Spontaneous Mesotheliomas in Germline Bap1 Heterozygous Mice from Different Genetic Backgrounds
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Generation of the Original Bap1-Knockout Mouse Model in FVB/N Background
2.2. Generation of Bap1-Knockout Mice in 129/Sv and C57BL/6 Backgrounds
2.3. Generation of Bap1 W and Bap1 L Knock-In Mouse Models in FVB/N Background
2.4. Constitutive Heterozygous Bap1- Knockout Mouse Model in a C57BL/6 Background
2.5. Analysis of Spontaneous Tumors in Bap1-Mutant Mice
2.6. Genotyping
2.7. Identification of Published Datasets
2.8. Statistics
2.8.1. Fisher’s Exact Test
2.8.2. Bayesian Hierarchical Model
3. Results
3.1. Spontaneous Mesotheliomas in Bap1-Mutant Mice and WT Littermates
3.2. Historical Data Identified
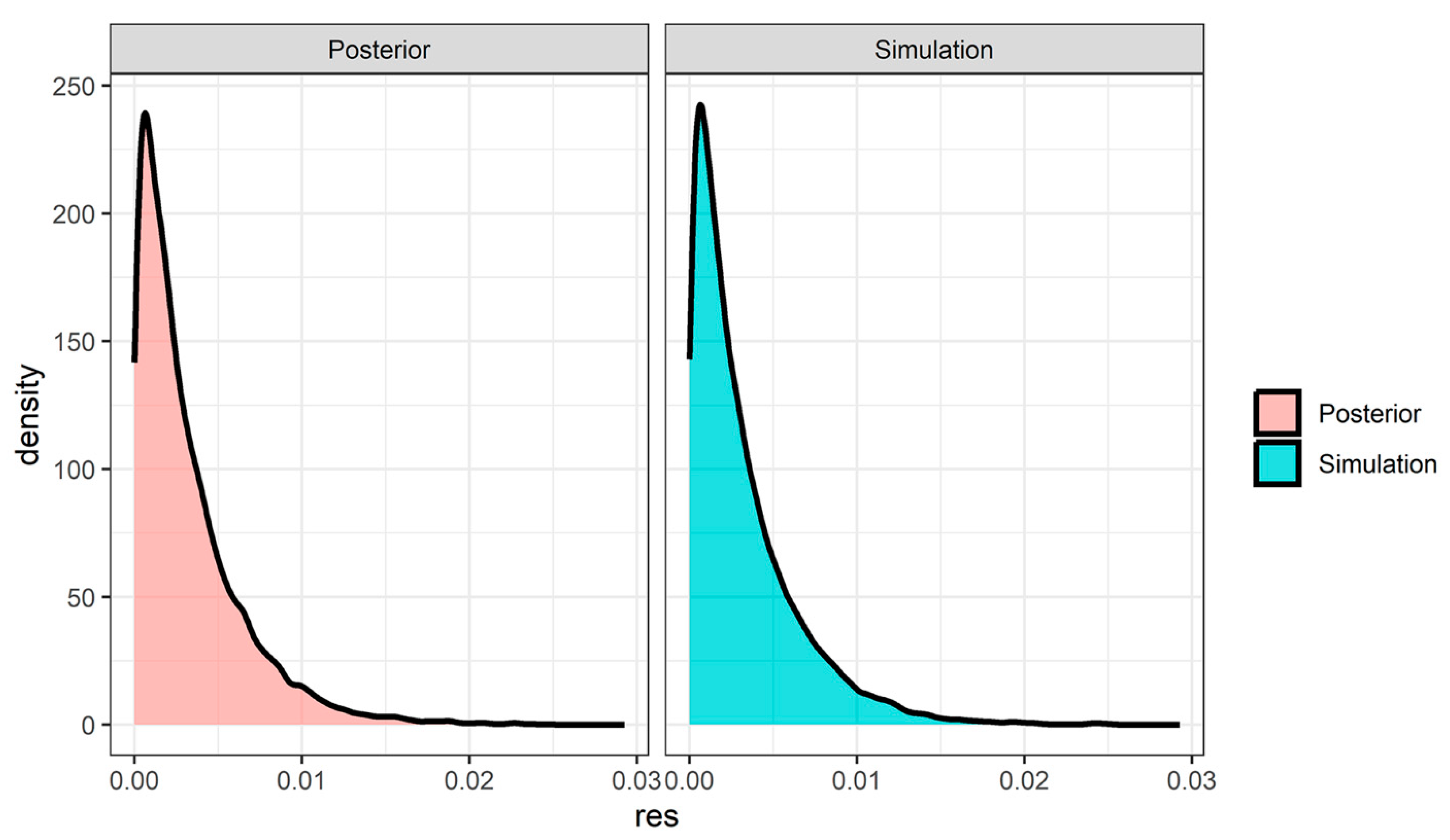
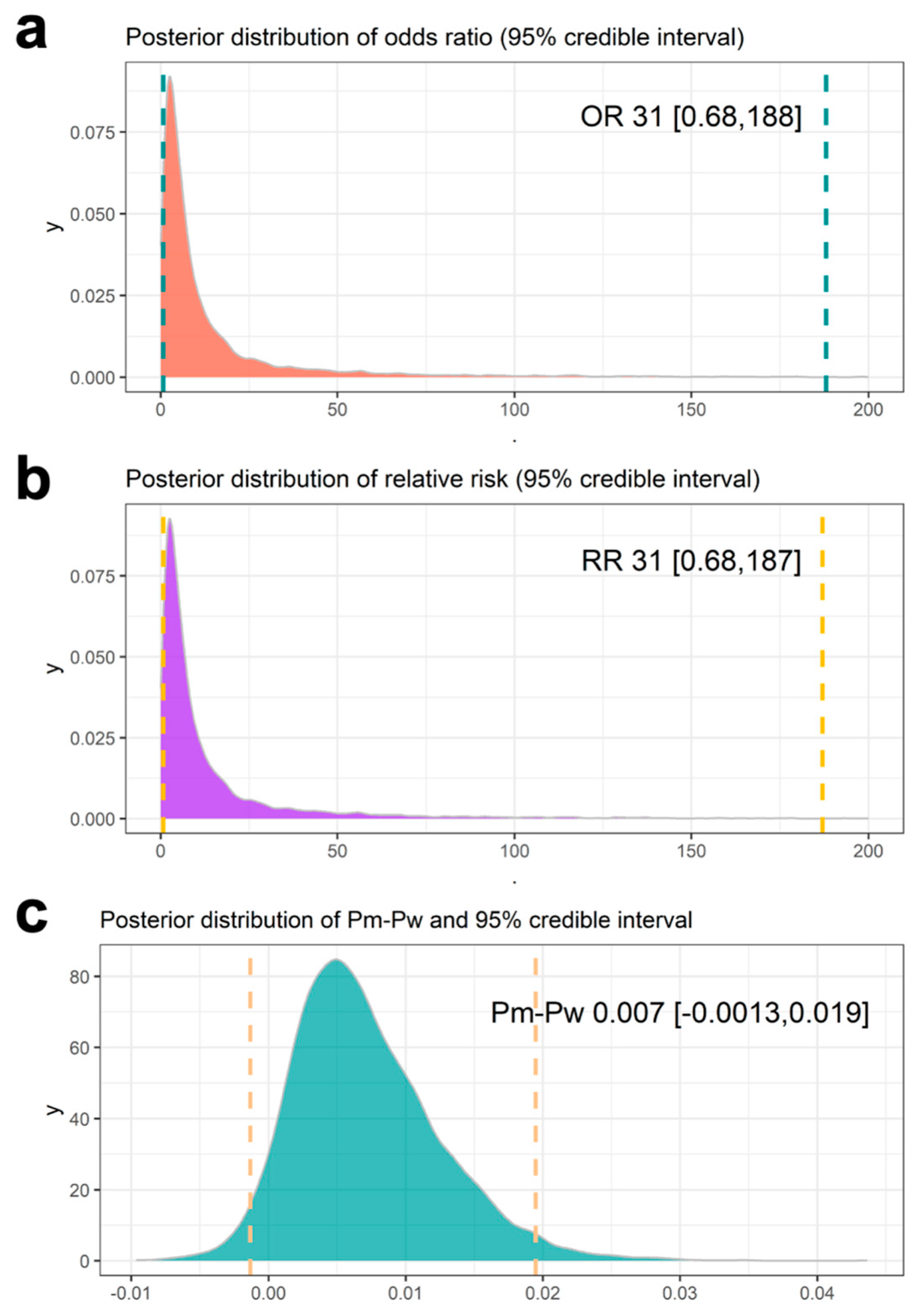
3.3. Results Using the Same Mesothelioma Occurrence Rate Across the Historical Data
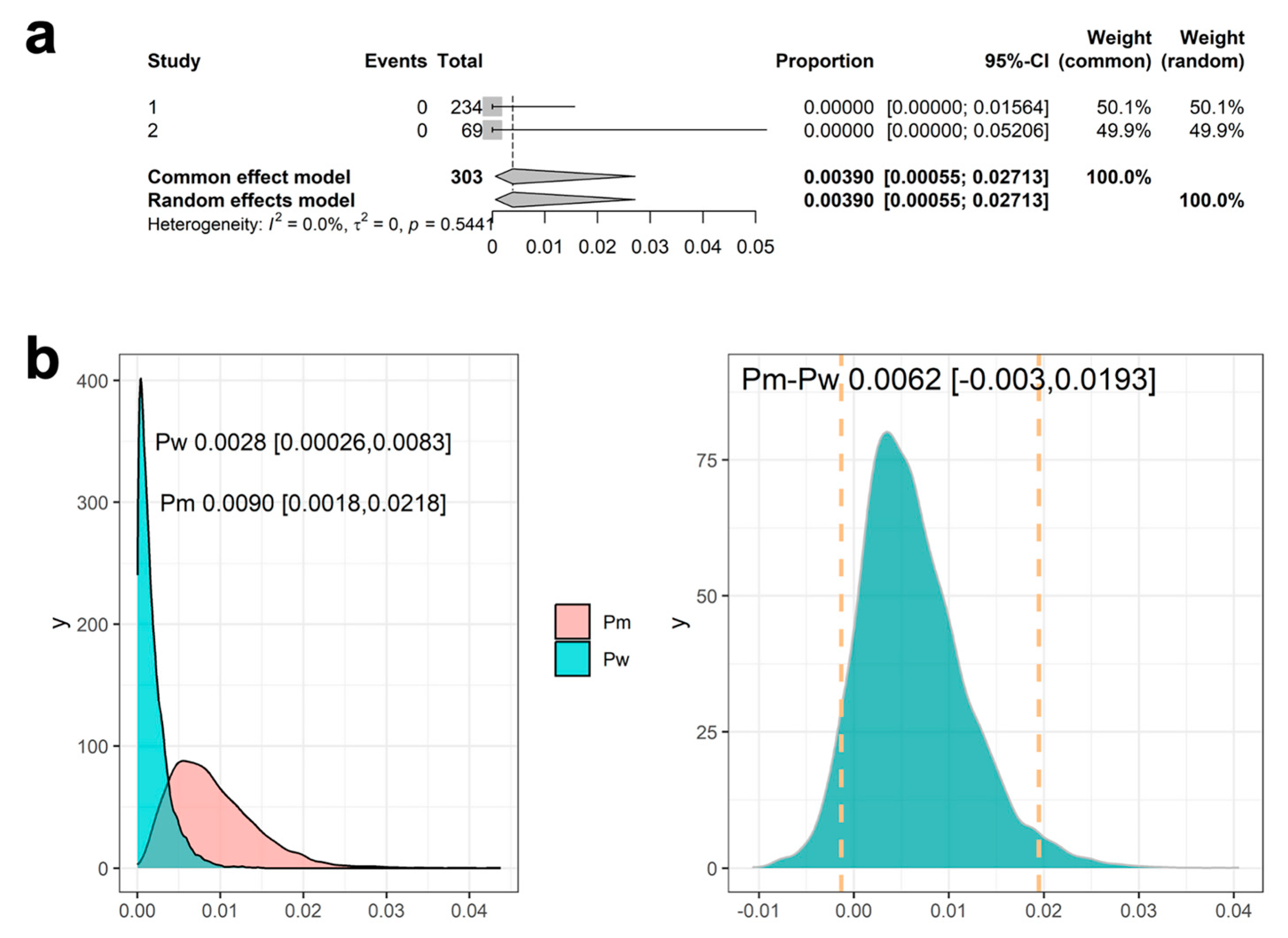
3.4. Results Using Meta-Analysis to Combine Mesothelioma Occurrence Rate in Historical Data
3.5. Results Using Meta-Analytic Predictive Priors for Historical Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| Bap1 | Mouse BRCA1-Associated Protein-1 Gene |
| BAP1 | Human BRCA1-Associated Protein-1 Gene |
| BAP1-TPDS | BAP1 Tumor Predisposition Syndrome |
| CI | Confidence Interval |
| GEMMs | Genetically Engineered Mouse Models |
| HCD | Historical Control Dataset |
| IACUC | Institutional Animal Care and Use Committee |
| IHC | Immunohistochemical |
| MAP | Meta-Analytic Predictive |
| MCMC | Markov Chain Monte Carlo |
| ML | Maximum-Likelihood |
| MPM | Malignant Pleural Mesothelioma |
| OR | Odds Ratio |
| PV | Pathogenic Variant |
| RD | Risk Difference |
| RR | Risk Ratio |
| WT | Wild-Type |
| ZFN | Zinc Finger Nuclease |
References
- Pilarski, R.; Rai, K.; Cebulla, C.; Abdel-Rahman, M. BAP1 Tumor Predisposition Syndrome. In GeneReviews(R); [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mefford, H.C., Stephens, K., Amemiya, A., Ledbetter, N., Eds.; University of Washington: Seattle, WA, USA, 2016. [Google Scholar]
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; de la Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. [Google Scholar] [CrossRef]
- Xu, J.; Kadariya, Y.; Cheung, M.; Pei, J.; Talarchek, J.; Sementino, E.; Tan, Y.; Menges, C.W.; Cai, K.Q.; Litwin, S.; et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014, 74, 4388–4397. [Google Scholar] [CrossRef]
- Napolitano, A.; Pellegrini, L.; Dey, A.; Larson, D.; Tanji, M.; Flores, E.G.; Kendrick, B.; Lapid, D.; Powers, A.; Kanodia, S.; et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016, 35, 1996–2002. [Google Scholar] [CrossRef]
- Kadariya, Y.; Cheung, M.; Xu, J.; Pei, J.; Sementino, E.; Menges, C.W.; Cai, K.Q.; Rauscher, F.J.; Klein-Szanto, A.J.; Testa, J.R. Bap1 is a bona fide tumor suppressor: Genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res. 2016, 76, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, Y.; Sementino, E.; Ruan, M.; Cheung, M.; Hadikhani, P.; Osmanbeyoglu, H.U.; Klein-Szanto, A.J.; Cai, K.; Testa, J.R. Low exposures to amphibole or serpentine asbestos in germline Bap1-mutant mice induce mesothelioma characterized by an immunosuppressive tumor microenvironment. Cancer Res. Commun. 2024, 4, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef]
- Kadariya, Y.; Sementino, E.; Hua, X.; Kappes, D.J.; Testa, J.R. Modeling malignant mesothelioma in genetically engineered mice. Curr. Protoc. 2025, 5, e70086. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Seshasayee, D.; Noubade, R.; French, D.M.; Liu, J.; Chaurushiya, M.S.; Kirkpatrick, D.S.; Pham, V.C.; Lill, J.R.; Bakalarski, C.E.; et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 2012, 337, 1541–1546. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis, 3rd ed.; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mayer, B.; Allgoewer, A.; Muche, R. Essential standards of biometrical sample size calculation for animal experiments in preclinical research in terms of the 3R. Berl. Munch. Tierarztl. Wochenschr. 2018, 131, 272–278. [Google Scholar]
- Rover, C.; Bender, R.; Dias, S.; Schmid, C.H.; Schmidli, H.; Sturtz, S.; Weber, S.; Friede, T. On weakly informative prior distributions for the heterogeneity parameter in Bayesian random-effects meta-analysis. Res. Synth. Methods 2021, 12, 448–474. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Stijnen, T.; Hamza, T.H.; Ozdemir, P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010, 29, 3046–3067. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, B.; Capkun-Niggli, G.; Branson, M.; Spiegelhalter, D.J. Summarizing historical information on controls in clinical trials. Clin. Trials 2010, 7, 5–18. [Google Scholar] [CrossRef]
- Schmidli, H.; Gsteiger, S.; Roychoudhury, S.; O’Hagan, A.; Spiegelhalter, D.; Neuenschwander, B. Robust meta-analytic-predictive priors in clinical trials with historical control information. Biometrics 2014, 70, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Team, S.D. RStan: The R interface to Stan. 2023. Available online: https://mc-stan.org/rstan/ (accessed on 11 June 2025).
- Nielsen, D.M.; Hsu, M.; Zapata, M., 3rd; Ciavarra, G.; van Zyl, L. Bayesian analysis of the rate of spontaneous malignant mesothelioma among BAP1 mutant mice in the absence of asbestos exposure. Sci. Rep. 2025, 15, 169. [Google Scholar] [CrossRef]
- Huang, P.; Duda, D.G.; Jain, R.K.; Fukumura, D. Histopathologic findings and establishment of novel tumor lines from spontaneous tumors in FVB/N mice. Comp. Med. 2008, 58, 253–263. [Google Scholar]
- Panchenko, A.V.; Popovich, I.G.; Trashkov, A.P.; Egormin, P.A.; Yurova, M.N.; Tyndyk, M.L.; Gubareva, E.A.; Artyukin, I.N.; Vasiliev, A.G.; Khaitsev, N.V.; et al. Biomarkers of aging, life span and spontaneous carcinogenesis in the wild type and HER-2 transgenic FVB/N female mice. Biogerontology 2016, 17, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Minaai, M.; Takinishi, Y.; Pagano, I.; Yang, H. Preventive and therapeutic opportunities: Targeting BAP1 and/or HMGB1 pathways to diminish the burden of mesothelioma. J. Transl. Med. 2023, 21, 749. [Google Scholar] [CrossRef]
- Everett, R. Factors affecting spontaneous tumor incidence rates in mice: A literature review. Crit. Rev. Toxicol. 1984, 13, 235–251. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, J.; Xu, S.; She, T.; Zhang, Y.; Sun, Y.; Wen, M.; Jiang, T.; Xiong, Y.; Lei, J. Experimental mouse models for translational human cancer research. Front. Immunol. 2023, 14, 1095388. [Google Scholar] [CrossRef]
- Giknis, M.L.A.; Clifford, C.B. Spontaneous Neoplastic Lesions in the CrI:CD-1(ICR) Mouse in Control Groups from 18 Month to 2 Year Studies; Charles River Laboratories: Wilmington, MA, USA, 2005. [Google Scholar]
- Maita, K.; Hirano, M.; Harada, T.; Mitsumori, K.; Yoshida, A.; Takahashi, K.; Nakashima, N.; Kitazawa, T.; Enomoto, A.; Inui, K.; et al. Mortality, major cause of moribundity, and spontaneous tumors in CD-1 mice. Toxicol. Pathol. 1988, 16, 340–349. [Google Scholar] [CrossRef]
- Betti, M.; Aspesi, A.; Ferrante, D.; Sculco, M.; Righi, L.; Mirabelli, D.; Napoli, F.; Rondon-Lagos, M.; Casalone, E.; Vignolo Lutati, F.; et al. Sensitivity to asbestos is increased in patients with mesothelioma and pathogenic germline variants in BAP1 or other DNA repair genes. Genes Chromosomes Cancer 2018, 57, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Sculco, M.; La Vecchia, M.; Aspesi, A.; Pinton, G.; Clavenna, M.G.; Casalone, E.; Allione, A.; Grosso, F.; Libener, R.; Muzio, A.; et al. Malignant pleural mesothelioma: Germline variants in DNA repair genes may steer tailored treatment. Eur. J. Cancer 2022, 163, 44–54. [Google Scholar] [CrossRef]
- Panou, V.; Gadiraju, M.; Wolin, A.; Weipert, C.M.; Skarda, E.; Husain, A.N.; Patel, J.D.; Rose, B.; Zhang, S.R.; Weatherly, M.; et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J. Clin. Oncol. 2018, 36, 2863–2871. [Google Scholar] [CrossRef]
- Hassan, R.; Morrow, B.; Thomas, A.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Gadiraju, M.; Panou, V.; Gao, S.; Mian, I.; et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 9008–9013. [Google Scholar] [CrossRef]
- Carbone, M.; Harbour, J.W.; Brugarolas, J.; Bononi, A.; Pagano, I.; Dey, A.; Krausz, T.; Pass, H.I.; Yang, H.; Gaudino, G. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov. 2020, 10, 1103–1120. [Google Scholar] [CrossRef]
- Field, M.G.; Durante, M.A.; Anbunathan, H.; Cai, L.Z.; Decatur, C.L.; Bowcock, A.M.; Kurtenbach, S.; Harbour, J.W. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat. Commun. 2018, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.A.; Field, M.G.; Sanchez, M.I.; Covington, K.R.; Decatur, C.L.; Dubovy, S.R.; Harbour, J.W. Genomic evolution of uveal melanoma arising in ocular melanocytosis. Cold Spring Harb. Mol. Case Stud. 2019, 5, a004051. [Google Scholar] [CrossRef] [PubMed]


| Mice with Various Bap1 Heterozygous Mutations in Different Genetic Backgrounds | Total No. Mice | No. Mice with Mesothelioma | % Mice with Mesothelioma |
|---|---|---|---|
| Bap1+/−, Bap1+/L, Bap1+/W FVB/N [5] | 93 | 2 | 2.15 |
| Bap1+/− FVB/N | 54 | 0 | 0.00 |
| Bap1+/− C57BL/6 | 62 | 0 | 0.00 |
| Bap1+/− 129/Sv | 59 | 0 | 0.00 |
| Bap1+/− C57BL/6 (Genentech) | 61 | 0 | 0.00 |
| Total Bap1-mutant mice | 329 | 2 | 0.61 |
| Bap1+/+ (WT) littermates of mice listed above | |||
| Bap1+/+ FVB/N [5] | 43 | 0 | 0.00 |
| Bap1+/+ FVB/N | 20 | 0 | 0.00 |
| Bap1+/+ C57BL/6 | 57 | 0 | 0.00 |
| Bap1+/+ 129/Sv | 43 | 0 | 0.00 |
| Bap1+/+ C57BL/6 (Genentech) | 64 | 0 | 0.00 |
| Total WT littermates | 227 | 0 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadariya, Y.; Zhang, L.; Sementino, E.; Ross, E.; Testa, J.R. Spontaneous Mesotheliomas in Germline Bap1 Heterozygous Mice from Different Genetic Backgrounds. Cancers 2025, 17, 2692. https://doi.org/10.3390/cancers17162692
Kadariya Y, Zhang L, Sementino E, Ross E, Testa JR. Spontaneous Mesotheliomas in Germline Bap1 Heterozygous Mice from Different Genetic Backgrounds. Cancers. 2025; 17(16):2692. https://doi.org/10.3390/cancers17162692
Chicago/Turabian StyleKadariya, Yuwaraj, Li Zhang, Eleonora Sementino, Eric Ross, and Joseph R. Testa. 2025. "Spontaneous Mesotheliomas in Germline Bap1 Heterozygous Mice from Different Genetic Backgrounds" Cancers 17, no. 16: 2692. https://doi.org/10.3390/cancers17162692
APA StyleKadariya, Y., Zhang, L., Sementino, E., Ross, E., & Testa, J. R. (2025). Spontaneous Mesotheliomas in Germline Bap1 Heterozygous Mice from Different Genetic Backgrounds. Cancers, 17(16), 2692. https://doi.org/10.3390/cancers17162692






