Advances in the Molecular Biology of Chondrosarcoma for Drug Discovery and Precision Medicine
Simple Summary
Abstract
1. Introduction
2. Amplified or Deleted Genes in Chondrosarcoma
3. Mutated Genes and Aberrant Signaling Pathways in Chondrosarcoma
3.1. IDH1/2
3.2. TP53
3.3. RB1
3.4. COL2A1
3.5. Hedgehog
3.6. TGF-β1 Signaling
4. Fusion Genes in Chondrosarcoma
4.1. HEY1-NCOA2
4.2. IRF2BP2-CDX1
5. Epigenetic Alterations in Chondrosarcoma
5.1. DNA Methylation
5.2. Non-Coding RNAs
6. Approaches for In-Depth Analysis and Comprehensive Understanding of Chondrosarcoma
6.1. Integrated Multi-Omics and Multi-Layered Profiling in Chondrosarcoma
6.2. RNA Sequencing Approaches for Transcriptomic Profiling of Chondrosarcoma
7. In Vitro Preclinical Modeling of Chondrosarcoma
7.1. Chondrosarcoma Cell Lines
7.2. Chondrosarcoma Organoid Models and Other 3D Modeling Techniques
8. In Vivo Preclinical Modeling of Chondrosarcoma in Mice
8.1. Cell Line-Derived Xenograft Models of Chondrosarcoma
8.2. Genetically Engineered Xenograft Models of Chondrosarcoma
8.3. Patient-Derived Xenograft Models of Chondrosarcoma
9. Potential Targeted Therapies and Treatment Strategies for Chondrosarcoma
9.1. IDH
9.2. Hedgehog
9.3. CDK4 and Rb
9.4. HIF-2α and SIRT1
9.5. Death Receptor 5
9.6. Chondroitin Sulfate Proteoglycan 4
9.7. Therapeutic Angiogenesis Inhibition
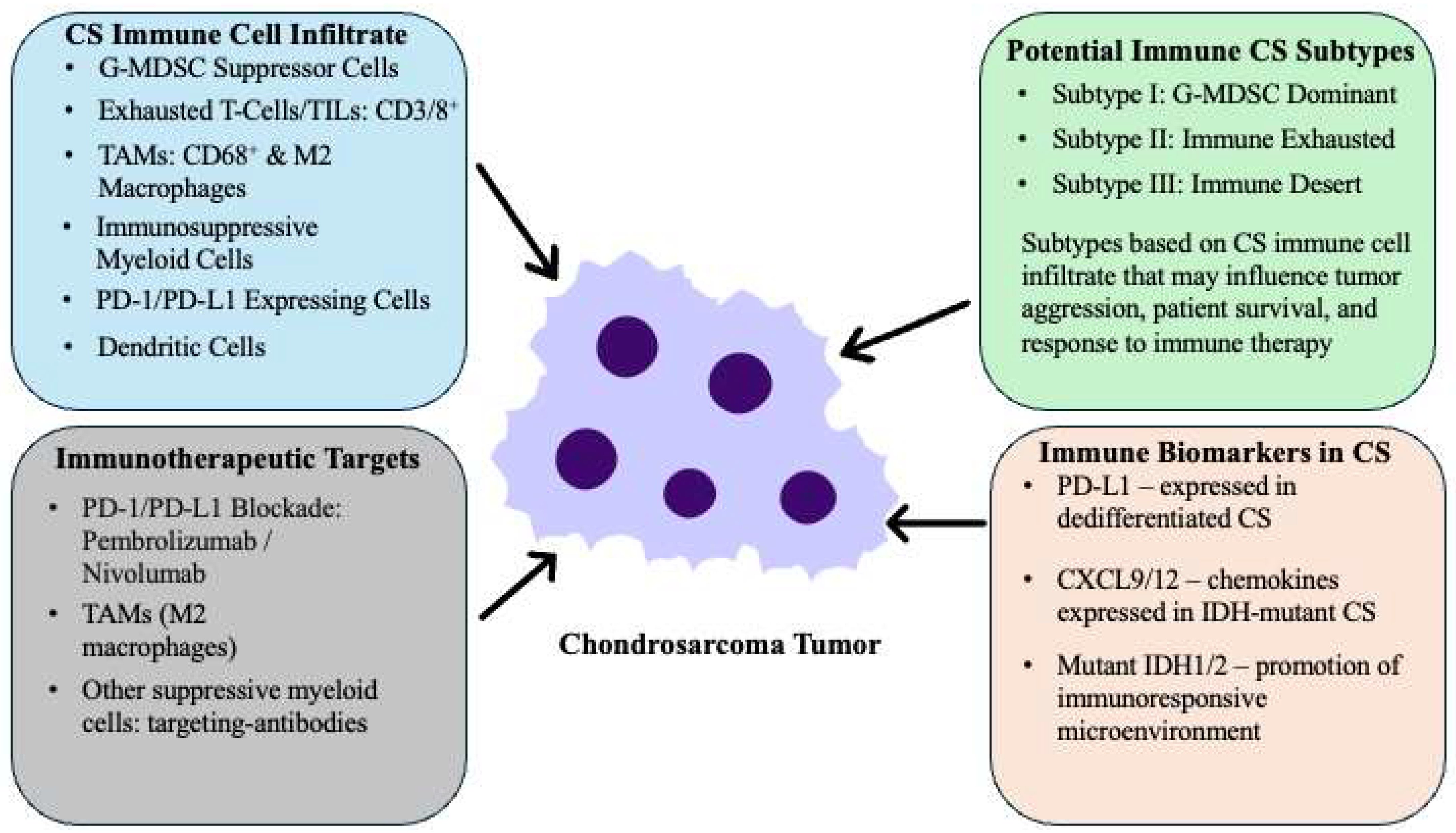
10. Immune-Based Therapies for Chondrosarcoma
11. Epigenetic-Based Therapies for Chondrosarcoma
Metabolic Alterations and Potential Targets in Chondrosarcoma
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hogendoorn, P.C.W.; Bovée, J.V.M.G.; Nielsen, G.P. Chondrosarcoma (grades I-III), including primary and secondary variants and periosteal chondrosarcoma. In WHO Classification of Tumours of Soft Tissue and Bone (IARC Lyon 2013): WHO Classification of Tumours, 4th ed.; World Health Organization: Geneva, Switzerland, 2013; Volume 5, pp. 262–264. [Google Scholar]
- Mery, B.; Espenel, S.; Guy, J.B.; Rancoule, C.; Vallard, A.; Aloy, M.T.; Rodriguez-Lafrasse, C.; Magne, N. Biological aspects of chondrosarcoma: Leaps and hurdles. Crit. Rev. Oncol. Hematol. 2018, 126, 32–36. [Google Scholar] [CrossRef]
- Yang, J.; Lou, S.; Yao, T. Trends in primary malignant bone cancer incidence and mortality in the United States, 2000–2017: A population-based study. J. Bone Oncol. 2024, 46, 100607. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, F.; Zhang, Y.; Yin, M.; Han, X.; Feng, J.; Wang, G. Twenty-year outcome of prevalence, incidence, mortality and survival rate in patients with malignant bone tumors. Int. J. Cancer 2024, 154, 226–240. [Google Scholar] [CrossRef]
- Whelan, J.S.; Davis, L.E. Osteosarcoma, Chondrosarcoma, and Chordoma. J. Clin. Oncol. 2018, 36, 188–193. [Google Scholar] [CrossRef]
- Fletcher, C.D.M. (Ed.) Pathology and Genetics of Tumours of Soft Tissue and Bone; No. 4; IARC Press: Lyon, France, 2002. [Google Scholar]
- Murphey, M.D.; Walker, E.A.; Wilson, A.J.; Kransdorf, M.J.; Temple, H.T.; Gannon, F.H. From the archives of the AFIP: Imaging of primary chondrosarcoma: Radiologic-pathologic correlation. Radiographics 2003, 23, 1245–1278. [Google Scholar] [CrossRef]
- Kim, M.J.; Cho, K.J.; Ayala, A.G.; Ro, J.Y. Chondrosarcoma: With updates on molecular genetics. Sarcoma 2011, 2011, 405437. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.E. WHO Classification of Soft Tissue and Bone, fourth edition: Summary and commentary. Curr. Opin. Oncol. 2013, 25, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Doyle, L.A. Refinements in Sarcoma Classification in the Current 2013 World Health Organization Classification of Tumours of Soft Tissue and Bone. Surg. Oncol. Clin. N. Am. 2016, 25, 621–643. [Google Scholar] [CrossRef] [PubMed]
- Landuzzi, L.; Ruzzi, F.; Lollini, P.L.; Scotlandi, K. Chondrosarcoma: New Molecular Insights, Challenges in Near-Patient Preclinical Modeling, and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1542. [Google Scholar] [CrossRef]
- Leddy, L.R.; Holmes, R.E. Chondrosarcoma of bone. Cancer Treat. Res. 2014, 162, 117–130. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Kim, H.J. Biomarkers of chondrosarcoma. J. Clin. Pathol. 2018, 71, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.; Radmacher, M.; Mohammed, N.; Suster, D.; Auer, H.; Jones, S.; Riggenbach, J.; Kelbick, N.; Bos, G.; Mayerson, J. MYC amplification and polysomy 8 in chondrosarcoma: Array comparative genomic hybridization, fluorescent in situ hybridization, and association with outcome. J. Clin. Oncol. 2005, 23, 9369–9376. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, L.B.; Szuhai, K.; Schrage, Y.M.; Rosenberg, C.; Tanke, H.J.; Taminiau, A.H.; Cleton-Jansen, A.M.; Bovee, J.V.; Hogendoorn, P.C. Array-comparative genomic hybridization of central chondrosarcoma: Identification of ribosomal protein S6 and cyclin-dependent kinase 4 as candidate target genes for genomic aberrations. Cancer 2006, 107, 380–388. [Google Scholar] [CrossRef]
- Sandberg, A.A.; Bridge, J.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: Chondrosarcoma and other cartilaginous neoplasms. Cancer Genet. Cytogenet. 2003, 143, 1–31. [Google Scholar] [CrossRef]
- Sjogren, H.; Orndal, C.; Tingby, O.; Meis-Kindblom, J.M.; Kindblom, L.G.; Stenman, G. Cytogenetic and spectral karyotype analyses of benign and malignant cartilage tumours. Int. J. Oncol. 2004, 24, 1385–1391. [Google Scholar] [PubMed]
- Ouyang, Z.; Wang, S.; Zeng, M.; Li, Z.; Zhang, Q.; Wang, W.; Liu, T. Therapeutic effect of palbociclib in chondrosarcoma: Implication of cyclin-dependent kinase 4 as a potential target. Cell Commun. Signal 2019, 17, 17. [Google Scholar] [CrossRef]
- Vanni, S.; Miserocchi, G.; Gallo, G.; Fausti, V.; Gabellone, S.; Liverani, C.; Spadazzi, C.; Cocchi, C.; Calabrese, C.; De Luca, G.; et al. Role of CDK4 as prognostic biomarker in Soft Tissue Sarcoma and synergistic effect of its inhibition in dedifferentiated liposarcoma sequential treatment. Exp. Hematol. Oncol. 2024, 13, 74. [Google Scholar] [CrossRef]
- Bovee, J.V.; van den Broek, L.J.; Cleton-Jansen, A.M.; Hogendoorn, P.C. Up-regulation of PTHrP and Bcl-2 expression characterizes the progression of osteochondroma towards peripheral chondrosarcoma and is a late event in central chondrosarcoma. Lab. Investig. 2000, 80, 1925–1934. [Google Scholar] [CrossRef]
- Rozeman, L.B.; Hameetman, L.; Cleton-Jansen, A.M.; Taminiau, A.H.; Hogendoorn, P.C.; Bovee, J.V. Absence of IHH and retention of PTHrP signalling in enchondromas and central chondrosarcomas. J. Pathol. 2005, 205, 476–482. [Google Scholar] [CrossRef]
- Kriajevska, M.; Fischer-Larsen, M.; Moertz, E.; Vorm, O.; Tulchinsky, E.; Grigorian, M.; Ambartsumian, N.; Lukanidin, E. Liprin beta 1, a member of the family of LAR transmembrane tyrosine phosphatase-interacting proteins, is a new target for the metastasis-associated protein S100A4 (Mts1). J. Biol. Chem. 2002, 277, 5229–5235. [Google Scholar] [CrossRef] [PubMed]
- van Beerendonk, H.M.; Rozeman, L.B.; Taminiau, A.H.; Sciot, R.; Bovee, J.V.; Cleton-Jansen, A.M.; Hogendoorn, P.C. Molecular analysis of the INK4A/INK4A-ARF gene locus in conventional (central) chondrosarcomas and enchondromas: Indication of an important gene for tumour progression. J. Pathol. 2004, 202, 359–366. [Google Scholar] [CrossRef]
- Asp, J.; Sangiorgi, L.; Inerot, S.E.; Lindahl, A.; Molendini, L.; Benassi, M.S.; Picci, P. Changes of the p16 gene but not the p53 gene in human chondrosarcoma tissues. Int. J. Cancer 2000, 85, 782–786. [Google Scholar] [CrossRef]
- Mulder, J.D.; Kroon, H.M.; Schutte, H.E.; Taconis, W.K. Radiologic Atlas of Bone Tumors, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- de Andrea, C.E.; Reijnders, C.M.; Kroon, H.M.; de Jong, D.; Hogendoorn, P.C.; Szuhai, K.; Bovee, J.V. Secondary peripheral chondrosarcoma evolving from osteochondroma as a result of outgrowth of cells with functional EXT. Oncogene 2012, 31, 1095–1104. [Google Scholar] [CrossRef]
- Gao, L.; Hong, X.; Guo, X.; Cao, D.; Gao, X.; DeLaney, T.F.; Gong, X.; Chen, R.; Ni, J.; Yao, Y.; et al. Targeted next-generation sequencing of dedifferentiated chondrosarcoma in the skull base reveals combined TP53 and PTEN mutations with increased proliferation index, an implication for pathogenesis. Oncotarget 2016, 7, 43557–43569. [Google Scholar] [CrossRef]
- Kim, H.; Cho, Y.; Kim, H.S.; Kang, D.; Cheon, D.; Kim, Y.J.; Chang, M.J.; Lee, K.M.; Chang, C.B.; Kang, S.B.; et al. A system-level approach identifies HIF-2alpha as a critical regulator of chondrosarcoma progression. Nat. Commun. 2020, 11, 5023. [Google Scholar] [CrossRef]
- Feng, H.; Wang, J.; Xu, J.; Xie, C.; Gao, F.; Li, Z. The expression of SIRT1 regulates the metastaticplasticity of chondrosarcoma cells by inducing epithelial-mesenchymal transition. Sci. Rep. 2017, 7, 41203. [Google Scholar] [CrossRef]
- Suh, J.; Kim, H.; Min, J.; Yeon, H.J.; Hemberg, M.; Scimeca, L.; Wu, M.R.; Kang, H.G.; Kim, Y.J.; Kim, J.H. Decoupling NAD(+) metabolic dependency in chondrosarcoma by targeting the SIRT1-HIF-2alpha axis. Cell Rep. Med. 2024, 5, 101342. [Google Scholar] [CrossRef]
- Meijer, D.; de Jong, D.; Pansuriya, T.C.; van den Akker, B.E.; Picci, P.; Szuhai, K.; Bovee, J.V. Genetic characterization of mesenchymal, clear cell, and dedifferentiated chondrosarcoma. Genes. Chromosomes Cancer 2012, 51, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fritchie, K.; Wei, S.; Ali, N.; Curless, K.; Shen, T.; Brini, A.T.; Latif, F.; Sumathi, V.; Siegal, G.P.; et al. Diagnostic utility of IDH1/2 mutations to distinguish dedifferentiated chondrosarcoma from undifferentiated pleomorphic sarcoma of bone. Hum. Pathol. 2017, 65, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.H.; Chen, S.C.; Wu, K.C.; Yang, C.B.; Fang, C.L.; Lai, W.F.; Tsai, Y.H. Differential effect of ECM molecules on re-expression of cartilaginous markers in near quiescent human chondrocytes. J. Cell Physiol. 2011, 226, 1981–1988. [Google Scholar] [CrossRef]
- Amary, M.F.; Ye, H.; Forbes, G.; Damato, S.; Maggiani, F.; Pollock, R.; Tirabosco, R.; Flanagan, A.M. Isocitrate dehydrogenase 1 mutations (IDH1) and p16/CDKN2A copy number change in conventional chondrosarcomas. Virchows Arch. 2015, 466, 217–222. [Google Scholar] [CrossRef]
- Kerr, D.A.; Lopez, H.U.; Deshpande, V.; Hornicek, F.J.; Duan, Z.; Zhang, Y.; Rosenberg, A.E.; Borger, D.R.; Nielsen, G.P. Molecular distinction of chondrosarcoma from chondroblastic osteosarcoma through IDH1/2 mutations. Am. J. Surg. Pathol. 2013, 37, 787–795. [Google Scholar] [CrossRef]
- Carosi, F.; Broseghini, E.; Fabbri, L.; Corradi, G.; Gili, R.; Forte, V.; Roncarati, R.; Filippini, D.M.; Ferracin, M. Targeting Isocitrate Dehydrogenase (IDH) in Solid Tumors: Current Evidence and Future Perspectives. Cancers 2024, 16, 2752. [Google Scholar] [CrossRef]
- Ivanov, S.; Nano, O.; Hana, C.; Bonano-Rios, A.; Hussein, A. Molecular Targeting of the Isocitrate Dehydrogenase Pathway and the Implications for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 7337. [Google Scholar] [CrossRef]
- Venneker, S.; Bovee, J. IDH Mutations in Chondrosarcoma: Case Closed or Not? Cancers 2023, 15, 3603. [Google Scholar] [CrossRef]
- Denu, R.A.; Yang, R.K.; Lazar, A.J.; Patel, S.S.; Lewis, V.O.; Roszik, J.; Livingston, J.A.; Wang, W.L.; Shaw, K.R.; Ratan, R.; et al. Clinico-Genomic Profiling of Conventional and Dedifferentiated Chondrosarcomas Reveals TP53 Mutation to Be Associated with Worse Outcomes. Clin. Cancer Res. 2023, 29, 4844–4852. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.A. Chondrosarcoma: Biology, genetics, and epigenetics. F1000Research 2018, 7, 1826. [Google Scholar] [CrossRef] [PubMed]
- Tarpey, P.S.; Behjati, S.; Cooke, S.L.; Van Loo, P.; Wedge, D.C.; Pillay, N.; Marshall, J.; O’Meara, S.; Davies, H.; Nik-Zainal, S.; et al. Frequent mutation of the major cartilage collagen gene COL2A1 in chondrosarcoma. Nat. Genet. 2013, 45, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Terek, R.M.; Healey, J.H.; Garin-Chesa, P.; Mak, S.; Huvos, A.; Albino, A.P. p53 mutations in chondrosarcoma. Diagn. Mol. Pathol. 1998, 7, 51–56. [Google Scholar] [CrossRef]
- Dobashi, Y.; Sugimura, H.; Sato, A.; Hirabayashi, T.; Kanda, H.; Kitagawa, T.; Kawaguchi, N.; Imamura, T.; Machinami, R. Possible association of p53 overexpression and mutation with high-grade chondrosarcoma. Diagn. Mol. Pathol. 1993, 2, 257–263. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. J. Am. Med. Assoc. Oncol. 2018, 4, 93–97. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, J.K.; Yu, Z.; Hornicek, F.J.; Kan, Q.; Duan, Z. Expression and therapeutic implications of cyclin-dependent kinase 4 (CDK4) in osteosarcoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1573–1582. [Google Scholar] [CrossRef]
- Schrage, Y.M.; Lam, S.; Jochemsen, A.G.; Cleton-Jansen, A.M.; Taminiau, A.H.; Hogendoorn, P.C.; Bovee, J.V. Central chondrosarcoma progression is associated with pRb pathway alterations: CDK4 down-regulation and p16 overexpression inhibit cell growth in vitro. J. Cell Mol. Med. 2009, 13, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Husar-Memmer, E.; Ekici, A.; Al Kaissi, A.; Sticht, H.; Manger, B.; Schett, G.; Zwerina, J. Premature osteoarthritis as presenting sign of type II collagenopathy: A case report and literature review. Semin. Arthritis Rheum. 2013, 42, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Gao, S.; Shen, J.; Hornicek, F.; Duan, Z. Three-dimensional (3D) culture in sarcoma research and the clinical significance. Biofabrication 2017, 9, 032003. [Google Scholar] [CrossRef] [PubMed]
- Totoki, Y.; Yoshida, A.; Hosoda, F.; Nakamura, H.; Hama, N.; Ogura, K.; Yoshida, A.; Fujiwara, T.; Arai, Y.; Toguchida, J.; et al. Unique mutation portraits and frequent COL2A1 gene alteration in chondrosarcoma. Genome Res. 2014, 24, 1411–1420. [Google Scholar] [CrossRef]
- Nicolle, R.; Ayadi, M.; Gomez-Brouchet, A.; Armenoult, L.; Banneau, G.; Elarouci, N.; Tallegas, M.; Decouvelaere, A.V.; Aubert, S.; Redini, F.; et al. Integrated molecular characterization of chondrosarcoma reveals critical determinants of disease progression. Nat. Commun. 2019, 10, 4622. [Google Scholar] [CrossRef]
- Cannonier, S.A.; Sterling, J.A. The Role of Hedgehog Signaling in Tumor Induced Bone Disease. Cancers 2015, 7, 1658–1683. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Y.; Sun, B.; McMahon, A.P.; Wang, Y. Hedgehog Signaling: From Basic Biology to Cancer Therapy. Cell Chem. Biol. 2017, 24, 252–280. [Google Scholar] [CrossRef]
- Palmini, G.; Marini, F.; Brandi, M.L. What Is New in the miRNA World Regarding Osteosarcoma and Chondrosarcoma? Molecules 2017, 22, 417. [Google Scholar] [CrossRef]
- Nugent, M. microRNA and Bone Cancer. Adv. Exp. Med. Biol. 2015, 889, 201–230. [Google Scholar] [CrossRef]
- Italiano, A.; Le Cesne, A.; Bellera, C.; Piperno-Neumann, S.; Duffaud, F.; Penel, N.; Cassier, P.; Domont, J.; Takebe, N.; Kind, M.; et al. GDC-0449 in patients with advanced chondrosarcomas: A French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann. Oncol. 2013, 24, 2922–2926. [Google Scholar] [CrossRef] [PubMed]
- Campbell, V.T.; Nadesan, P.; Ali, S.A.; Wang, C.Y.; Whetstone, H.; Poon, R.; Wei, Q.; Keilty, J.; Proctor, J.; Wang, L.W.; et al. Hedgehog pathway inhibition in chondrosarcoma using the smoothened inhibitor IPI-926 directly inhibits sarcoma cell growth. Mol. Cancer Ther. 2014, 13, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Jiang, T.; Guo, F.; Gong, C.; Yang, K.; Wu, Y.; Huang, X.; Cheng, W.; Xu, K. Hedgehog pathway inhibitor-4 suppresses malignant properties of chondrosarcoma cells by disturbing tumor ciliogenesis. Oncol. Rep. 2014, 32, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Samsa, W.E.; Zhou, X.; Zhou, G. Signaling pathways regulating cartilage growth plate formation and activity. Semin. Cell Dev. Biol. 2017, 62, 3–15. [Google Scholar] [CrossRef] [PubMed]
- van Oosterwijk, J.G.; Meijer, D.; van Ruler, M.A.; van den Akker, B.E.; Oosting, J.; Krenacs, T.; Picci, P.; Flanagan, A.M.; Liegl-Atzwanger, B.; Leithner, A.; et al. Screening for potential targets for therapy in mesenchymal, clear cell, and dedifferentiated chondrosarcoma reveals Bcl-2 family members and TGFbeta as potential targets. Am. J. Pathol. 2013, 182, 1347–1356. [Google Scholar] [CrossRef]
- El Beaino, M.; Roszik, J.; Livingston, J.A.; Wang, W.L.; Lazar, A.J.; Amini, B.; Subbiah, V.; Lewis, V.; Conley, A.P. Mesenchymal Chondrosarcoma: A Review with Emphasis on its Fusion-Driven Biology. Curr. Oncol. Rep. 2018, 20, 37. [Google Scholar] [CrossRef]
- Panagopoulos, I.; Gorunova, L.; Bjerkehagen, B.; Boye, K.; Heim, S. Chromosome aberrations and HEY1-NCOA2 fusion gene in a mesenchymal chondrosarcoma. Oncol. Rep. 2014, 32, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, K.B.; Panagopoulos, I.; Thorsen, J.; Haugom, L.; Gorunova, L.; Bjerkehagen, B.; Fossa, A.; Guriby, M.; Nome, T.; Lothe, R.A.; et al. Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS ONE 2012, 7, e49705. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Motoi, T.; Khanin, R.; Olshen, A.; Mertens, F.; Bridge, J.; Dal Cin, P.; Antonescu, C.R.; Singer, S.; Hameed, M.; et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes. Chromosomes Cancer 2012, 51, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Homme, M.; Teramura, Y.; Kumegawa, K.; Yamazaki, Y.; Yamashita, K.; Osato, M.; Maruyama, R.; Nakamura, T. HEY1-NCOA2 expression modulates chondrogenic differentiation and induces mesenchymal chondrosarcoma in mice. J. Clin. Investig. Insight 2023, 8, e160279. [Google Scholar] [CrossRef] [PubMed]
- Zavadil, J.; Cermak, L.; Soto-Nieves, N.; Bottinger, E.P. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004, 23, 1155–1165. [Google Scholar] [CrossRef]
- Miele, L.; Osborne, B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J. Cell Physiol. 1999, 181, 393–409. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef]
- Grogan, S.P.; Olee, T.; Hiraoka, K.; Lotz, M.K. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis Rheum. 2008, 58, 2754–2763. [Google Scholar] [CrossRef]
- Childs, K.S.; Goodbourn, S. Identification of novel co-repressor molecules for Interferon Regulatory Factor-2. Nucleic Acids Res. 2003, 31, 3016–3026. [Google Scholar] [CrossRef]
- Koeppel, M.; van Heeringen, S.J.; Smeenk, L.; Navis, A.C.; Janssen-Megens, E.M.; Lohrum, M. The novel p53 target gene IRF2BP2 participates in cell survival during the p53 stress response. Nucleic Acids Res. 2009, 37, 322–335. [Google Scholar] [CrossRef]
- Kang, J.M.; Lee, B.H.; Kim, N.; Lee, H.S.; Lee, H.E.; Park, J.H.; Kim, J.S.; Jung, H.C.; Song, I.S. CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer. J. Korean Med. Sci. 2011, 26, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.; Wong, N.A.; Liu, Y.; Bicknell, D.; Turley, H.; Hollins, L.; Miller, C.J.; Wilding, J.L.; Bodmer, W.F. Gastrointestinal differentiation marker Cytokeratin 20 is regulated by homeobox gene CDX1. Proc. Natl. Acad. Sci. USA 2009, 106, 1936–1941. [Google Scholar] [CrossRef]
- Wong, N.A.; Britton, M.P.; Choi, G.S.; Stanton, T.K.; Bicknell, D.C.; Wilding, J.L.; Bodmer, W.F. Loss of CDX1 expression in colorectal carcinoma: Promoter methylation, mutation, and loss of heterozygosity analyses of 37 cell lines. Proc. Natl. Acad. Sci. USA 2004, 101, 574–579. [Google Scholar] [CrossRef]
- Recillas-Targa, F. Cancer Epigenetics: An Overview. Arch. Med. Res. 2022, 53, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shen, J.K.; Xu, J.; Trahan, C.A.; Hornicek, F.J.; Duan, Z. Aberrant DNA methylations in chondrosarcoma. Epigenomics 2016, 8, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Ouadid-Ahidouch, H.; Rodat-Despoix, L.; Matifat, F.; Morin, G.; Ahidouch, A. DNA methylation of channel-related genes in cancers. Biochim. Biophys. Acta 2015, 1848, 2621–2628. [Google Scholar] [CrossRef]
- Ren, J.; Singh, B.N.; Huang, Q.; Li, Z.; Gao, Y.; Mishra, P.; Hwa, Y.L.; Li, J.; Dowdy, S.C.; Jiang, S.W. DNA hypermethylation as a chemotherapy target. Cell. Signal. 2011, 23, 1082–1093. [Google Scholar] [CrossRef]
- Liu, P.; Shen, J.K.; Hornicek, F.J.; Liu, F.; Duan, Z. Wnt inhibitory factor 1 (WIF1) methylation and its association with clinical prognosis in patients with chondrosarcoma. Sci. Rep. 2017, 7, 1580. [Google Scholar] [CrossRef]
- Martin-Orozco, E.; Sanchez-Fernandez, A.; Ortiz-Parra, I.; Ayala-San Nicolas, M. WNT Signaling in Tumors: The Way to Evade Drugs and Immunity. Front. Immunol. 2019, 10, 2854. [Google Scholar] [CrossRef]
- Rubin, E.M.; Guo, Y.; Tu, K.; Xie, J.; Zi, X.; Hoang, B.H. Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol. Cancer Ther. 2010, 9, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Poggi, L.; Casarosa, S.; Carl, M. An Eye on the Wnt Inhibitory Factor Wif1. Front. Cell Dev. Biol. 2018, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.; Pirzada, R.H.; Ain, Q.U.; Choi, S. Wnt Signaling in the Regulation of Immune Cell and Cancer Therapeutics. Cells 2019, 8, 1380. [Google Scholar] [CrossRef]
- Losman, J.A.; Kaelin, W.G., Jr. What a difference a hydroxyl makes: Mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes. Dev. 2013, 27, 836–852. [Google Scholar] [CrossRef]
- Venneker, S.; Kruisselbrink, A.B.; Baranski, Z.; Palubeckaite, I.; Briaire-de Bruijn, I.H.; Oosting, J.; French, P.J.; Danen, E.H.J.; Bovee, J. Beyond the Influence of IDH Mutations: Exploring Epigenetic Vulnerabilities in Chondrosarcoma. Cancers 2020, 12, 3589. [Google Scholar] [CrossRef]
- Peterse, E.F.P.; van den Akker, B.; Niessen, B.; Oosting, J.; Suijker, J.; de Jong, Y.; Danen, E.H.J.; Cleton-Jansen, A.M.; Bovee, J. NAD Synthesis Pathway Interference Is a Viable Therapeutic Strategy for Chondrosarcoma. Mol. Cancer Res. 2017, 15, 1714–1721. [Google Scholar] [CrossRef]
- Jin, Z.; Han, Y.X.; Han, X.R. Loss of RUNX3 expression may contribute to poor prognosis in patients with chondrosarcoma. J. Mol. Histol. 2013, 44, 645–652. [Google Scholar] [CrossRef]
- Liu, P.; Garbutt, C.; Hornicek, F.J.; Liu, F.; Duan, Z. Aberration of p73 Promoter Methylation in Chondrosarcoma. Anticancer Res. 2017, 37, 2939–2945. [Google Scholar] [CrossRef]
- Chang, L.; Shrestha, S.; LaChaud, G.; Scott, M.A.; James, A.W. Review of microRNA in osteosarcoma and chondrosarcoma. Med. Oncol. 2015, 32, 613. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, Y.; Yu, H.; Machan, J.T.; Alladin, A.; Ramirez, J.; Taliano, R.; Hart, J.; Chen, Q.; Terek, R.M. Anti-miRNA Oligonucleotide Therapy for Chondrosarcoma. Mol. Cancer Ther. 2019, 18, 2021–2029. [Google Scholar] [CrossRef]
- Shui, X.; Zhou, C.; Lin, W.; Yu, Y.; Feng, Y.; Kong, J. Long non-coding RNA BCAR4 promotes chondrosarcoma cell proliferation and migration through activation of mTOR signaling pathway. Exp. Biol. Med. 2017, 242, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Ren, T.; Huang, Y.; Sun, K.; Wang, S.; Liu, K.; Zheng, B.; Guo, W. Knockdown of long non-coding RNA HOTAIR increases miR-454-3p by targeting Stat3 and Atg12 to inhibit chondrosarcoma growth. Cell Death Dis. 2017, 8, e2605. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, X.; Zuo, F.; Shi, H.; Jing, J. Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin. Cancer Biol. 2023, 88, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Ho, J.W.K.; Yau, R.C.H.; Lam, Y.L.; Shek, T.W.H.; Yeung, M.C.F.; Chen, H.; Oreffo, R.O.C.; Cheah, K.S.E.; Cheung, K.S.C. A single-cell atlas of conventional central chondrosarcoma reveals the role of endoplasmic reticulum stress in malignant transformation. Commun. Biol. 2024, 7, 124. [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, M.S.; Jiang, S.K.; Eaw, C.Y.; Han, Y.J.; Tang, C.H. Transcriptomic data integration and analysis revealing potential mechanisms of doxorubicin resistance in chondrosarcoma cells. Biochem. Pharmacol. 2025, 232, 116733. [Google Scholar] [CrossRef]
- Takigawa, M.; Tajima, K.; Pan, H.O.; Enomoto, M.; Kinoshita, A.; Suzuki, F.; Takano, Y.; Mori, Y. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 1989, 49, 3996–4002. [Google Scholar]
- Takigawa, M.; Pan, H.O.; Kinoshita, A.; Tajima, K.; Takano, Y. Establishment from a human chondrosarcoma of a new immortal cell line with high tumorigenicity in vivo, which is able to form proteoglycan-rich cartilage-like nodules and to respond to insulin in vitro. Int. J. Cancer 1991, 48, 717–725. [Google Scholar] [CrossRef]
- Kunisada, T.; Miyazaki, M.; Mihara, K.; Gao, C.; Kawai, A.; Inoue, H.; Namba, M. A new human chondrosarcoma cell line (OUMS-27) that maintains chondrocytic differentiation. Int. J. Cancer 1998, 77, 854–859. [Google Scholar] [CrossRef]
- Gil-Benso, R.; Lopez-Gines, C.; Lopez-Guerrero, J.A.; Carda, C.; Callaghan, R.C.; Navarro, S.; Ferrer, J.; Pellin, A.; Llombart-Bosch, A. Establishment and characterization of a continuous human chondrosarcoma cell line, ch-2879: Comparative histologic and genetic studies with its tumor of origin. Lab. Investig. 2003, 83, 877–887. [Google Scholar] [CrossRef]
- Huang, L.; Cao, J.; Cao, L.; Gao, L.; Yang, Y.; Xu, L. Puerarin induces cell apoptosis in human chondrosarcoma cell line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol. Lett. 2017, 14, 5585–5590. [Google Scholar] [CrossRef][Green Version]
- Jeong, J.Y.; Jeong, W.; Kim, H.J. Promotion of Chondrosarcoma Cell Survival, Migration and Lymphangiogenesis by Periostin. Anticancer Res. 2020, 40, 5463–5469. [Google Scholar] [CrossRef]
- Clark, J.C.; Akiyama, T.; Dass, C.R.; Choong, P.F. New clinically relevant, orthotopic mouse models of human chondrosarcoma with spontaneous metastasis. Cancer Cell Int. 2010, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- de Jong, Y.; Ingola, M.; Briaire-de Bruijn, I.H.; Kruisselbrink, A.B.; Venneker, S.; Palubeckaite, I.; Heijs, B.; Cleton-Jansen, A.M.; Haas, R.L.M.; Bovee, J. Radiotherapy resistance in chondrosarcoma cells; a possible correlation with alterations in cell cycle related genes. Clin. Sarcoma Res. 2019, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; van Oosterwijk, J.G.; Sicinska, E.; Moss, S.; Remillard, S.P.; van Wezel, T.; Buhnemann, C.; Hassan, A.B.; Demetri, G.D.; Bovee, J.V.; et al. Functional profiling of receptor tyrosine kinases and downstream signaling in human chondrosarcomas identifies pathways for rational targeted therapy. Clin. Cancer Res. 2013, 19, 3796–3807. [Google Scholar] [CrossRef]
- Hoyt, A.K.; Moran, A.; Granger, C.; Sedani, A.; Saigh, S.; Brown, J.; Galoian, K.A. PRP-1 significantly decreases the ALDHhigh cancer stem cell population and regulates the aberrant Wnt/beta-catenin pathway in human chondrosarcoma JJ012 cells. Oncol. Rep. 2019, 42, 103–114. [Google Scholar] [CrossRef] [PubMed]
- van Oosterwijk, J.G.; Plass, J.R.; Meijer, D.; Que, I.; Karperien, M.; Bovee, J.V. An orthotopic mouse model for chondrosarcoma of bone provides an in vivo tool for drug testing. Virchows Arch. 2015, 466, 101–109. [Google Scholar] [CrossRef]
- Li, X.; Dean, D.C.; Ferreira, A.; Nelson, S.D.; Hornicek, F.J.; Yu, S.; Duan, Z. Establishment and Characterization of a Novel Dedifferentiated Chondrosarcoma Cell Line DDCS2. Cancer Control 2021, 28, 10732748211045274. [Google Scholar] [CrossRef]
- Veys, C.; Benmoussa, A.; Contentin, R.; Duchemin, A.; Brotin, E.; Lafont, J.E.; Saintigny, Y.; Poulain, L.; Denoyelle, C.; Demoor, M.; et al. Tumor Suppressive Role of miR-342-5p in Human Chondrosarcoma Cells and 3D Organoids. Int. J. Mol. Sci. 2021, 22, 5590. [Google Scholar] [CrossRef]
- Al Shihabi, A.; Tebon, P.J.; Nguyen, H.T.L.; Chantharasamee, J.; Sartini, S.; Davarifar, A.; Jensen, A.Y.; Diaz-Infante, M.; Cox, H.; Gonzalez, A.E.; et al. The landscape of drug sensitivity and resistance in sarcoma. Cell Stem Cell 2024, 31, 1524–1542.e4. [Google Scholar] [CrossRef]
- Munoz-Garcia, J.; Jubelin, C.; Loussouarn, A.; Goumard, M.; Griscom, L.; Renodon-Corniere, A.; Heymann, M.F.; Heymann, D. In vitro three-dimensional cell cultures for bone sarcomas. J. Bone Oncol. 2021, 30, 100379. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, V.A.; Mironov, V.A.; van Kampen, K.A.; Karalkin, P.A.; Koudan, E.V.; Pereira, F.D.; Petrov, S.V.; Nezhurina, E.K.; Petrov, O.F.; Myasnikov, M.I.; et al. Scaffold-free and label-free biofabrication technology using levitational assembly in a high magnetic field. Biofabrication 2020, 12, 045022. [Google Scholar] [CrossRef] [PubMed]
- Palubeckaite, I.; Venneker, S.; van den Akker, B.; Briaire-de Bruijn, I.H.; Bovee, J. Does PARP Inhibition Sensitize Chondrosarcoma Cell Lines to Chemotherapy or Radiotherapy? Results From a Three-dimensional Spheroid Cell Model. Clin. Orthop. Relat. Res. 2023, 481, 608–619. [Google Scholar] [CrossRef]
- Minopoli, M.; Sarno, S.; Di Carluccio, G.; Azzaro, R.; Costantini, S.; Fazioli, F.; Gallo, M.; Apice, G.; Cannella, L.; Rea, D.; et al. Inhibiting Monocyte Recruitment to Prevent the Pro-Tumoral Activity of Tumor-Associated Macrophages in Chondrosarcoma. Cells 2020, 9, 1062. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Cai, C.; Zhang, H.; Shen, H.; Han, Y. Patient-derived xenograft models in cancer therapy: Technologies and applications. Signal Transduct. Target. Ther. 2023, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Safaric Tepes, P.; Segovia, D.; Jevtic, S.; Ramirez, D.; Lyons, S.K.; Sordella, R. Patient-derived xenografts and in vitro model show rationale for imatinib mesylate repurposing in HEY1-NCoA2-driven mesenchymal chondrosarcoma. Lab. Investig. 2022, 102, 1038–1049. [Google Scholar] [CrossRef]
- Monderer, D.; Luseau, A.; Bellec, A.; David, E.; Ponsolle, S.; Saiagh, S.; Bercegeay, S.; Piloquet, P.; Denis, M.G.; Lode, L.; et al. New chondrosarcoma cell lines and mouse models to study the link between chondrogenesis and chemoresistance. Lab. Investig. 2013, 93, 1100–1114. [Google Scholar] [CrossRef]
- Pathmanapan, S.; Ilkayeva, O.; Martin, J.T.; Loe, A.K.H.; Zhang, H.; Zhang, G.F.; Newgard, C.B.; Wunder, J.S.; Alman, B.A. Mutant IDH and non-mutant chondrosarcomas display distinct cellular metabolomes. Cancer Metab. 2021, 9, 13. [Google Scholar] [CrossRef]
- Sun, H.; Cao, S.; Mashl, R.J.; Mo, C.K.; Zaccaria, S.; Wendl, M.C.; Davies, S.R.; Bailey, M.H.; Primeau, T.M.; Hoog, J.; et al. Comprehensive characterization of 536 patient-derived xenograft models prioritizes candidatesfor targeted treatment. Nat. Commun. 2021, 12, 5086. [Google Scholar] [CrossRef]
- Subbiah, V.; Chawla, S.P.; Conley, A.P.; Wilky, B.A.; Tolcher, A.; Lakhani, N.J.; Berz, D.; Andrianov, V.; Crago, W.; Holcomb, M.; et al. Preclinical Characterization and Phase I Trial Results of INBRX-109, A Third-Generation, Recombinant, Humanized, Death Receptor 5 Agonist Antibody, in Chondrosarcoma. Clin. Cancer Res. 2023, 29, 2988–3003. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Merlini, A.; Ferrero, G.; Mesiano, G.; Fiorino, E.; Brusco, S.; Centomo, M.L.; Leuci, V.; D’Ambrosio, L.; Aglietta, M.; et al. EphA2 Expression in Bone Sarcomas: Bioinformatic Analyses and Preclinical Characterization in Patient-Derived Models of Osteosarcoma, Ewing’s Sarcoma and Chondrosarcoma. Cells 2021, 10, 2893. [Google Scholar] [CrossRef]
- Baumhoer, D.; Amary, F.; Flanagan, A.M. An update of molecular pathology of bone tumors. Lessons learned from investigating samples by next generation sequencing. Genes. Chromosomes Cancer 2019, 58, 88–99. [Google Scholar] [CrossRef]
- Li, L.; Hu, X.; Eid, J.E.; Rosenberg, A.E.; Wilky, B.A.; Ban, Y.; Sun, X.; Galoian, K.; DeSalvo, J.; Yue, J.; et al. Mutant IDH1 Depletion Downregulates Integrins and Impairs Chondrosarcoma Growth. Cancers 2020, 12, 141. [Google Scholar] [CrossRef]
- Norsworthy, K.J.; Luo, L.; Hsu, V.; Gudi, R.; Dorff, S.E.; Przepiorka, D.; Deisseroth, A.; Shen, Y.L.; Sheth, C.M.; Charlab, R.; et al. FDA Approval Summary: Ivosidenib for Relapsed or Refractory Acute Myeloid Leukemia with an Isocitrate Dehydrogenase-1 Mutation. Clin. Cancer Res. 2019, 25, 3205–3209. [Google Scholar] [CrossRef]
- Casak, S.J.; Pradhan, S.; Fashoyin-Aje, L.A.; Ren, Y.; Shen, Y.L.; Xu, Y.; Chow, E.C.Y.; Xiong, Y.; Zirklelbach, J.F.; Liu, J.; et al. FDA Approval Summary: Ivosidenib for the Treatment of Patients with Advanced Unresectable or Metastatic, Chemotherapy Refractory Cholangiocarcinoma with an IDH1 Mutation. Clin. Cancer Res. 2022, 28, 2733–2737. [Google Scholar] [CrossRef]
- Eisenstein, M. FDA approves first IDH-targeted glioma drug. Nat. Biotechnol. 2024, 42, 1325. [Google Scholar] [CrossRef]
- Tap, W.D.; Villalobos, V.M.; Cote, G.M.; Burris, H.; Janku, F.; Mir, O.; Beeram, M.; Wagner, A.J.; Jiang, L.; Wu, B.; et al. Phase I Study of the Mutant IDH1 Inhibitor Ivosidenib: Safety and Clinical Activity in Patients With Advanced Chondrosarcoma. J. Clin. Oncol. 2020, 38, 1693–1701. [Google Scholar] [CrossRef]
- Dermawan, J.K.T.; Nafa, K.; Mohanty, A.; Xu, Y.; Rijo, I.; Casanova, J.; Villafania, L.; Benhamida, J.; Kelly, C.M.; Tap, W.D.; et al. Distinct IDH1/2-associated Methylation Profile and Enrichment of TP53 and TERT Mutations Distinguish Dedifferentiated Chondrosarcoma from Conventional Chondrosarcoma. Cancer Res. Commun. 2023, 3, 431–443. [Google Scholar] [CrossRef]
- Tiet, T.D.; Hopyan, S.; Nadesan, P.; Gokgoz, N.; Poon, R.; Lin, A.C.; Yan, T.; Andrulis, I.L.; Alman, B.A.; Wunder, J.S. Constitutive hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am. J. Pathol. 2006, 168, 321–330. [Google Scholar] [CrossRef]
- Bovee, J.V.; Hogendoorn, P.C.; Wunder, J.S.; Alman, B.A. Cartilage tumours and bone development: Molecular pathology and possible therapeutic targets. Nat. Rev. Cancer 2010, 10, 481–488. [Google Scholar] [CrossRef]
- Pacey, S.; Wilson, R.H.; Walton, M.; Eatock, M.M.; Hardcastle, A.; Zetterlund, A.; Arkenau, H.T.; Moreno-Farre, J.; Banerji, U.; Roels, B.; et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin. Cancer Res. 2011, 17, 1561–1570. [Google Scholar] [CrossRef]
- Griffith, T.S.; Stokes, B.; Kucaba, T.A.; Earel, J.K., Jr.; VanOosten, R.L.; Brincks, E.L.; Norian, L.A. TRAIL gene therapy: From preclinical development to clinical application. Curr. Gene Ther. 2009, 9, 9–19. [Google Scholar] [CrossRef]
- Subbiah, V.; Brown, R.E.; Buryanek, J.; Trent, J.; Ashkenazi, A.; Herbst, R.; Kurzrock, R. Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (dulanermin), a dual proapoptotic receptor (DR4/DR5) agonist. Mol. Cancer Ther. 2012, 11, 2541–2546. [Google Scholar] [CrossRef]
- Wang, B.T.; Kothambawala, T.; Wang, L.; Matthew, T.J.; Calhoun, S.E.; Saini, A.K.; Kotturi, M.F.; Hernandez, G.; Humke, E.W.; Peterson, M.S.; et al. Multimeric Anti-DR5 IgM Agonist Antibody IGM-8444 Is a Potent Inducer of Cancer Cell Apoptosis and Synergizes with Chemotherapy and BCL-2 Inhibitor ABT-199. Mol. Cancer Ther. 2021, 20, 2483–2494. [Google Scholar] [CrossRef]
- Palmerini, E.; Lopez Pousa, A.; Grignani, G.; Redondo, A.; Hindi, N.; Provenzano, S.; Sebio, A.; Lopez Martin, J.A.; Valverde, C.; Martinez Trufero, J.; et al. Nivolumab and sunitinib in patients with advanced bone sarcomas: A multicenter, single-arm, phase 2 trial. Cancer 2025, 131, e35628. [Google Scholar] [CrossRef]
- Nota, S.; Osei-Hwedieh, D.O.; Drum, D.L.; Wang, X.; Sabbatino, F.; Ferrone, S.; Schwab, J.H. Chondroitin sulfate proteoglycan 4 expression in chondrosarcoma: A potential target for antibody-based immunotherapy. Front. Oncol. 2022, 12, 939166. [Google Scholar] [CrossRef]
- Tarone, L.; Giacobino, D.; Camerino, M.; Maniscalco, L.; Iussich, S.; Parisi, L.; Giovannini, G.; Dentini, A.; Bolli, E.; Quaglino, E.; et al. A chimeric human/dog-DNA vaccine against CSPG4 induces immunity with therapeutic potential in comparative preclinical models of osteosarcoma. Mol. Ther. 2023, 31, 2342–2359. [Google Scholar] [CrossRef]
- Kalinski, T.; Krueger, S.; Sel, S.; Werner, K.; Ropke, M.; Roessner, A. Differential expression of VEGF-A and angiopoietins in cartilage tumors and regulation by interleukin-1beta. Cancer 2006, 106, 2028–2038. [Google Scholar] [CrossRef]
- Chow, W.; Frankel, P.; Ruel, C.; Araujo, D.M.; Milhem, M.; Okuno, S.; Hartner, L.; Undevia, S.; Staddon, A. Results of a prospective phase 2 study of pazopanib in patients with surgically unresectable or metastatic chondrosarcoma. Cancer 2020, 126, 105–111. [Google Scholar] [CrossRef]
- Duffaud, F.; Italiano, A.; Bompas, E.; Rios, M.; Penel, N.; Mir, O.; Piperno-Neumann, S.; Chevreau, C.; Delcambre, C.; Bertucci, F.; et al. Efficacy and safety of regorafenib in patients with metastatic or locally advanced chondrosarcoma: Results of a non-comparative, randomised, double-blind, placebo controlled, multicentre phase II study. Eur. J. Cancer 2021, 150, 108–118. [Google Scholar] [CrossRef]
- Ingangi, V.; De Chiara, A.; Ferrara, G.; Gallo, M.; Catapano, A.; Fazioli, F.; Di Carluccio, G.; Peranzoni, E.; Marigo, I.; Carriero, M.V.; et al. Emerging Treatments Targeting the Tumor Microenvironment for Advanced Chondrosarcoma. Cells 2024, 13, 977. [Google Scholar] [CrossRef]
- Wei, R.; Dean, D.C.; Thanindratarn, P.; Hornicek, F.J.; Guo, W.; Duan, Z. Cancer testis antigens in sarcoma: Expression, function and immunotherapeutic application. Cancer Lett. 2020, 479, 54–60. [Google Scholar] [CrossRef]
- Bluman, E.M.; Coulie, P.G.; Xiaojuan, S.; Machan, J.; Lin, C.; Meitner, P.A.; Block, J.A.; Terek, R.M. Lysis of human chondrosarcoma cells by cytolytic T lymphocytes recognizing a MAGE-A3 antigen presented by HLA-A1 molecules. J. Orthop. Res. 2007, 25, 678–684. [Google Scholar] [CrossRef]
- Pollack, S.M.; Li, Y.; Blaisdell, M.J.; Farrar, E.A.; Chou, J.; Hoch, B.L.; Loggers, E.T.; Rodler, E.; Eary, J.F.; Conrad, E.U., 3rd; et al. NYESO-1/LAGE-1s and PRAME are targets for antigen specific T cells in chondrosarcoma following treatment with 5-Aza-2-deoxycitabine. PLoS ONE 2012, 7, e32165. [Google Scholar] [CrossRef]
- Torabi, A.; Amaya, C.N.; Wians, F.H., Jr.; Bryan, B.A. PD-1 and PD-L1 expression in bone and soft tissue sarcomas. Pathology 2017, 49, 506–513. [Google Scholar] [CrossRef]
- Kostine, M.; Cleven, A.H.; de Miranda, N.F.; Italiano, A.; Cleton-Jansen, A.M.; Bovee, J.V. Analysis of PD-L1, T-cell infiltrate and HLA expression in chondrosarcoma indicates potential for response to immunotherapy specifically in the dedifferentiated subtype. Mod. Pathol. 2016, 29, 1028–1037. [Google Scholar] [CrossRef]
- Paoluzzi, L.; Cacavio, A.; Ghesani, M.; Karambelkar, A.; Rapkiewicz, A.; Weber, J.; Rosen, G. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin. Sarcoma Res. 2016, 6, 24. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Chimeric antigen receptor T (CAR-T) cell immunotherapy for sarcomas: From mechanisms to potential clinical applications. Cancer Treat. Rev. 2020, 82, 101934. [Google Scholar] [CrossRef]
- Li, B.; Li, G.; Yan, X.; Zhu, D.; Lin, P.P.; Wang, Z.; Qu, H.; He, X.; Fu, Y.; Zhu, X.; et al. Fresh Tissue Multi-omics Profiling Reveals Immune Classification and Suggests Immunotherapy Candidates for Conventional Chondrosarcoma. Clin. Cancer Res. 2021, 27, 6543–6558. [Google Scholar] [CrossRef]
- Iseulys, R.; Anne, G.B.; Corinne, B.; Gonzague, D.B.P.; Marie, K.; Jean-Yves, B.; Aurelie, D. The immune landscape of chondrosarcoma reveals an immunosuppressive environment in the dedifferentiated subtypes and exposes CSFR1+ macrophages as a promising therapeutic target. J. Bone Oncol. 2020, 20, 100271. [Google Scholar] [CrossRef]
- Sheikh, T.N.; Chen, X.; Xu, X.; McGuire, J.T.; Ingham, M.; Lu, C.; Schwartz, G.K. Growth Inhibition and Induction of Innate Immune Signaling of Chondrosarcomas with Epigenetic Inhibitors. Mol. Cancer Ther. 2021, 20, 2362–2371. [Google Scholar] [CrossRef]
- Lacuna, K.P.; Ingham, M.; Chen, L.; Das, B.; Lee, S.M.; Ge, L.; Druta, M.; Conley, A.P.; Keohan, M.L.; Agulnik, M.; et al. Correlative results from NCI CTEP/ETCTN 10330: A phase 2 study of belinostat with SGI-110 (guadecitabine) or ASTX727 (decitabine/cedazuridine) for advanced conventional chondrosarcoma (cCS). J. Clin. Oncol. 2024, 42, 11526. [Google Scholar] [CrossRef]
- Strauss, S.J.; Hindi, N.; Palmerini, E.; Martínez-Trufero, J.; Lopez-Pousa, A.; Carrasco-Garcia, I.; Gonzalez-Billalabeitia, E.; Moura, D.S.; Ramos, R.; Tirabosco, R.; et al. ImmunoSarc II master trial (phase II of sunitinib and nivolumab): Results from the dedifferentiated chondrosarcoma (DDCS) cohort—A GEIS, ISG and UCL study. J. Clin. Oncol. 2024, 42, 11506. [Google Scholar] [CrossRef]
- Micaily, I.; Roche, M.; Ibrahim, M.Y.; Martinez-Outschoorn, U.; Mallick, A.B. Metabolic Pathways and Targets in Chondrosarcoma. Front. Oncol. 2021, 11, 772263. [Google Scholar] [CrossRef] [PubMed]
- Pathmanapan, S.; Poon, R.; De Renshaw, T.B.; Nadesan, P.; Nakagawa, M.; Seesankar, G.A.; Loe, A.K.H.; Zhang, H.H.; Guinovart, J.J.; Duran, J.; et al. Mutant IDH regulates glycogen metabolism from early cartilage development to malignant chondrosarcoma formation. Cell Rep. 2023, 42, 112578. [Google Scholar] [CrossRef]

| Genetic Alteration | Frequency | CS Subtype(s) | Functional Impact | Therapeutic Implications |
|---|---|---|---|---|
| IDH1/2 mutations | ~80% | Conventional, Dedifferentiated | Epigenetic reprogramming via D-2HG accumulation | Targeted by IDH inhibitors |
| TP53 mutations | ~25–30% (high-grade) | Dedifferentiated | Loss of tumor suppressor function, genomic instability | Potential MDM2 inhibitors |
| PTEN Deletion | Associated with a TP53 mutation | Recurrent dedifferentiated | Increased proliferative index | Research is ongoing |
| COL2A1 mutations | Frequent in conventional CS | Conventional | ECM dysregulation and excess type II collagen | Research is ongoing |
| HEY1–NCOA2 fusion | ~85% in mesenchymal CS | Mesenchymal | Oncogenic transcriptional program activation | Targeting fusion-specific pathways (research ongoing) |
| IRF2BP2-CDX1 fusion | Rare | Mesenchymal | Tumorigenesis likely via TP53 and IRF2 | Research is ongoing |
| CDK4 amplification | Common in high-grade and dedifferentiated CS | High-grade and dedifferentiated | Cell cycle dysregulation via inhibition of the Rb pathway | CDK4 inhibitors (e.g., Palbociclib) |
| RB1 deletion | Common in high-grade and dedifferentiated CS | High-grade and dedifferentiated | Loss of cell cycle progression regulation | CDK4 inhibitors (e.g., Palbociclib); Research is ongoing |
| MYC amplification | Detected in high-grade CS | High-grade | Promotes proliferation and survival | MYC-targeted strategies (experimental) |
| CDKN2A deletion | Common in dedifferentiated CS | Dedifferentiated | Loss of cell cycle checkpoint control | Potential for CDK4/6 inhibitors |
| SAS amplification | Rare | High-grade and dedifferentiated | Tumorigenesis via an unknown mechanism | Research is ongoing |
| GLI amplification | Rare | Conventional and dedifferentiated | Enhances survival and differentiation via the HH pathway activation | HH pathways inhibitors (e.g., Vismodegib) |
| PTHLH amplification | Observed in high-grade CS | Dedifferentiated | Promotes chondrocyte growth, differentiation, and possibly tumor progression | Research is ongoing |
| PPFIBP1 amplification | Observed in high-grade CS | Dedifferentiated | Invasiveness and metastasis via S100A4 upregulation | Research is ongoing |
| INK4A/INK4A-ARF deletion | Common in dedifferentiated CS | Dedifferentiated | Loss of tumor suppressors CDKN2A/CDKN2C and p16, results in unchecked cell cycle progression | Potential for CDK4/6 inhibitors |
| RPS6 deletion | Rare | Dedifferentiated | Disrupts cell growth regulation through the ribosomal protein pathway | Research is ongoing |
| EXTL2 deletion | Observed in secondary peripheral CS | Secondary peripheral | Contributes to osteochondroma transformation into secondary peripheral CS | Research is ongoing |
| EXT1/2 mutations | Observed in secondary peripheral CS | Secondary peripheral | Impaired heparan sulfate biosynthesis; Facilitates osteochondroma transformation into secondary peripheral CS | Research is ongoing |
| EPAS1 amplification | Reported in high-grade CS | High-grade | Promotes drug resistance via HIF-2α | HIF-2α inhibitors (e.g., PT2385, PT2977) |
| SIRT1 | Highly expressed in high-grade and dedifferentiated CS | High-grade and dedifferentiated | HIF-2α activation, metastasis | Research is ongoing |
| Hedgehog mutations | Rare | Conventional and dedifferentiated | Activates GLI TFs, enhancing survival and differentiation | HH pathway inhibitors (e.g., Vismodegib) |
| Therapeutic Class | Agent(s) | Target | Preclinical/Clinical Status | CS Subtype/Context |
|---|---|---|---|---|
| IDH Inhibitors | Ivosidenib, Enasidenib | IDH1/2 mutations | Phase I/II/III clinical trials in IDH-mutant tumors (NCT04278781; NCT04278781; NCT06127407) | IDH1/2-mutant conventional and dedifferentiated CS |
| HDAC Inhibitors | Panobinostat, Belinostat, Vorinostat, Romidepsin | Epigenetic dysregulation (histone deacetylation) | Preclinical; Phase II clinical trial (NCT04340843) | Conventional and dedifferentiated CS; unresectable or metastatic conventional CS |
| DMNT Inhibitors | 5-aza-2′-deoxycytidine, Decitabine, Guadecitabine | Epigenetic dysregulation (DNA methyltransferase) | Phase II clinical trial (NCT04340843) | Unresectable or metastatic conventional CS |
| CDK4/6 Inhibitors | Palbociclib | CDK4 amplification, pRB pathway | Preclinical evidence in CS | Dedifferentiated CS |
| HIF-2α Inhibitors | TC-S7009 | HIF-2α | Preclinical | High-grade CS expressing HIF-2α |
| SIRT1 Inhibitors | EX527 | SIRT1 | Preclinical | Conventional CS |
| Immune Checkpoint Inhibitors | Pembrolizumab, Nivolumab, Sintilimab | PD-1/PD-L1 axis | Clinical trial (NCT02301039); in vivo study | Dedifferentiated and immune-enriched CS subtypes |
| CAR-T Cells | CSPG4-directed CAR-T cells | CSPG4 antigen | Preclinical efficacy demonstrated in CS models | CSPG4-expressing CS |
| TRAIL Receptor Agonists | TRAIL/DR5 agonists (e.g., Dulanermin) | TRAIL receptors, e.g., DR5 | Preclinical studies | Metastasis CS patient |
| DR5 | DR5 Ab agonists, e.g., anti-DR5 IgM, INBRX-109 | DR5 | Phase I/II clinical trials (NCT04553692; NCT03715933; NCT03277924; NCT04950075) | Conventional CS; unresectable or metastatic conventional CS |
| Hedgehog Pathway Inhibitors | HPI-4, IPI-929 | SMO; Hedgehog-GLI signaling | Preclinical | Conventional CS |
| VEGFR Inhibitors | Pazopanib, Regorafenib | VEGFRs | Phase II clinical trial: NCT01330966 | Unresectable and metastatic conventional CS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, R.L.; Hornicek, F.J.; Duan, Z. Advances in the Molecular Biology of Chondrosarcoma for Drug Discovery and Precision Medicine. Cancers 2025, 17, 2689. https://doi.org/10.3390/cancers17162689
Walker RL, Hornicek FJ, Duan Z. Advances in the Molecular Biology of Chondrosarcoma for Drug Discovery and Precision Medicine. Cancers. 2025; 17(16):2689. https://doi.org/10.3390/cancers17162689
Chicago/Turabian StyleWalker, Robert Lee, Francis J. Hornicek, and Zhenfeng Duan. 2025. "Advances in the Molecular Biology of Chondrosarcoma for Drug Discovery and Precision Medicine" Cancers 17, no. 16: 2689. https://doi.org/10.3390/cancers17162689
APA StyleWalker, R. L., Hornicek, F. J., & Duan, Z. (2025). Advances in the Molecular Biology of Chondrosarcoma for Drug Discovery and Precision Medicine. Cancers, 17(16), 2689. https://doi.org/10.3390/cancers17162689







