C.; Morrione, A.; Scotlandi, K. EphA2 in Cancer: Molecular Complexity and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 12191. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph Receptors and Ephrins in Cancer Progression. Nat. Rev. Cancer 2023, 24, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, I.Y.; You, H.J. Interactions between EGFR and EphA2 Promote Tumorigenesis through the Action of Ephexin1. Cell Death Dis. 2022, 13, 528. [Google Scholar] [CrossRef]
- Song, W.; Kim, L.C.; Han, W.; Hou, Y.; Edwards, D.N.; Wang, S.; Blackwell, T.S.; Cheng, F.; Brantley-Sieders, D.M.; Chen, J. Phosphorylation of PLCg1 by EphA2 Receptor Tyrosine Kinase Promotes Tumor Growth in Lung Cancer. Mol. Cancer Res. 2020, 18, 1735–1743. [Google Scholar] [CrossRef]
- Amato, K.R.; Wang, S.; Hastings, A.K.; Youngblood, V.M.; Santapuram, P.R.; Chen, H.; Cates, J.M.; Colvin, D.C.; Ye, F.; Brantley-Sieders, D.M.; et al. Genetic and Pharmacologic Inhibition of EPHA2 Promotes Apoptosis in NSCLC. J. Clin. Investig. 2014, 124, 2037–2049. [Google Scholar] [CrossRef]
- Amato, K.R.; Wang, S.; Tan, L.; Hastings, A.K.; Song, W.; Lovly, C.M.; Meador, C.B.; Ye, F.; Lu, P.; Balko, J.M.; et al. EPHA2 Blockade Overcomes Acquired Resistance to EGFR Kinase Inhibitors in Lung Cancer. Cancer Res. 2016, 76, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Iwahori, K.; Kakarla, S.; Velasquez, M.P.; Yu, F.; Yi, Z.; Gerken, C.; Song, X.T.; Gottschalk, S. Engager T Cells: A New Class of Antigen-Specific T Cells That Redirect Bystander T Cells. Mol. Ther. 2015, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, S.; Sun, M.; Chen, W.; Xu, X.; Zeng, Z.; Tang, Y.; Dong, Y.; Chang, A.H.; Zhao, Q. Chimeric Antigen Receptor-Modified T Cells Redirected to EphA2 for the Immunotherapy of Non-Small Cell Lung Cancer. Transl. Oncol. 2018, 11, 11–17. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.Y.; Kim, S.I.; Bae, J.Y.; Hong, J.T.; Jo, S.; Kim, J.H.; Chung, H.Y.; Kim, T.D. Developing CAR-T/NK Cells That Target EphA2 for Non-Small Cell Lung Cancer Treatment. Front. Immunol. 2025, 16, 1448438. [Google Scholar] [CrossRef]
- Taylor, H.; Campbell, J.; Nobes, C.D. Ephs and Ephrins. Curr. Biol. 2017, 27, R90–R95. [Google Scholar] [CrossRef]
- Wilson, K.; Shiuan, E.; Brantley-Sieders, D.M. Oncogenic Functions and Therapeutic Targeting of EphA2 in Cancer. Oncogene 2021, 40, 2483–2495. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph Receptors and Ephrins in Cancer: Bidirectional Signalling and Beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Shiuan, E.; Chen, J. Eph Receptor Tyrosine Kinases in Tumor Immunity. Cancer Res. 2016, 76, 6452–6457. [Google Scholar] [CrossRef]
- Hyeon, J.; Cho, S.Y.; Hong, M.E.; Kang, S.Y.; Do, I.; Im, Y.H.; Cho, E.Y. NanoString NCounter® Approach in Breast Cancer: A Comparative Analysis with Quantitative Real-Time Polymerase Chain Reaction, In Situ Hybridization, and Immunohistochemistry. J. Breast Cancer 2017, 20, 286–296. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, M.; Ihle, M.A.; Hein, R.; Merkelbach-Bruse, S.; Scheel, A.H.; Siemanowski, J.; Brägelmann, J.; Kron, A.; Abedpour, N.; Ueckeroth, F.; et al. K-Ras Mutation Subtypes in NSCLC and Associated Co-Occuring Mutations in Other Oncogenic Pathways. J. Thorac. Oncol. 2019, 14, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Markosyan, N.; Li, J.; Sun, Y.H.; Richman, L.P.; Lin, J.H.; Yan, F.; Quinones, L.; Sela, Y.; Yamazoe, T.; Gordon, N.; et al. Tumor Cell-Intrinsic EPHA2 Suppresses Antitumor Immunity by Regulating PTGS2 (COX-2). J. Clin. Investig. 2019, 129, 3594–3609. [Google Scholar] [CrossRef]
- Kim, S.I.; Cassella, C.R.; Byrne, K.T. Tumor Burden and Immunotherapy: Impact on Immune Infiltration and Therapeutic Outcomes. Front. Immunol. 2021, 11, 629722. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-Cell Exhaustion in the Tumor Microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef]
- Pauken, K.E.; Wherry, E.J. Overcoming T Cell Exhaustion in Infection and Cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Barquilla, A.; Pasquale, E.B. Eph Receptors and Ephrins: Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 465–487. [Google Scholar] [CrossRef]
- Takasugi, M.; Okada, R.; Takahashi, A.; Virya Chen, D.; Watanabe, S.; Hara, E. Small Extracellular Vesicles Secreted from Senescent Cells Promote Cancer Cell Proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Sun, C.; Pan, Y. Whole-exome Sequencing Reveals Lewis Lung Carcinoma Is a Hypermutated Kras/Nras–Mutant Cancer with Extensive Regional Mutation Clusters in Its Genome. Sci. Rep. 2024, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Yeddula, N.; Xia, Y.; Ke, E.; Beumer, J.; Verma, I.M. Screening for Tumor Suppressors: Loss of Ephrin Receptor A2 Cooperates with Oncogenic KRas in Promoting Lung Adenocarcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, E6476–E6485. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.; Zaragkoulias, A.; Salvador-Barbero, B.; Parfitt, G.J.; Alatsatianos, M.; Padilha, A.; Porazinski, S.; Woolley, T.E.; Morton, J.P.; Sansom, O.J.; et al. EPHA2-Dependent Outcompetition of KRASG12D Mutant Cells by Wild-Type Neighbors in the Adult Pancreas. Curr. Biol. 2021, 31, 2550–2560.e5. [Google Scholar] [CrossRef] [PubMed]
- Brantley-Sieders, D.M. Clinical Relevance of Ephs and Ephrins in Cancer: Lessons from Breast, Colorectal, and Lung Cancer Profiling. Semin. Cell Dev. Biol. 2012, 23, 102–108. [Google Scholar] [CrossRef]
- Kania, A.; Klein, R. Mechanisms of Ephrin-Eph Signalling in Development, Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef]
- Miao, H.; Burnett, E.; Kinch, M.; Simon, E.; Wang, B. Activation of EphA2 Kinase Suppresses Integrin Function and Causes Focal-Adhesion-Kinase Dephosphorylation. Nat. Cell Biol. 2000, 2, 62–69. [Google Scholar] [CrossRef]
- Beauchamp, A.; Lively, M.O.; Mintz, A.; Gibo, D.; Wykosky, J.; Debinski, W. EphrinA1 Is Released in Three Forms from Cancer Cells by Matrix Metalloproteases. Mol. Cell Biol. 2012, 32, 3253–3264. [Google Scholar] [CrossRef]
- Stuart, J.A.; Harper, J.A.; Brindle, K.M.; Jekabsons, M.B.; Brand, M.D. Physiological Levels of Mammalian Uncoupling Protein 2 Do Not Uncouple Yeast Mitochondria. J. Biol. Chem. 2001, 276, 18633–18639. [Google Scholar] [CrossRef]
- Verhagen, A. Using FLAG Epitope-Tagged Proteins for Coimmunoprecipitation of Interacting Proteins. Cold Spring Harb. Protoc. 2006, 2006, pdb.prot4557. [Google Scholar] [CrossRef] [PubMed]
- Mui, M.Z.; Kucharski, M.; Miron, M.-J.; Hur, W.S.; Berghuis, A.M.; Blanchette, P.; Branton, P.E. Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication. PLoS Pathog. 2013, 9, e1003742. [Google Scholar] [CrossRef]
- Saito, T.; Matsuba, Y.; Yamazaki, N.; Hashimoto, S.; Saido, T.C. Calpain Activation in Alzheimer’s Model Mice Is an Artifact of APP and Presenilin Overexpression. J. Neurosci. 2016, 36, 9933–9936. [Google Scholar] [CrossRef]
- Liu, L.; Ito, W.; Morozov, A. Overexpression of Channelrhodopsin-2 Interferes with the GABAb Receptor-Mediated Depression of GABA Release from the Somatostatin-Containing Interneurons of the Prefrontal Cortex. Neurophotonics 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.R.; Lingerak, R.; Kim, S.; Wang, B. Abstract A084: Regulation of Anti-Tumor Immune Response by EphA2 Receptor Tyrosine Kinase. Cancer Immunol. Res. 2025, 13, A084. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiuan, E.; Wang, S.; Brantley-Sieders, D.M. Tumor-Specific EphA2 Receptor Tyrosine Kinase Inhibits Anti-Tumor Immunity by Recruiting Suppressive Myeloid Populations in Murine Models of Non-Small Cell Lung Cancer. Cancers 2025, 17, 2693. https://doi.org/10.3390/cancers17162693
Shiuan E, Wang S, Brantley-Sieders DM. Tumor-Specific EphA2 Receptor Tyrosine Kinase Inhibits Anti-Tumor Immunity by Recruiting Suppressive Myeloid Populations in Murine Models of Non-Small Cell Lung Cancer. Cancers. 2025; 17(16):2693. https://doi.org/10.3390/cancers17162693
Chicago/Turabian StyleShiuan, Eileen, Shan Wang, and Dana M. Brantley-Sieders. 2025. "Tumor-Specific EphA2 Receptor Tyrosine Kinase Inhibits Anti-Tumor Immunity by Recruiting Suppressive Myeloid Populations in Murine Models of Non-Small Cell Lung Cancer" Cancers 17, no. 16: 2693. https://doi.org/10.3390/cancers17162693
APA StyleShiuan, E., Wang, S., & Brantley-Sieders, D. M. (2025). Tumor-Specific EphA2 Receptor Tyrosine Kinase Inhibits Anti-Tumor Immunity by Recruiting Suppressive Myeloid Populations in Murine Models of Non-Small Cell Lung Cancer. Cancers, 17(16), 2693. https://doi.org/10.3390/cancers17162693





