Laparoscopic Versus Robotic Completely Intracorporeal Jejunal Pouch Reconstruction After Gastrectomy: A Single-Center Analysis from Germany
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Patient in French position. The surgeon stands between the patient’s legs. First assistant stands on the left side of the patient, second assistant stands on the right side.
- Port trocar placement:
- -
- supraumbilical incision, insertion of an optical trocar and establishment of pneumoperitoneum
- -
- intraabdominal inspection for exclusion of signs of peritoneal carcinosis as well as obvious liver metastasis
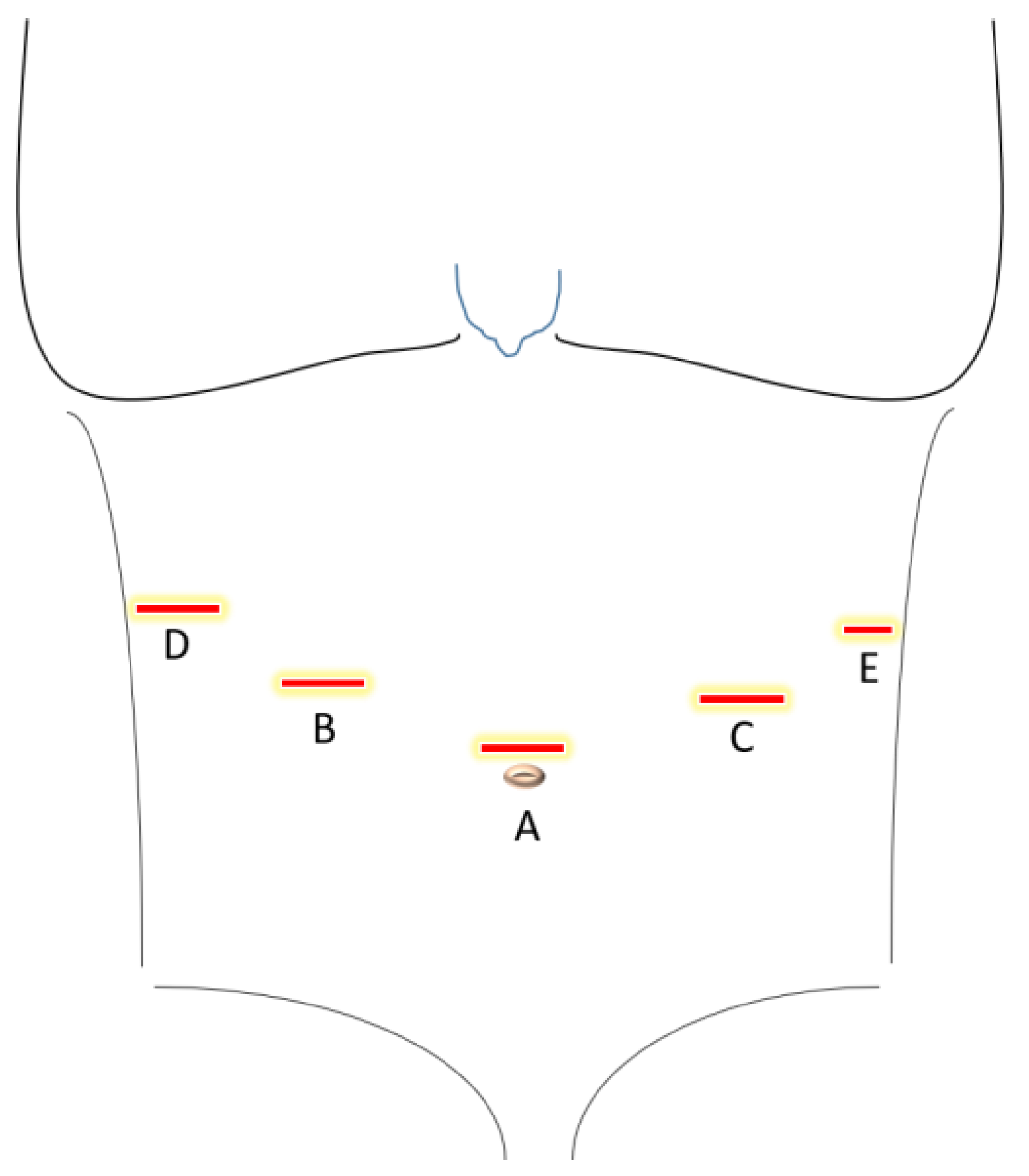
- Main and assistant trocar placement: three 12 mm and one 5 mm trocars in the upper abdomen as described in Figure 1.
- Mobilization of the greater omentum from the transverse colon.
- Isolation and ligation of the left gastroepiploic vessels near the splenic vessels using Hem-o-lok clips.
- Dissection of the short gastric vessels near the spleen.
- Mobilization of the gastric fundus and exposure of the left diaphragmatic crus.
- Lymphadenectomy along the splenic artery (lymph node (LN) stations 10 and 11).
- Mobilization of the hepatic flexure and duodenum.
- Ligation of the right gastroepiploic vessels (LN station 6).
- Suprapyloric dissection:
- -
- liver retraction with a liver paddle. Incision in the lesser omentum and exclusion of an aberrant liver artery. Mobilization of the abdominal esophagus.
- -
- lymphadenectomy along the hepatic artery, left gastric artery, and celiac trunk (LN stations 7 and 9).
- -
- completion of the lymphadenectomy along the splenic artery (LN station 11) and extension of the dissection to the hepatoduodenal ligament (LN stations 5, 8, and 12).
- Ligation of the coronary gastric vein (LN station 8).
- Stapling of the duodenum 2 cm post pylorus.
- Ligation of the left gastric artery (LN station 2).
- Tying of the esophagus with a Mersilene band.
- Specimen retrieval:
- -
- mini-laparotomy and insertion of an Alexis retractor. Division of the esophagus at the abdominal portion and specimen retrieval. Frozen section for conformation of tumor-free margins.
- Cholecystectomy.
- Reconstruction:
- -
- a 20 cm long Roux jejunal limb is used for the establishment of a 15 cm long Hunt-Lawrence pouch.
- -
- End-to-side esophagojejunostomy using a 29 mm circular stapler.
- -
- Side-to-side jejunojejunostomy 45 cm distal to the esophagojejunostomy for the biliary limb (Roux-en-Y reconstruction).
- Final steps:
- -
- check for hemostasis and inspection of the anastomoses.
- -
- placement of a drain at the esophagojejunostomy.
- -
- fascia and wound closure.
3. Results
3.1. Patient Demographics
3.2. Operative Outcomes
3.3. Oncologic Aspects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| ARDS | Acute respiratory distress syndrome |
| BMI | Body mass index |
| CDH1 | Cadherin-1 gene |
| ICU | Intensive care unit |
| EA | Ethics approval code |
| MOT | Mean operating time |
| LN | Lymph node |
| pN | Pathologic nodal Stage |
| pT | Pathologic tumor Stage |
| R0 | Complete tumor resection with negative margins |
| R1 | Microscopic residual tumor |
| R2 | Macroscopic residual tumor |
| yr | years |
References
- Lin, J.-L.; Lin, J.-X.; Lin, G.-T.; Huang, C.-M.; Zheng, C.-H.; Xie, J.-W.; Wang, J.; Lu, J.; Chen, Q.-Y.; Li, P. Global Incidence and Mortality Trends of Gastric Cancer and Predicted Mortality of Gastric Cancer by 2035. BMC Public. Health 2024, 24, 1763. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Arawker, M.H.; Sun, Q.; Jiang, M.; Wang, L.; Wang, Y.; Ali, M.; Wang, D. Robotic Versus Laparoscopic Gastrectomy for Gastric Cancer: A Mega Meta-Analysis. Front. Surg. 2022, 9, 895976. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Esposito, G.; Magistri, P.; Serra, V.; Guidetti, C.; Olivieri, T.; Catellani, B.; Assirati, G.; Ballarin, R.; Di Sandro, S.; et al. Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: The Largest Meta-Analysis. Int. J. Surg. 2020, 82, 210–228. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Liu, Y.; Wang, Z. Perioperative Outcomes of Robotic versus Laparoscopic Distal Gastrectomy for Gastric Cancer: A Meta-Analysis of Propensity Score-Matched Studies and Randomized Controlled Trials. BMC Surg. 2022, 22, 427. [Google Scholar] [CrossRef]
- Buhl, K.; Lehnert, T.; Schlag, P.; Herfarth, C. Reconstruction after Gastrectomy and Quality of Life. World J. Surg. 1995, 19, 558–564. [Google Scholar] [CrossRef]
- Poorman, C.E.; Patel, A.D.; Davis, S.S.; Lin, E. Laparoscopic Hunt–Lawrence Jejunal Pouch for Reconstruction After Total Gastrectomy for Gastric Cancer. J. Laparoendosc. Adv. Surg. Tech. 2021, 31, 1051–1054. [Google Scholar] [CrossRef]
- Li, Z.; Wei, B.; Zhou, Y.; Li, T.; Li, J.; Zhou, Z.; She, J.; Qin, X.; Hu, J.; Li, Y.-X.; et al. Long-Term Oncological Outcomes of Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: Multicentre Cohort Study. Br. J. Surg. 2024, 111, znad435. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.L.; Pawlak, M.M.; Simons, M.P.; Aufenacker, T.; Balla, A.; Berger, C.; Berrevoet, F.; de Beaux, A.C.; East, B.; Henriksen, N.A.; et al. Midline Incisional Hernia Guidelines: The European Hernia Society. Br. J. Surg. 2023, 110, 1732–1768. [Google Scholar] [CrossRef]
- Luijendijk, R.W.; Jeekel, J.; Storm, R.K.; Schutte, P.J.; Hop, W.C.; Drogendijk, A.C.; Huikeshoven, F.J. The Low Transverse Pfannenstiel Incision and the Prevalence of Incisional Hernia and Nerve Entrapment. Ann. Surg. 1997, 225, 365–369. [Google Scholar] [CrossRef]
- Stoyanova, A.; Berg, A.-K.; Beyer, K. A Robotic Completely Intercorporeal Jejunal Pouch Reconstruction after Gastrectomy. Curr. Oncol. 2022, 29, 8600–8608. [Google Scholar] [CrossRef]
- Brenkman, H.J.F.; Correa-Cote, J.; Ruurda, J.P.; van Hillegersberg, R. A Step-Wise Approach to Total Laparoscopic Gastrectomy with Jejunal Pouch Reconstruction: How and Why We Do It. J. Gastrointest. Surg. 2016, 20, 1908–1915. [Google Scholar] [CrossRef]
- Rivero-Moreno, Y.; Echevarria, S.; Vidal-Valderrama, C.; Pianetti, L.; Cordova-Guilarte, J.; Navarro-Gonzalez, J.; Acevedo-Rodríguez, J.; Dorado-Avila, G.; Osorio-Romero, L.; Chavez-Campos, C.; et al. Robotic Surgery: A Comprehensive Review of the Literature and Current Trends. Cureus 2023, 15, e42370. [Google Scholar] [CrossRef]
- Wong, S.W.; Crowe, P. Visualisation Ergonomics and Robotic Surgery. J. Robot. Surg. 2023, 17, 1873–1878. [Google Scholar] [CrossRef]
- Bradley, E.L.; Isaacs, J.; Hersh, T.; Davidson, E.D.; Millikan, W. Nutritional Consequences of Total Gastrectomy. Ann. Surg. 1975, 182, 415–429. [Google Scholar] [CrossRef]
- Roberts, G.; Hardwick, R.; Fitzgerald, R.C. Decision Making, Quality of Life and Prophylactic Gastrectomy in Carriers of Pathogenic CDH1 Mutations. Transl. Gastroenterol. Hepatol. 2017, 2, 21. [Google Scholar] [CrossRef]
- Worster, E.; Liu, X.; Richardson, S.; Hardwick, R.H.; Dwerryhouse, S.; Caldas, C.; Fitzgerald, R.C. The Impact of Prophylactic Total Gastrectomy on Health-Related Quality of Life: A Prospective Cohort Study. Ann. Surg. 2014, 260, 87–93. [Google Scholar] [CrossRef]
- Kim, A.; Kim, B.S.; Yook, J.H.; Kim, B.S. Optimal proximal resection margin distance for gastrectomy in advanced gastric cancer. World J. Gastroenterol. 2020, 26, 2232–2246. [Google Scholar] [CrossRef] [PubMed]
- Squires, M.H.; Kooby, D.A.; Pawlik, T.M.; Weber, S.M.; Poultsides, G.; Schmidt, C.; Votanopoulos, K.; Fields, R.C.; Ejaz, A.; Acher, A.W.; et al. Utility of the Proximal Margin Frozen Section for Resection of Gastric Adenocarcinoma: A 7-Institution Study of the US Gastric Cancer Collaborative. Ann. Surg. Oncol. 2014, 21, 4202–4210. [Google Scholar] [CrossRef] [PubMed]
- Squires Iii, M.H.; Kooby, D.A.; Poultsides, G.A.; Pawlik, T.M.; Weber, S.M.; Schmidt, C.R.; Votanopoulos, K.I.; Fields, R.C.; Ejaz, A.; Acher, A.W.; et al. Is It Time to Abandon the 5-cm Margin Rule During Resection of Distal Gastric Adenocarcinoma? A Multi-Institution Study of the U.S. Gastric Cancer Collab. Ann. Surg. Oncol. 2015, 22, 1243–1251. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Lin, G.-T.; Zhong, Q.; Zheng, C.-H.; Li, P.; Xie, J.-W.; Wang, J.-B.; Lin, J.-X.; Lu, J.; Cao, L.-L.; et al. Laparoscopic Total Gastrectomy for Upper-Middle Advanced Gastric Cancer: Analysis Based on Lymph Node Noncompliance. Gastric Cancer 2020, 23, 184–194. [Google Scholar] [CrossRef]
- Nishizaki, D.; Ganeko, R.; Hoshino, N.; Hida, K.; Obama, K.; Furukawa, T.A.; Sakai, Y.; Watanabe, N. Roux-En-Y versus Billroth-I Reconstruction after Distal Gastrectomy for Gastric Cancer. Cochrane Database Syst. Rev. 2021, 9, CD012998. [Google Scholar] [CrossRef]
- Masui, T.; Kubora, T.; Nakanishi, Y.; Aoki, K.; Sugimoto, S.; Takamura, M.; Takeda, H.; Hashimoto, K.; Tokuka, A. The Flow Angle beneath the Gastrojejunostomy Predicts Delayed Gastric Emptying in Roux-En-Y Reconstruction after Distal Gastrectomy. Gastric Cancer 2012, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Realis Luc, M.; Bonomi, A.M.; Carbone, F.; Ascari, F.; de Pascale, S.; Fumagalli Romario, U. Roux-En-Y with or without Jejunal J-Pouch Reconstruction after Total Gastrectomy for Gastric Cancer: Systematic Review and Meta-Analysis of Long-Term Functional Outcomes. J. Gastrointest. Surg. 2024, 28, 291–300. [Google Scholar] [CrossRef] [PubMed]
- El Halabi, H.M.; Lawrence, W. Clinical Results of Various Reconstructions Employed after Total Gastrectomy. J. Surg. Oncol. 2008, 97, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Tuma, F. Incisional Hernia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Muysoms, F.E.; Antoniou, S.A.; Bury, K.; Campanelli, G.; Conze, J.; Cuccurullo, D.; de Beaux, A.C.; Deerenberg, E.B.; East, B.; Fortelny, R.H.; et al. European Hernia Society Guidelines on the Closure of Abdominal Wall Incisions. Hernia 2015, 19, 1–24. [Google Scholar] [CrossRef]
- Widmar, M.; Aggarwal, P.; Keskin, M.; Strombom, P.D.; Patil, S.; Smith, J.J.; Nash, G.M.; Garcia-Aguilar, J. Intracorporeal Anastomoses in Minimally Invasive Right Colectomies Are Associated With Fewer Incisional Hernias and Shorter Length of Stay. Dis. Colon. Rectum 2020, 63, 685–692. [Google Scholar] [CrossRef]
- Calini, G.; Abdalla, S.; Aziz, M.A.A.E.; Behm, K.T.; Shawki, S.F.; Mathis, K.L.; Larson, D.W. Incisional Hernia Rates between Intracorporeal and Extracorporeal Anastomosis in Minimally Invasive Ileocolic Resection for Crohn’s Disease. Langenbecks Arch. Surg. 2023, 408, 251. [Google Scholar] [CrossRef]
- Brown, R.F.; Cleary, R.K. Intracorporeal Anastomosis versus Extracorporeal Anastomosis for Minimally Invasive Colectomy. J. Gastrointest. Oncol. 2020, 11, 500–507. [Google Scholar] [CrossRef]
- Selznick, S.; Levy, J.; Bogdan, R.-M.; Hawel, J.; Elnahas, A.; Alkhamesi, N.A.; Schlachta, C.M. Laparoscopic Right Colectomies with Intracorporeal Compared to Extracorporeal Anastomotic Techniques Are Associated with Reduced Post-Operative Incisional Hernias. Surg. Endosc. 2023, 37, 5500–5508. [Google Scholar] [CrossRef]
- Frigault, J.; Avoine, S.; Drolet, S.; Letarte, F.; Bouchard, A.; Gagné, J.-P.; Thibault, C.; Grégoire, R.C.; Bouthillette, N.J.; Gosselin, M.; et al. Intracorporeal versus Extracorporeal Anastomosis in Laparoscopic Right Hemicolectomy: A Retrospective Cohort Study of Anastomotic Complications. Ann. Coloproctol. 2022, 39, 147–155. [Google Scholar] [CrossRef]
| Laparoscopic (n = 15) | Robotic (n = 12) | |
|---|---|---|
| Age (yr) | 62.2 ± 15.0 | 64.4 ± 9.0 |
| Gender male (n/%) | 9/60% | 8/66.7% |
| Body mass index (kg/m2) | 26.25 ± 4.97 | 24.8 ± 4.19 |
| Regular alcohol consumption | 3/20% | 2/16.7% |
| Smokers | 2/13.3% | 3/25% |
| Laparoscopic (n = 15) | Robotic (n = 12) | |
|---|---|---|
| MOT (minutes) | 329.8 ± 79.21 | 275.45 ± 63.74 |
| Intraoperative complications | 1 | 1 |
| Major postoperative complications | 8 | 3 |
| Pulmonary complications | 6 | 5 |
| Reintubation | 2 | 1 |
| Length of ICU stay (days) | 4.5 ± 9.6 | 1.9 ± 2.7 |
| Length of hospital stay (days) | 21.8 ± 18.2 | 15.8 ± 13.5 |
| Anastomotic leakage | 2 | 1 |
| Wound infections | 0 | 0 |
| Mortality | 0 | 1 |
| Laparoscopic (n = 15) | Robotic (n = 12) | |
|---|---|---|
| adenocarcinoma | 9 | 10 |
| signet ring carcinoma | 3 | 2 |
| other * | 3 | 0 |
| neoadjuvant therapy | 13 | 9 |
| number of harvested lymph nodes | 35.27 ± 12.3 | 35.33 ± 7.3 |
| re-resection needed | 0 | 2 |
| Laparoscopic (n = 15) | Robotic (n = 12) | ||
|---|---|---|---|
| Pathologic T-stage | pT0 pTis pT1 pT2 pT3 pT4 | 1 0 2 0 9 3 | 0 0 1 3 5 3 |
| Pathologic nodal status (pN) | pN0 pN1 pN2 pN3 | 10 1 1 3 | 6 1 4 1 |
| Disease-free gross margins (R2) Disease-free microscopic margins (R1) | 15 15 | 12 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, A.K.; Speichinger, F.; Pozios, I.; Beyer, K.; Berg, A.-K. Laparoscopic Versus Robotic Completely Intracorporeal Jejunal Pouch Reconstruction After Gastrectomy: A Single-Center Analysis from Germany. Cancers 2025, 17, 2690. https://doi.org/10.3390/cancers17162690
Stoyanova AK, Speichinger F, Pozios I, Beyer K, Berg A-K. Laparoscopic Versus Robotic Completely Intracorporeal Jejunal Pouch Reconstruction After Gastrectomy: A Single-Center Analysis from Germany. Cancers. 2025; 17(16):2690. https://doi.org/10.3390/cancers17162690
Chicago/Turabian StyleStoyanova, Ani K., Fiona Speichinger, Ioannis Pozios, Katharina Beyer, and Ann-Kathrin Berg. 2025. "Laparoscopic Versus Robotic Completely Intracorporeal Jejunal Pouch Reconstruction After Gastrectomy: A Single-Center Analysis from Germany" Cancers 17, no. 16: 2690. https://doi.org/10.3390/cancers17162690
APA StyleStoyanova, A. K., Speichinger, F., Pozios, I., Beyer, K., & Berg, A.-K. (2025). Laparoscopic Versus Robotic Completely Intracorporeal Jejunal Pouch Reconstruction After Gastrectomy: A Single-Center Analysis from Germany. Cancers, 17(16), 2690. https://doi.org/10.3390/cancers17162690






